Abstract
Background
Excess body mass index (BMI) is associated with increased risk of cancer. To inform public health policyand future research, we estimated the global burden of cancer attributable to excess BMI.
Methods
Population attributable fractions (PAFs) were derived using relative risks and BMI estimates in adults by age, sex and country. Assuming a10-year lag-period, PAFs were calculated using BMI estimates in 2002. GLOBOCAN2012 was used to compute numbers of new cancer cases attributable to excess BMI. In an alternative scenario, we computed the proportion of potentially avoidable cancers assuming that populations maintained their BMI-level observed in 1982. Secondary analyses were performed to test the model and estimate the impactof hormone replacement therapy (HRT) and smoking.
Findings
Worldwide, we estimated that 481,000 or 3·6% of all new cancer cases in 2012 were attributable to excess BMI. PAFs were greater in women compared with men (5·4% versus 1·9%). The burden was concentrated in countries with very high and high human development index (HDI, PAF: 5·3% and 4·8%) compared with countries with moderate and low HDI (PAF: 1·6% and 1·0%). Corpus uteri, post-menopausal breast and colon cancers accounted for approximately two-thirds (64%) of excess BMI attributable cancers. One fourth (~118,000) of all cases related to excess BMI in 2012 could be attributed to the rising BMI since 1982.
Interpretation
These findings further underpin the need for a global effort to abate the rising trends in population-level excess weight. Assuming that the relationship between excess BMI and cancer is causal and the current pattern of population weight gain continues, this will likely augment the future burden of cancer.
Funding
World Cancer Research Fund, Marie Currie Fellowship, the National Health and Medical Research Council Australia and US NIH.
Keywords: cancer incidence, global, obesity, population attributable fraction
Introduction
Excess body mass index (BMI≥25kg/m2) is a known risk factor for various chronic diseases and mortality. Although wide variations exist in its prevalence, overweight and obesity have been increasing globally, raising concerns of their impacts on health. Recent global statistics showed that 35%of the adult population (age 20+)is overweight (BMI ≥25kg/m2) and 12% obese (BMI ≥30 kg/m2).1While the current prevalence of excess BMI is around 10% in many Asian and African countries, the highest prevalence of over 90% has been reported in Pacific Nations such as Cook island and Nauru followed by other developed countries. According to recent estimates,1,2 the global prevalence of excess BMI in adults has increased by 27.5% between 1980 and 2013, although the upward tendency may have slowed down in recent years in some European countries and in the US.3-7
Continuous updates of the literature have confirmed the association between excess BMI and risk of oesophageal adenocarcinoma, colon, rectal, kidney, pancreas, gallbladder (females only), post-menopausal breast, ovarian and endometrial cancer.8-13 The estimated increase in risk of these cancers due to excess BMI ranged from 3 to 10% per unit increase in BMI.14 A recent estimate from Global Burden of Disease project reported that 3·9% of cancer mortality in 2010 can be attributed to high BMI.15 Yet this estimate did nottake into account lag-time for the excess BMI to lead to the development of a new cancer case. In addition, relatingrisk factor to mortality in the estimation of disease burden may be problematic due to the potential role of reverse causation.16 Consideration should also be given to confounders and effect modifiers of the BMI and cancer association such as the use of hormone replacement therapy (HRT) and smoking and their impact on both BMI and cancer.17,18
This study aims to estimate the global population attributable fraction (PAF) of cancer incidence in 2012 attributable to excess BMI in 2002, acknowledging the time-lag factor between the exposure (excess BMI) and outcomes (cancer incidence). The robustness of the estimates will be tested in a series of sensitivity analyses, amongst which assess the role of smoking and HRT as potential effect modifiers and/or confounders.
Methods
Body mass index (BMI)
This study used the estimated BMI reported by Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (GBMRF). The details of the applied model and its assumptions in estimating mean BMI have been published elsewhere.19 For this study, we obtained the annual estimates of mean BMI and the corresponding standard deviations for adults aged 20+ years for each country by sex and age group (20-34, 35-44, 45-54, 55-64, 65-74, 75+ years) in 1982and 2002 (see appendix i for more details).
Relative risk estimates
In our primary analysis, we included only cancers reported by the World Cancer Research Fund (WCRF) as having sufficient evidence to be associated with excess BMI.8-13 These include oesophageal adenocarcinoma, colon, rectal, kidney, pancreatic, gallbladder, postmenopausal breast, corpus uteri and ovarian cancers and are collectivelydefined here as obesity-related cancers.
Given the differences in risk of colon and rectal cancer associated with obesity, PAFs were estimated separately for the two sites. Similarly, only adenocarcinoma of the oesophagus was included because of lack of association between excess BMI and oesophageal squamous cell carcinoma. The sex-specific relative risks (RR) for the sites included in the analysis were obtained from the published standardized meta-analysis estimates by Renehan et al.14 and the WCRF Continuous Update Project (CUP). In these meta-analyses, risk estimates were pooled from cohort studies and also for studies that have used cancer incidence as outcome i.e. excluding mortality from cancer.
Our secondary analysis included thyroid cancer and Non-Hodgkin lymphoma as additional cancer sites, which have recently been suggested to be associated with excess BMI but were not listed by WCRF as having sufficient evidence. The exact source and size of relative risks (RR) are described in appendix ii.
Population attributable fraction (PAF)
The population attributable fraction (PAF) was calculated based on the approach suggested by the Comparative Risk Assessment Collaborative Group, using the following formula20:
Where P(x) is the population distribution of BMI, P*(x) is the distribution of theoretical minimum BMI and RR(x) the relative risk of cancer associated with BMI at level x. The theoretical minimum distribution of BMI was defined as a BMI distribution with a mean of 22 kg/m2 and a standard deviation of 1, where the disease burden is assumed to be lowest at the population level.15,21
A log-logit function was used to characterize the shape of the RR across BMI units. Furthermore, since RR estimates beyond these points were scant, no risk for BMI below 22 and no risk increase above BMI 40 were assumed. A pictorial illustration and more detailed description of these assumptions of the risk function are presented in appendix ii.
Age-, sex- and country-specific PAFs were calculated for individual obesity- related cancer sites. The number of cancer cases attributable to excess BMI was then derived by multiplying age-, sex-, country-, and cancer-specific PAFs by the corresponding incident cancers in 2012. Overall country, region and global estimates of the total attributable proportion of cancer related to excess BMI were calculated by summing up the number of attributable incident cases and dividing them by the total number of cancer cases in each subgroup.
Uncertainty limits for PAF were estimated using Monte-Carlo simulation. We also computed a counterfactual scenario to provide a more realistic view regarding the preventable fraction of the current burden of cancers which was caused by excess BMI. The analysis was done by replacing the theoretical minimum distribution with the BMI distribution that was observed in 1982, an attainable value in the past in each country and probably a more realistic goal for prevention. Here we estimated the answer to the question: what would PAF be if BMI level had stayed constant at its 1982 value. A more detailed description of PAF inputs and calculation is presented in appendix i-v.
Cancer incidence and attributable cancer burden
The numbers of incident cancers in 2012 by age (30+), sex and country were obtained from GLOBOCAN 2012.22 Countries were grouped into 12 geographical regions: sub-Saharan Africa(eastern, middle, southern, and western Africa); Middle East and north Africa (western Asia and northern Africa);23 Latin America and the Caribbean (central and southern America and the Caribbean); North America; East Asia (eastern Asia, including China); southeast Asia; south-central Asia (southern Asia, including India); eastern Europe; northern Europe; southern Europe; western Europe; and Oceania (including Australia and New Zealand). Furthermore, countries were grouped into four categories according to their human development index in 2012 (very high, high, moderate, and low HDI).24 As the incidence of colon and rectal cancers (separately) and oesophageal cancer by histological subtypes are not reported in GLOBOCAN,22 the number of cancersby subtypes were estimated based on country-and sex-specific proportions of subtypes reported in Cancer Incidence in Five Continents volume X (CI5 X),25,26 see also appendix iii.
The precise lag-time between the exposureto excess BMI and the occurrence of cancer is not well-established. However, there is a general perception that excess BMI is not an initiator of cancer but rather a promoter of cancer to clinical presentation over several years. Renehan et al.27assumed a 10-year lag time based on theliterature where the averagefollow-up time of 10 years demonstrated the beneficial effect of weight loss onsubsequent cancer.28,29 With no additional information available, a 10-year lag period was assumed in this study,mapping BMI prevalence in 2002 (by sex, age and country) to the cancer incidence in 2012.
Sensitivity analyses
While estimating the PAF of cancers attributable to excess BMI, several assumptions regarding the population BMI distribution and relative risks functionwere made. In order to assess their influence on the results, analyses were repeated by changing thefollowing assumptions: exposure definition (categorical vs. continuous BMI, appendix vi); BMI distribution (normal vs. log-normal, appendix vii); shape of the RR (linear/log-linear vs. log-logit, appendix viii); region-specific vs. global RR estimates (appendix ix). As smoking30-33 and hormone replacement therapy (HRT)8,11,34 are known effect modifiers of the BMI-cancer association, we estimated PAF stratified by current smoking status (for pancreatic cancer) or HRT use (for postmenopausal breast, ovarian and endometrial cancer)and assessed the bias that may have occurred when ignoring these interactions (appendix x). Furthermore, as studies have shown a protective effect of high BMI on premenopausal breast cancer8,14, we also assessed the potentially adverse effects of decreasing population BMI on premenopausal breast cancer incidence and its impact on the total PAF (appendix xi).
Role of the funding source
The sponsor of the study had no role in study design,data collection, data analysis, data interpretation, orwriting of the report. The corresponding author had fullaccess to all the data in the study and had finalresponsibility for the decision to submit for publication.
Results
Globally,in 2012, there were an estimated3·6% or 481,000 new cancer cases of all cancers attributable to excess body mass index in adults, 1·9% or 136,000 cases in males and 5·4% or 345,000 cases in females (Table 1a and 1b). The burden was larger in countries with very high and high HDI (PAF: 5·3% and 4·8% respectively) compared to countries with moderate and low HDI (PAF: 1·6% and 1·0% respectively). Region-specific estimates showthat all three Asian regions and Sub-Saharan Africa had the lowest PAF ranging from0·4 to 0·9% of total cancers (4% to 6% of total obesity-related cancers) in males and 1·7 to 3·0% of total cancers (5% to 8% of total obesity-related cancers) in females, while it was highest for Northern America (PAF: 3·5% and 9·4% of total cancers; 21 % and 19% of obesity-related cancers, in male and female respectively). For the remaining regions such as Middle East and Northern Africa, Latin America and Caribbean, Oceania and all European regions, the PAF was in the upper range of 2·0 to 9·9% of total cancers (14 to 18% of obesity-related cancers) in both sexes.
Table 1a.
Estimated population attributable fraction (PAF, %) and cancer cases (N) and their 90% uncertainty intervals associated with excess body mass index by world region and cancer site in 2012, males.
| Region | Oesophageal adenocarcinoma |
Colon | Rectum | Pancreas | Kidney | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAF | N | PAF | N | PAF | N | PAF | N | PAF | N | N | PAF obesitya | PAF allb | |
| Sub-Saharan Africa | 15% (11 %-21 %) | 125 (88-169) | 5% (4%-7%) | 330 (235-457) | 3% (2%-4%) | 144 (103-199) | 4% (3%-6%) | 175 (132-227) | 6% (4%-8%) | 94 (68-129) | 867 (625-1181) | 5% (3%-6%) | 0-4% (0-3%-0-5%) |
| Middle East and Northern Africa | 34% (28%-41%) | 391 (323-462) | 16% (14%-18%) | 2111 (1831-2395) | 8% (7%-9%) | 631 (547-719) | 11% (9%-12%) | 697 (608-788) | 20% (17%-22%) | 1150 (1018-1282) | 4980 (4327-5646) | 14% (13%-16%) | 2-0% (1-7%-2-2%) |
| Latin America & Caribbean | 36% (32%-39%) | 1193 (1063-1312) | 15% (13%-17%) | 4058 (3520-4590) | 8% (7%-9%) | 1099 (953-1248) | 10% (9%-11%) | 1294 (1123-1470) | 19% (17%-21%) | 2366 (2080-2639) | 10009 (8739-11258) | 14% (13%-16%) | 2-0% (1-7%-2-2%) |
| North America | 44% (46%-50%) | 4293 (4456-4804) | 21% (20%-22%) | 11453 (10861-12002) | 11% (10%-12%) | 2792 (2638-2941) | 14% (13%-15%) | 3390 (3208-3578) | 25% (24%-26%) | 9801 (9307-10270) | 31729 (30470-33595) | 21% (20%-22%) | 3-5% (3-4%-3-7%) |
| East Asia | 18% (15%-20%) | 1390 (1200-1587) | 7% (6%-8%) | 9120 (7797-10534) | 3% (3%-4%) | 3386 (2888-3926) | 4% (4%-5%) | 2625 (2246-3041) | 8% (7%-9%) | 4878 (4201-5632) | 21398 (18332-24720) | 6% (5%-7%) | 0-9% (0-8%-1-1%) |
| South-East Asia | 14% (10%-19%) | 91 (66-128) | 4% (3%-6%) | 951 (659-1325) | 2% (1%-3%) | 301 (208-421) | 3% (2%-4%) | 165 (111-234) | 6% (4%-8%) | 248 (177-337) | 1756 (1221-2444) | 4% (2%-5%) | 0-5% (0-3%-0-7%) |
| South-Central Asia | 10% (7%-13%) | 389 (266-527) | 4% (3%-5%) | 997 (736-1277) | 2% (1%-2%) | 353 (246-476) | 3% (2%-4%) | 255 (185-332) | 5% (4%-7%) | 481 (350-623) | 2474 (1783-3235) | 4% (3%-5%) | 0-4% (0-3%-0-5%) |
| Eastern Europe | 38% (33%-43%) | 637 (549-716) | 16% (14%-19%) | 6082 (5141-7021) | 8% (7%-10%) | 2586 (2144-3049) | 10% (9%-12%) | 1829 (1538-2130) | 19% (16%-21%) | 4382 (3744-5016) | 15517 (13115-17931) | 14% (12%-16%) | 3-1% (2-6%-3-6%) |
| Northern Europe | 44% (42%-46%) | 2262 (2139-2369) | 18% (17%-20%) | 3756 (3447-4046) | 9% (9%-10%) | 1337 (1221-1452) | 12% (11%-13%) | 845 (769-919) | 22% (20%-23%) | 2042 (1867-2202) | 10242 (9443-10989) | 18% (17%-20%) | 3-8% (3-5%-4-1%) |
| Southern Europe | 43% (40%-46%) | 527 (484-564) | 18% (16%-20%) | 7002 (6295-7667) | 9% (8%-10%) | 1930 (1717-2142) | 12% (11%-13%) | 1379 (1223-1533) | 21% (19%-23%) | 3206 (2873-3514) | 14044 (12593-15421) | 16% (14%-18%) | 3-3% (3-0%-3-6%) |
| Western Europe | 43% (38%-46%) | 1911 (1705-2083) | 19% (17%-21%) | 8536 (7586-9447) | 10% (9%-11%) | 2847 (2529-3167) | 12% (11%-14%) | 1959 (1742-2178) | 22% (20%-24%) | 4984 (4461-5488) | 20236 (18023-22364) | 17% (15%-19%) | 3-3% (3-0%-3-7%) |
| Oceania | 44% (42%-46%) | 361 (340-379) | 19% (18%-20%) | 1212 (1131-1292) | 10% (9%-11%) | 399 (371-427) | 13% (12%-14%) | 233 (216-250) | 23% (21%-24%) | 600 (560-637) | 2804 (2619-2985) | 18% (17%-19%) | 3-4% (3-2%-3-6%) |
| Low HDIc | 8% (3%-15%) | 175 (64-326) | 3% (2%-6%) | 341 (165-634) | 2% (1%-3%) | 127 (61-239) | 2% (1%-4%) | 117 (63-199) | 4% (2%-7%) | 134 (62-243) | 895 (414-1642) | 3% (1%-6%) | 0-3% (0-1%-0-5%) |
| Medium HDI | 16% (14%-19%) | 1703 (1425-1991) | 6% (5%-7%) | 7399 (6037-8894) | 3% (2%-3%) | 2778 (2268-3346) | 4% (3%-5%) | 2340 (1939-2788) | 8% (6%-9%) | 4365 (3675-5135) | 18584 (15343-22154) | 5% (4%-6%) | 0-7% (0-6%-0-8%) |
| High HDI | 34% (30%-38%) | 1735 (1527-1926) | 14% (12%-17%) | 8533 (7289-9775) | 7% (6%-9%) | 3104 (2610-3620) | 10% (8%-11%) | 2717 (2312-3132) | 17% (15%-20%) | 5437 (4695-6178) | 21527 (18433-24630) | 13% (11%-15%) | 2-1% (1-8%-2-4%) |
| Very high HDI | 43% (42%-47%) | 9955 (9664-10857) | 17% (15%-18%) | 39335 (35748-42749) | 8% (8%-9%) | 11795 (10626-12963) | 11% (10%-12%) | 9672 (8787-10560) | 21% (19%-23%) | 24295 (22275-26213) | 95052 (87100-103344) | 16% (15%-17%) | 3-2% (2-9%-3-4%) |
| World | 33% (31%-37%) | 13569 (12680-15100) | 13% (11%-14%) | 55608 (49239-62052) | 6% (5%-7%) | 17804 (15565-20168) | 8% (7%-9%) | 14845 (13100-16680) | 17% (15%-18%) | 34231 (30707-37769) | 136058 (121291-151769) | 12% (11%-13%) | 1-9% (1-7%-2-1%) |
PAF obesity: proportion of obesity-related cancers (i.e. oesophageal adenocarcinoma, pancreas, kidney, colon, rectum)attributable to excess BMI
PAF all cancers: proportion of all cancer (excluding non-melanoma skin cancers) attributable to excess BMI
HDI: Human development index
Table 1b.
Estimated population attributable fraction (PAF, %) and cancer cases (N) and their 90% uncertainty intervals associated with excess body mass index by world region and cancer site in 2012, females.
| Region | Oesophageal adenocarcinoma |
Colon | Rectum | Gallbladder | Pancreas | Breast (postmenopausal) |
Corpus uteri | Ovary | Kidney | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAF | N | PAF | N | PAF | N | PAF | N | PAF | N | PAF | N | PAF | N | PAF | N | PAF | N | N | PAF obesitya | PAF allb | |
| Sub-Saharan Africa | 27% (22%-33%) | 151 (122-182) | 5% (4%-6%) | 302 (235-381) | 2% (2%-3%) | 128 (96-167) | 20% (14%-27%) | 279 (195-373) | 6% (5%-7%) | 216 (176-262) | 6% (5%-8%) | 2759 (2079-3526) | 25% (19%-30%) | 2142 (1678-2619) | 2% (2%-3%) | 252 (178-339) | 11% (8%-15%) | 184 (127-252) | 6413 (4886-8102) | 8% (6%-10%) | 2-0% (1-5%-2-5%) |
| Middle East and Northern Africa | 44% (39%-48%) | 172 (153-189) | 11% (10%-12%) | 1254 (1145-1359) | 6% (5%-6%) | 334 (304-366) | 53% (48%-58%) | 1501 (1364-1631) | 12% (11%-13%) | 514 (470-556) | 15% (13%-17%) | 6760 (5912-7598) | 48% (45%-51%) | 4609 (4346-4881) | 6% (6%-7%) | 617 (547-691) | 35% (33%-37%) | 1256 (1181-1332) | 17018 (15421-18602) | 18% (16%-20%) | 7-0% (6-4%-7-7%) |
| Latin America & Caribbean | 41% (38%-44%) | 507 (473-537) | 9% (8%-10%) | 2670 (2415-2913) | 5% (4%-5%) | 603 (546-662) | 48% (44%-53%) | 4930 (4504-5361) | 10% (9%-10%) | 1377 (1255-1497) | 12% (11%-13%) | 12401 (11024-13797) | 42% (39%-44%) | 8022 (7424-8554) | 5% (5%-6%) | 900 (811-991) | 30% (28%-32%) | 2269 (2102-2425) | 33678 (30553-36736) | 16% (14%-17%) | 6-4% (5-8%-6-9%) |
| North America | 48% (47%-49%) | 706 (686-723) | 11% (11%-11%) | 6052 (5801-6276) | 6% (5%-6%) | 1009 (963-1053) | 53% (50%-57%) | 3131 (2959-3311) | 11% (11%-12%) | 2625 (2527-2715) | 14% (13%-15%) | 29741 (27095-32111) | 48% (47%-49%) | 26082 (25397-26677) | 7% (6%-7%) | 1554 (1469-1633) | 34% (33%-35%) | 8084 (7848-8296) | 78984 (74746-82796) | 19% (18%-20%) | 9-4% (8-9%-9-8%) |
| East Asia | 22% (19%-23%) | 582 (524-632) | 4% (4%-4%) | 4229 (3807-4668) | 2% (2%-2%) | 1228 (1103-1360) | 25% (22%-28%) | 10285 (9018-11500) | 4% (4%-4%) | 1796 (1617-1981) | 5% (4%-6%) | 8143 (7105-9252) | 20% (17%-21%) | 16920 (15093-18511) | 2% (2%-2%) | 965 (852-1087) | 14% (12%-15%) | 4045 (3657-4445) | 48192 (42775-53435) | 8% (7%-9%) | 3-0% (2-6%-3-3%) |
| South-East Asia | 22% (18%-26%) | 54 (45-64) | 4% (3%-4%) | 694 (581-820) | 2% (1%-2%) | 174 (146-208) | 23% (17%-29%) | 819 (616-1034) | 3% (3%-4%) | 200 (166-238) | 5% (4%-6%) | 2959 (2447-3511) | 19% (16%-22%) | 2842 (2415-3265) | 2% (2%-3%) | 399 (333-473) | 13% (11%-15%) | 321 (271-375) | 8465 (7019-9987) | 6% (5%-7%) | 2-2% (1-9%-2-6%) |
| South-Central Asia | 18% (14%-21%) | 289 (237-346) | 3% (3%-4%) | 636 (529-754) | 1% (1%-2%) | 227 (182-278) | 13% (10%-17%) | 2249 (1708-2916) | 3% (3%-4%) | 237 (191-288) | 4% (3%-5%) | 4736 (3755-5796) | 17% (13%-20%) | 3241 (2593-3908) | 2% (1%-2%) | 540 (426-667) | 12% (10%-14%) | 526 (440-617) | 12682 (10061-15569) | 5% (4%-7%) | 1-7% (1-3%-2-1%) |
| Eastern Europe | 46% (43%-50%) | 228 (209-243) | 11% (10%-12%) | 4543 (4121-4939) | 5% (5%-6%) | 1371 (1216-1527) | 53% (48%-57%) | 3450 (3133-3737) | 11% (10%-12%) | 1796 (1622-1961) | 13% (12%-15%) | 13337 (11582-15010) | 45% (41%-48%) | 18745 (17264-19981) | 6% (5%-7%) | 1639 (1450-1827) | 33% (30%-35%) | 5317 (4906-5705) | 50425 (45503-54931) | 18% (16%-20%) | 9-9% (8-9%-10-7%) |
| Northern Europe | 44% (42%-45%) | 579 (558-598) | 9% (9%-10%) | 1829 (1716-1936) | 5% (4%-5%) | 441 (413-471) | 47% (44%-51%) | 656 (607-707) | 10% (9%-10%) | 695 (654-733) | 12% (11%-13%) | 7513 (6790-8246) | 42% (40%-43%) | 5707 (5440-5947) | 6% (5%-6%) | 544 (504-584) | 30% (28%-31%) | 1728 (1640-1808) | 19693 (18321-21031) | 15% (14%-16%) | 7-9% (7-4%-8-4%) |
| Southern Europe | 44% (42%-46%) | 111 (105-116) | 10% (9%-10%) | 2943 (2737-3138) | 5% (5%-5%) | 647 (596-698) | 49% (45%-53%) | 2193 (2027-2356) | 10% (9%-10%) | 1122 (1042-1197) | 12% (11%-13%) | 9096 (8103-10074) | 41% (39%-43%) | 8013 (7509-8452) | 5% (5%-6%) | 672 (610-733) | 30% (28%-31%) | 2231 (2089-2359) | 27028 (24818-29123) | 15% (14%-17%) | 8-1% (7-5%-8-7%) |
| Western Europe | 42% (39%-45%) | 416 (388-443) | 9% (9%-10%) | 3707 (3416-4019) | 5% (4%-5%) | 875 (802-956) | 49% (45%-53%) | 2631 (2438-2843) | 10% (9%-10%) | 1533 (1420-1654) | 11% (10%-13%) | 14582 (12956-16283) | 41% (38%-43%) | 9562 (8951-10162) | 5% (5%-6%) | 758 (687-835) | 29% (27%-31%) | 3791 (3603-4105) | 37854 (34662-41301) | 15% (13%-16%) | 7-8% (7-1%-8-5%) |
| Oceania | 44% (42%-45%) | 67 (64-70) | 10% (9%-10%) | 592 (561-622) | 5% (5%-5%) | 122 (115-129) | 49% (46%-53%) | 222 (208-237) | 10% (9%-10%) | 158 (150-165) | 12% (11%-14%) | 1740 (1575-1908) | 42% (41%-44%) | 1287 (1231-1339) | 6% (5%-6%) | 108 (101-116) | 31% (29%-32%) | 428 (411-445) | 4722 (4416-5031) | 15% (14%-16%) | 7-2% (6-7%-7-6%) |
| Low HDIc | 14% (8%-20%) | 150 (86-221) | 3% (2%-4%) | 253 (143-384) | 1% (1%-2%) | 104 (60-158) | 12% (6%-19%) | 882 (444-1394) | 3% (2%-5%) | 138 (84-202) | 4% (3%-6%) | 2980 (1955-4152) | 17% (11%-24%) | 2005 (1289-2755) | 2% (1%-2%) | 301 (174-455) | 9% (5%-14%) | 234 (136-350) | 7048 (4370-10070) | 5% (3%-8%) | 1-5% (0-9%-2-1%) |
| Medium HDI | 23% (20%-25%) | 850 (760-932) | 4% (4%-5%) | 4112 (3615-4644) | 2% (2%-2%) | 1279 (1119-1452) | 23% (20%-26%) | 10150 (8776-11546) | 4% (4%-5%) | 1774 (1561-1996) | 6% (5%-7%) | 15466 (13194-17864) | 21% (18%-23%) | 21979 (19414-24325) | 2% (2%-3%) | 1814 (1563-2084) | 14% (13%-16%) | 4052 (3622-4500) | 61478 (53624-69342) | 8% (7%-9%) | 2-7% (2-4%-3-1%) |
| High HDI | 41% (39%-44%) | 796 (741-844) | 10% (9%-11%) | 6539 (5945-7100) | 5% (4%-6%) | 1845 (1647-2043) | 49% (45%-53%) | 6285 (5732-6823) | 10% (9%-11%) | 2692 (2446-2927) | 13% (11%-14%) | 24317 (21339-27228) | 44% (40%-46%) | 25125 (23184-26784) | 6% (5%-6%) | 2424 (2160-2691) | 32% (29%-34%) | 6604 (6115-7061) | 76626 (69310-83501) | 17% (15%-18%) | 7-5% (6-8%-8-2%) |
| Very high HDI | 44% (42%-46%) | 2066 (1976-2148) | 9% (8%-9%) | 18547 (17361-19696) | 4% (4%-5%) | 3933 (3654-4221) | 42% (38%-45%) | 15029 (13823-16244) | 9% (8%-10%) | 7665 (7198-8123) | 12% (11 %-13%) | 71005 (63936-77866) | 42% (40%-44%) | 58061 (55455-60433) | 5% (5%-6%) | 4409 (4071-4749) | 30% (28%-31%) | 19288 (18401-20251) | 200003 (185876-213731) | 15% (14%-17%) | 7-8% (7-2%-8-3%) |
| World | 34% (31%-36%) | 3862 (3564-4144) | 8% (7%-8%) | 29451 (27065-31824) | 4% (3%-4%) | 7160 (6480-7874) | 32% (29%-36%) | 32346 (28776-36007) | 8% (7%-8%) | 12269 (11289-13248) | 10% (9%-11%) | 113767 (100424-127111) | 34% (32%-36%) | 107172 (99342-114297) | 4% (4%-4%) | 8948 (7967-9978) | 26% (24%-28%) | 30179 (28273-32162) | 345154 (313180-376644) | 13% (12%-14%) | 5-4% (4-9%-5-9%) |
PAF obesity: proportion of obesity-related cancers (i.e. oesophageal adenocarcinoma, pancreas, kidney, postmenopausal breast, ovary, corpus uteri, gallbladder, colon, rectum)attributable to excess BMI
PAF all cancers: proportion of all cancer (excluding non-melanoma skin cancers) attributable to excess BMI
HDI: Human development index
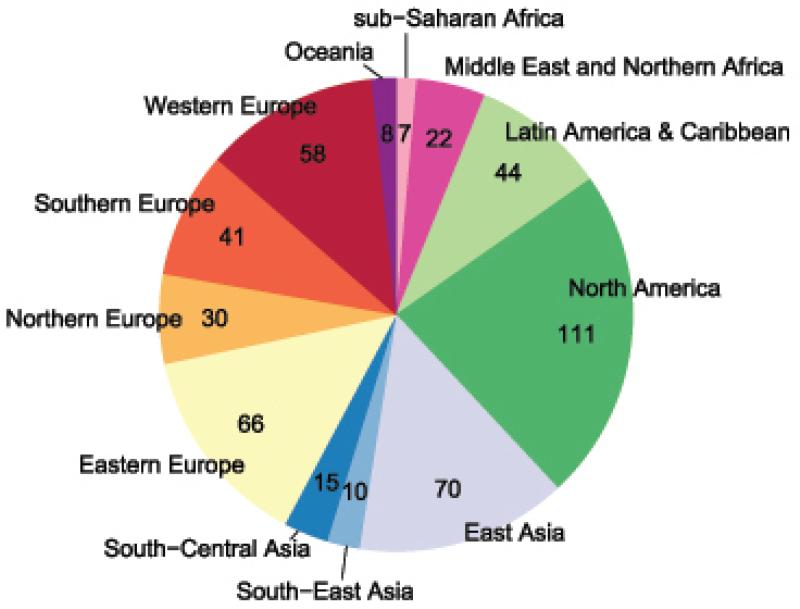
In terms of regional contribution to current obesity related-cancer, the North American region contributed by far the most cases with 111,000 cancers (23% of the total global cancer attributable to excess BMI)and Sub-Saharan Africa contributed the least (7,300 cancers or 1·5% of the total cancers attributable to excess BMI, Figure 1). Eastern Europe had the greatest share of burden (66,000 cancers) among the European regions (34% of the total European cases attributable to excess BMI). Despite the low PAF (1·8%), East Asia ranked second in the share of the regional cases attributable to excess BMI (70,000 cancers).
Figure 1.
Cancer cases (in thousands) attributable to excess body mass index by world region in 2012
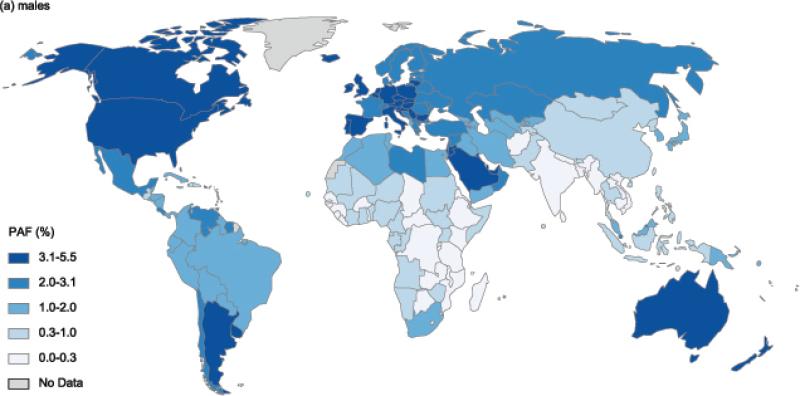
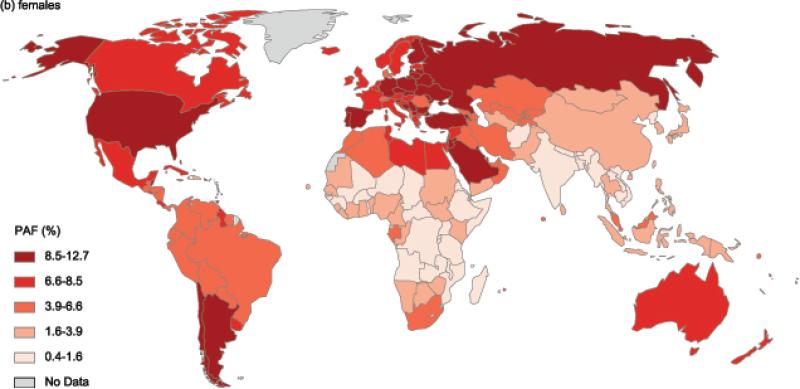
Country-specific PAFs for males and females are illustrated in Figure 2a and Figure 2b (see also appendix xiii for specific estimates). In males, the highest PAF of 5·5% was observed in Czech Republic, followed by 4·5% in Jordan and Argentina and 4·4% in the UK and Malta. The greatest cross country differences within a region were observed in the Latin American region, where PAF ranged from 4·5% (in Argentina) to 0·7% (in Haiti and Jamaica). In females, Barbados led all countries with 12·7% of cancers attributable to excess BMI; other countries or territories where PAFs in females were remarkably high were Czech Republic (12·0%) and Puerto Rico (11·6%). As in males, cross country differences were largest in Latin America, where PAF ranged from as high as 12·7% (in Barbados) to as low as 1·6% (in Haiti). Meanwhile, countries within the sub-Saharan African region had consistently lower overall PAF of below 2% in males and below 4% (with the exception of Mauritius and South Africa) in females.
Figure 2.
Population attributable fractionofnew cancer cases due to excess body mass index in 2012 in (a) males and (b) females
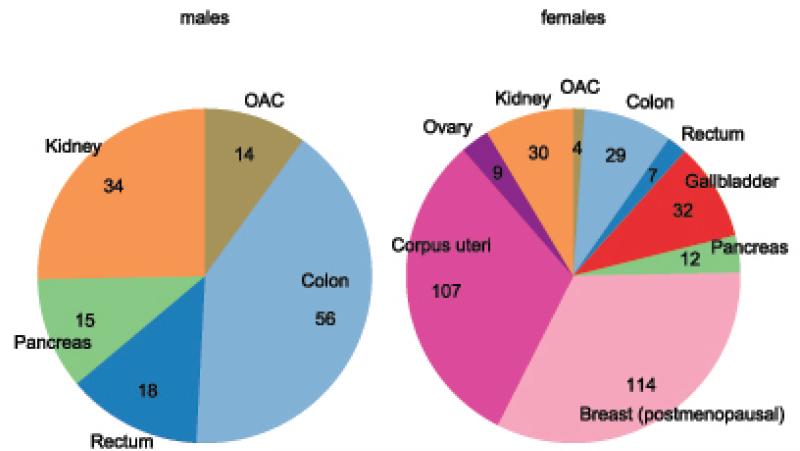
Population attributable fraction also varied greatly by cancer sites ranging from 6%for rectal cancer to 33% for oesophageal adenocarcinoma in males and 4%for rectal cancer to 34% for cancer of the corpus uteriand oesophageal adenocarcinoma in females (Table 1a and 1b). Despite having alarge estimated PAF, oesophageal adenocarcinoma contributed only 10% of the total attributable cases in males and 1% in females (Figure 3a and 3b).Meanwhile, colon cancer in malesand postmenopausal breast cancerin females contributed the largest number of cancer cases attributable to excess BMI. In men, colon and kidney cancer together contributed two thirdsof attributed cancers (90,000cases) and in females, post-menopausal breast cancer and corpus uteri contributed about two thirds of the total cancer burden attributed to excess BMI (221,000 cases).
Figure 3.
Estimated number of cancer cases (in thousands) attributable to excess body mass index in 2012 by cancer site in males and female OAC = oesophageal adenocarcinoma
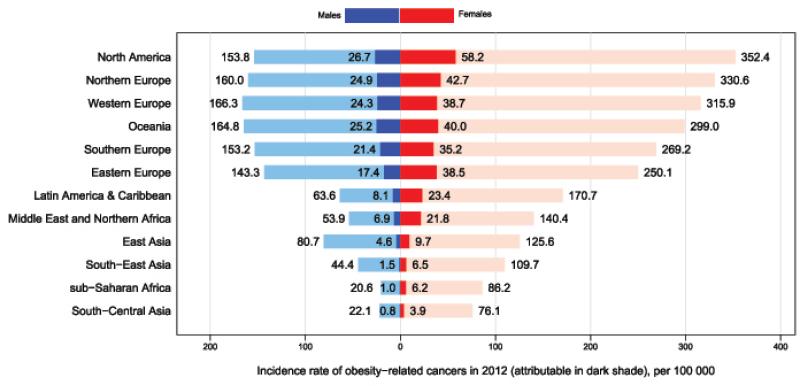
Marked gender differences in PAF were observed for colon cancer (males:13% and females:8%). Meanwhile, gender differentials in the number of cases were largest for colon cancer and oesophageal adenocarcinoma with 56,000 and 14,000 attributable cases respectively in males and only 29,000 and 4,000 attributable cases in females (Table 1a and 1b). The rate of cancer burden attributed to excess BMI was relatively higher for women compared to men in all regions (Figure 4). In particular, regions with lower burden of obesity-related cancers such as Asia and sub-Saharan Africa, the cancer rate attributable to excess BMI was 2-3 folds greater among females compared to males.
Figure 4.
Age-standardised incidence rate of obesity-related cancers (per 100,000, standardised to the world standard population, light bars) and the fraction attributable to excess body mass index (in rates, dark bars) by world region and sex in 2012 Obesity-related cancers: oesophageal adenocarcinoma, pancreas, kidney, postmenopausal breast, ovary, corpus uteri, gallbladder (females only), colon, rectum
Counterfactual scenario: Maintaining the 1982 BMI
If BMI had remained as recorded in 1982, about one fourth (118,000 cases) of all obesity-related cancer cases in 2012 could have been averted. In other words, a quarter of all obesity-related cancers could be attributed to the increase in BMI between 1982 and 2002 (appendix xii). About 0·9% (0.5% in males and 1·3% in females) of all cancers diagnosed in 2012 were therefore considered realistically avoidable. The realistically attributable fraction was greatest in countries with a very high and high HDI, where 83% of all potentially avoidable cancers occurred. In a high burden region such as North America, this translated into more than 40,000 or 38% of all attributable cancer cases that could be linked to the increase in BMI since 1982. As for the specific cancer sites about 11% of all oesophageal adenocarcinomas (5,600), 8·5% of all corpus uteri (27,000), 5% of all kidney (15,000) and 2·5% of all postmenopausal breast cancers (28,000) could have been avoided if BMI had not increased between 1982 and 2002.
Effect of smoking and hormonal replacement therapy
When PAF for pancreatic cancer was corrected for smoking status,it was estimated to range between 1-18% for both males and females (appendix x), depending on the country. This is a 0-5% point difference in males and 0-9% point difference in females as compared to the unadjusted PAF. This difference was largest in the UK for both males and females. HRT non-users had a substantially higher PAF when compared to HRT users, ranging from 50-65% for corpus uteri cancer and from 8-12% for postmenopausal breast and ovarian cancer. When comparing the correctedPAF to the unadjustedone, the difference was small for most countries ranging from 0-14 percent points for corpus uteri cancer. In countries where prevalence of HRT non-users was high (>45%) and a large proportion of women with excess BMI, e.g. in Germany or Russia, the difference was larger.
Discussion
In 2012, approximately 3·6% of all cancers (excluding non-melanoma skin cancer) or 13% of all obesity-related cancers could be attributed to excess BMI in adults. These translated into an estimated 481,000 new cancers in men and women in 2012 that might be due to excess BMI. Postmenopausal breast, corpus uteri and colon cancer contributed 73% of the total attributable cases in females, whereas in males kidney and colon cancer contributed 66% of all attributable cases. While 64% of all global cancer cases related to excess BMI were mainly found in the Northern American and European regions, the PAF was also large in Oceania, Latin America and Caribbean and Middle East and Northern Africa.Most importantly, about one fourth of the total cases attributable to excess BMI (118,000 cancers) might have potentially been avoided if the population mean BMI had remained at the same level since 1982.
This study fills in the gap of information on cancer incidence related to excess BMI in particular for non-Caucasian populations in low and middle income countries. It remains evident that for the moment this issue mainly affects higher resource regions, e.g. 64% of all global cancer cases related to excess BMI were found in North America and Europe. Besides the notable unequal distribution of cancer cases attributable to excess BMI globally, marked differences were observedwithin regions; for instance in Latin America PAFs ranged from 12·7% (in Barbados) to 1·6% (in Haiti) in females. Although obesity-related cancers have become a global issue,35this transition occurs at different speed for each country or region. In a few countries such as the UK or the US where BMI has markedly increased in the 1980s and1990s, the BMI increase has since slowed down, yet in most countries average BMI has continued to raise steadily since the 1980s.2 The results of our secondary analysis, where historical BMI was used as achievable BMI level, could also be used to measure the changing impact of BMI on the burden of cancer. Taking into account both the current level of BMI and its change over time, the increase in PAF has been greatest in the Middle East and Northern Africa, Latin America and Caribbean, North America and Oceania. In contrast, Eastern Europe has retained a similar (high) BMI level between 1982 and 2002, so despite the large current PAF, only a very few cases are attributable to the change in BMI in that period. The varying pattern in BMI distribution and trends across countries highlights the need for future research on the cumulative effects of obesity on the burden of cancer, and also other chronic diseases.
Independent pooled studies30-32 have reported an attenuated risk of excess BMI in smokers for pancreatic and thyroid cancer. Taking into account the differential effect by smoking status produced different estimates depending on the country smoking prevalence. In highly developed countries, due to the high past prevalence of tobacco smoking36 and high current BMI,1 the attributable fraction of pancreatic cancer related to excess BMI was slightly underestimated. On the other hand, in less developed countries, where smoking has only started to rise,37 the effect of high BMI on pancreatic cancer was slightly overestimated. Another important effect modifier in the relation between BMI and cancer is HRT use, where the risk of female hormonally-driven cancers related to excess BMI is largely attenuated or even eliminated among HRT users.8,11,34 In the sensitivity analysis, we showed that the majority of postmenopausal breast, ovary and corpus uteri cancers attributable to excess BMI occurred among HRT non-users. The declining use of HRT since the early 2000s38,39 has contributed to a decrease in breast cancer incidence where usage was high, on the other hand it will probably translate into a higher proportion of cases being attributable to excess BMI and therefore amenable to prevention by weight loss.
This study adds important insights to the contribution of different lifestyle and exogenous risk factors on disease, specifically cancer risk. Previous studies have quantified the global cancer burden attributable to infections (2 million new cases in 2008, PAF 16.1%) 40 and to smoking (1.4 million cancer deaths in 2000, PAF 21%) 41. Given the growing number of cancer cases on the global scale and the prevalence of obesity still rising in almost all countries, the cancer burden due to obesity is likely to increase.
Strengths and Limitations
To-date,this is the most comprehensive study on the global scale estimating the current global burden of cancer due to excess BMI. A previously published report by the Global burden of disease project15 estimated burden of cancer due to excess BMI. Those results are not directly comparable to ours because PAF was presented as a proportion of deaths or disability-adjusted life years attributable to excess BMI, whereas in our study incidence was the reported outcome. Furthermore, information on the exposure prevalence and the incidence of cancer was obtained from the same year, not allowing for a lag-time between the exposure and cancer development. In addition, this current study also provided estimates for a more realistic scenario. A few other studies have estimated cancer incidence associated with excess BMI,27,42-44 however these were constrained to European populations. Differences in estimates are to be expected given that our estimates reflect more current data in both the prevalence of excess BMI and the incidence of cancer, which have increased greatly over the past decade.19,45
Another strength of this study includes detailed input of age-, sex- and country-specific BMI prevalence and the latest available cancer incidence estimates. These data were estimates, therefore careful interpretation of the results is advised, yet to-date these are the best available estimates. Many assumptions were made when estimating the PAF, however the large series of sensitivity analyses that were performed showed that changing these assumptions made no appreciable difference in the reported PAF. One of the assumptions that we tested was related to theevidence of non-linear associations between BMI and several cancers e.g. oesophageal, colon, breast and endometrial cancer.42 We opted for a log-logit RR function for all cancer sites included in this study instead of a linear function which partly addressed this issue. In the sensitivity analyses, we tested different RR functions and found that it has only minor impacts on the final PAF estimates.
Alongside point estimates for the PAF, we presented uncertainty intervals to provide a measure of reliability. Yet, these uncertainties do not take into account uncertainties in the cancer data from use of the GLOBOCAN 2012 estimates. The GLOBOCAN 2012 database provides a qualitative ranking of data quality for each country-specific estimate.22 Quantifying this uncertainty and incorporating additional uncertainties from the modelling and estimation processes remains a significant challenge and therefore was not incorporated in our analysis.46,47
Another limitation of this study includes the assumption of constant RRs across very diverse populations. Risk of some cancers associated with excess BMI have been reported to vary by ethnic group and geographic location.14,48Variation exists in the distribution of body fatness across ethnic groups and how this is reflected in the BMI measure. For example, within the USA, African or Hispanic are more likely to be obese than Caucasian and Asian women, yet the latter have been shown to have higher body fatness at similar BMI levels.49 Other anthropometric measures, such as waist circumference or waist-to-hip ratio, have been suggested as better predictors of obesity-related healthoutcomes when compared to BMI.50,51 Furthermore, rural and urban differences in the prevalence of obesity have been described.52-54 In our study, some variation in the distribution of BMI across ethnic groups may have been captured by using country-specific BMI estimates. Residual variation, i.e. within countries, was however not contemplated in the models. Given the very limited information available on these sub-groups and the lack of comparable global prevalence data of other anthropometric measures as well as their risk estimates, additional analyses for these factors were not performed.
Another drawback is the assumption of the absence of time-dependent effects of excess BMI on cancer risk, which cannot be completely captured by age-specific BMI data and lag-time. We assumed a 10-year lag in our modelling and recognized that the time-related effects of excess adiposity are likely to vary between cancer types. Recent studies have shown that the risk of cancer from excess weight accumulates with the number of years lived with excess weight, suggesting that the risk can better be predicted using years of life lived with excess weight.55,56 Longer obesity duration has also been linked to other diseases and conditions, such as coronary artery calcification, a precursor of coronary heart disease.57 Although this is in line with the biologic mechanisms underlying the relationship between obesity and the development cancer, studies examining this aspect of obesity are newly emerging and neither risk estimates nor the exposure information are available for each obesity-related cancer site.
Lastly, the estimation of the PAF is based on the assumption that the association between excess BMI and each cancer site included in our study is causal.58 We thus assume that reducing BMI will lead to a decline in the incidence of these cancers. Excess BMI has been shown to increase circulating levels of oestrogens and bioactivity of insulin-like growth factor 1and hence promoting the development of cancer.59 It should also be noted that epidemiological studies that report risk associations between BMI and cancer are prone to several limitations. It remains possible that residual confounding may explain the association between obesity and some types of cancer and this was not accounted for in our analysis. We have tried to overcome this issue by exclusively using risk estimates based on large meta-analyses that included only high quality studies and, whenever possible, only cohort studies.
In 2012, excess BMI among adults was associated with 3·6% or 481,000 cases of newly diagnosed cancer globally. If the world population BMI remained as it was in 1982, a quarter of this burden might have been potentially avoided. Historical and ongoing increases in the global prevalence of excess BMI, especially in younger cohorts, are expected to translate into further increases in the burden of cancer related to obesity in the near future. Changes in the prevalence of strong effect modifiers such as HRT are expected to heighten the total burden of cancer attributable to excess BMI, in particular among females. The large burden of cancer attributable to excess BMI in North America, Europe, Oceania, South America, the Middle East and North Africa points to the importance of weight control programs in these regions. This study informs health policy in terms of targets for prevention programs and highlights existing gaps in our knowledge of the relationship between BMI and cancer. It also underlines the need for research on effective interventions to control weight gain to avoid further rise in the burden of cancer related to excess BMI.
Panel: Research in Context
Systematic Review
Global and country-specific estimates of theincidence and mortality burden for cancers associated with obesity were obtained from the GLOBOCAN 2012 database.22The relevant cancers were those identified by the World Cancer Research Fund (WCRF) as having sufficient evidence to be associated with excess BMI.13 For each cancer site, we obtained sex-specific relative risk estimates from a published meta-analysis and the WCRF Continuous Update Project (CUP).8-12,14To estimate the population attributable fraction (PAF) for excess body weight, we used comparative data on mean body mass index (BMI) from the global burden of disease study.1,19 These estimates were obtained using systematic analysis of published and unpublished data from health examination surveys and epidemiological studies identified through systematic reviews of the literature, the WHO Global Infobase and data and studies known to the investigators. In sensitivity analyses, we used additional risk estimates specific for different world regions9,14,60 and three more cancer sites, namely thyroid cancer, Non-Hodgkin lymphoma and premenopausal breast cancer.8,14,61 The relevantinformation was derived from reports and articles mainly obtained from a series ofMedline searches using MeSH terms of above mentioned topics published before Jan 1,2014. No language restriction was employed. Finally, we searched, reviewed, and compared key papers on previous calculations of the PAF of excess body weight 15,17,27,43,44 with our estimates.
Interpretation
Other studies have estimated the burden of cancer attributable to obesity in specific countries or regions 27,42-44, but this is the first to provide a global estimate using a comprehensive set of data sources and sensitivity analyses. The findings show that 3.6% of all cancers (a total of 481,000 new cases in 2012) are attributable to excess BMI. The findings further underpin the need for a global effort to abate the rising trends in population-level obesity. If the current pattern of population weight gain continues, it will certainly augment the future burden of cancer especially in regions of the world, such as South America and North Africa, where the largest increases in the rate of obesity have been observed in the past three decades.2This study also informs health policy in terms of targets for prevention programs and highlights existing gaps in our knowledge of the relationship between BMI and cancer.
Supplementary Material
Acknowledgements
This project was funded by the World Cancer Research Fund International (grant nr. SG 2012/619). NP was funded by National Health and Medical Research Council (NHMRC project 31691). IS was funded by Marie Currie IEF Fellowship (project number: 302050). We would like to thank Dr. Rachel Thompson and Dr. Martin Wiseman from World Cancer Research Fund Internationalfor their critical input in several stages of the project. JJM is with the CRONICAS Centre of Excellence in Chronic Diseases at Universidad Peruana Cayetano Heredia, supported by federal funds of the National Heart, Lung And Blood Institute, United States National Institutes of Health, Department of Health and Human Services under contract number HHSN268200900033C. GS is a staff member of the World Health Organization. The author alone is responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy, or views of the World Health Organization.
Data on BMI by HRT status have been collected by the WHO MONICA investigators (for list see http://www.thl.fi/publications/monica/investigators.htm) and have been made available for this publication by the WHO MONICA Project. Views expressed in this paper are those of the authors and may not reflect those of individual WHO MONICA Principal Investigators.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
MA, NP, and IS contributed to data collection, study design, analysis, and wrote the first draft of the paper. GB and AR contributed to study design, analysis, and finalising the report. GS and MEcontributed to study design, data collection, analysis, and finalising the report. JF contributed to data collection and finalising the report. DF, RD, IR and JJM contributed to study design and drafting of the report. All authors read and approved the final report.
Declaration of interests
None declared.
References
- 1.Stevens GA, Singh GM, Lu Y, et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 2012;10(1):22. doi: 10.1186/1478-7954-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014. 384(9945):766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García-Alvarez A, Serra-Majem L, Ribas-Barba L, et al. Obesity and overweight trends in Catalonia, Spain (1992-2003): gender and socio-economic determinants. Public Health Nutr. 2007;10(11A):1368–78. doi: 10.1017/S1368980007000973. [DOI] [PubMed] [Google Scholar]
- 4.Sperrin M, Marshall AD, Higgins V, Buchan IE, Renehan AG. Slowing down of adult body mass index trend increases in England: a latent class analysis of cross-sectional surveys (1992-2010). International journal of obesity. 2014;38(6):818–24. doi: 10.1038/ijo.2013.161. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 6.Norberg M, Lindvall K, Stenlund H, Lindahl B. The obesity epidemic slows among the middle-aged population in Sweden while the socioeconomic gap widens. Glob Health Action. 2010;3 doi: 10.3402/gha.v3i0.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faeh D, Bopp M. Excess weight in the canton of Zurich, 1992-2009: harbinger of a trend reversal in Switzerland? Swiss Med Wkly. 2010;140:w13090. doi: 10.4414/smw.2010.13090. [DOI] [PubMed] [Google Scholar]
- 8.Continuous Update Project Report. World Cancer Research Fund / American Institute for Cancer Research; 2010. Food, Nutrition, Physical activity, and the Prevention of Breast Cancer. [Google Scholar]
- 9.Continuous Update Project Report. World Cancer Research Fund / American Institute for Cancer Research; 2011. Food, Nutrition, Physical activity, and the Prevention of Colorectal Cancer. [Google Scholar]
- 10.Continuous Update Project Report. World Cancer Research Fund / American Institute for Cancer Research; 2012. Food, Nutrition, Physical activity, and the Prevention of Pancreatic Cancer. [Google Scholar]
- 11.Continuous Update Project Report. World Cancer Research Fund / American Institute for Cancer Research; 2013. Food, Nutrition, Physical activity, and the Prevention of Endometrial Cancer. [Google Scholar]
- 12.Continuous Update Project Report. World Cancer Research Fund / American Institute for Cancer Research; 2014. Food, Nutrition, Physical activity, and the Prevention of Ovarian Cancer. [Google Scholar]
- 13.Food, nutrition, physical activity and the prevention of cancer : a global perspective : a project of World Cancer Research Fund International. American Institute for Cancer Research. World Cancer Research Fund; Washington, D.C.: 2007. [Google Scholar]
- 14.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 15.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012. 380(9859):2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parkin E, O'Reilly DA, Sherlock DJ, Manoharan P, Renehan AG. Excess adiposity and survival in patients with colorectal cancer: a systematic review. Obes Rev. 2014;15(5):434–51. doi: 10.1111/obr.12140. [DOI] [PubMed] [Google Scholar]
- 17.Renehan AG, Soerjomataram I, Leitzmann MF. Interpreting the epidemiological evidence linking obesity and cancer: A framework for population-attributable risk estimations in Europe. Eur J Cancer. 2010;46(14):2581–92. doi: 10.1016/j.ejca.2010.07.052. [DOI] [PubMed] [Google Scholar]
- 18.Luo J, Horn K, Ockene JK, et al. Interaction between smoking and obesity and the risk of developing breast cancer among postmenopausal women: the Women's Health Initiative Observational Study. Am J Epidemiol. 2011;174(8):919–28. doi: 10.1093/aje/kwr192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377(9765):557–67. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barendregt JJ, Veerman JL. Categorical versus continuous risk factors and the calculation of potential impact fractions. J Epidemiol Community Health. 2010;64(3):209–12. doi: 10.1136/jech.2009.090274. [DOI] [PubMed] [Google Scholar]
- 21.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. International Agency for Research on Cancer; Lyon, France: 2013. [Google Scholar]
- 23.World Population Prospects . The 2012 Revision. New York: United Nations. Department of Economic and Social Affairs, Population Division; 2013. [Google Scholar]
- 24.Human Development Report 2013 . The Rise of the South: Human Progress in a Diverse World. United Nations Development Programme (UNDP); New York: 2013. [Google Scholar]
- 25.Forman DBF, Brewster DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E, Swaminathan R, Ferlay J. Cancer Incidence in Five Continents, Vol. X (electronic version) IARC; Lyon: 2013. [DOI] [PubMed] [Google Scholar]
- 26.Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. GUT. 2014 doi: 10.1136/gutjnl-2014-308124. [DOI] [PubMed] [Google Scholar]
- 27.Renehan AG, Soerjomataram I, Tyson M, et al. Incident cancer burden attributable to excess body mass index in 30 European countries. Int J Cancer. 2010;126(3):692–702. doi: 10.1002/ijc.24803. [DOI] [PubMed] [Google Scholar]
- 28.Parker ED, Folsom AR. Intentional weight loss and incidence of obesity-related cancers: the Iowa Women's Health Study. Int J Obes Relat Metab Disord. 2003;27(12):1447–52. doi: 10.1038/sj.ijo.0802437. [DOI] [PubMed] [Google Scholar]
- 29.Elliott AM, Aucott LS, Hannaford PC, Smith WC. Weight change in adult life and health outcomes. Obes Res. 2005;13(10):1784–92. doi: 10.1038/oby.2005.217. [DOI] [PubMed] [Google Scholar]
- 30.Jiao L, Berrington de Gonzalez A, Hartge P, et al. Body mass index, effect modifiers, and risk of pancreatic cancer: a pooled study of seven prospective cohorts. Cancer Causes Control. 2010;21(8):1305–14. doi: 10.1007/s10552-010-9558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitahara CM, Platz EA, Freeman LE, et al. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev. 2011;20(3):464–72. doi: 10.1158/1055-9965.EPI-10-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steffen A, Schulze MB, Pischon T, et al. Anthropometry and esophageal cancer risk in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2009;18(7):2079–89. doi: 10.1158/1055-9965.EPI-09-0265. [DOI] [PubMed] [Google Scholar]
- 33.Whiteman DC, Sadeghi S, Pandeya N, et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57(2):173–80. doi: 10.1136/gut.2007.131375. [DOI] [PubMed] [Google Scholar]
- 34.Beral V, Hermon C, Peto R, et al. Ovarian cancer and body size: individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLoS medicine. 2012;9(4):e1001200. doi: 10.1371/journal.pmed.1001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. Lancet Oncol. 2012;13(8):790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 36.Lortet-Tieulent J, Renteria E, Sharp L, et al. Convergence of decreasing male and increasing female incidence rates in major tobacco-related cancers in Europe in 1988-2010. Eur J Cancer. 2013 doi: 10.1016/j.ejca.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 37.Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311(2):183–92. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 38.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–27. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 39.Soerjomataram I, Coebergh JW, Louwman MW, Visser O, van Leeuwen FE. Does the decrease in hormone replacement therapy also affect breast cancer risk in the Netherlands? J Clin Oncol. 2007;25(31):5038–9. doi: 10.1200/JCO.2007.13.7281. author reply 9-40. [DOI] [PubMed] [Google Scholar]
- 40.de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012. 13(6):607–15. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 41.Ezzati M, Henley SJ, Lopez AD, Thun MJ. Role of smoking in global and regional cancer epidemiology: current patterns and data needs. Int J Cancer. 2005;116(6):963–71. doi: 10.1002/ijc.21100. [DOI] [PubMed] [Google Scholar]
- 42.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384(9945):755–65. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergstrom A, Pisani P, Tenet V, Wolk A, Adami HO. Overweight as an avoidable cause of cancer in Europe. Int J Cancer. 2001;91(3):421–30. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1053>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 44.Parkin DM, Boyd L. 8. Cancers attributable to overweight and obesity in the UK in 2010. Br J Cancer. 2011;105(Suppl 2):S34–7. doi: 10.1038/bjc.2011.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arnold M, Karim-Kos HE, Coebergh JW, et al. Recent trends in incidence of five common cancers in 26 European countries since 1988: Analysis of the European Cancer Observatory. Eur J Cancer. 2013 doi: 10.1016/j.ejca.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2014 doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 47.Ferlay J, Forman D, Mathers CD, Bray F. Breast and cervical cancer in 187 countries between 1980 and 2010. Lancet 2012. 379(9824):1390–1. doi: 10.1016/S0140-6736(12)60595-9. [DOI] [PubMed] [Google Scholar]
- 48.Rush EC, Freitas I, Plank LD. Body size, body composition and fat distribution: comparative analysis of European, Maori, Pacific Island and Asian Indian adults. Br J Nutr. 2009;102(4):632–41. doi: 10.1017/S0007114508207221. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Beydoun MA. The obesity epidemic in the United States--gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 50.Moore LL, Bradlee ML, Singer MR, et al. BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham Study adults. Int J Obes Relat Metab Disord. 2004;28(4):559–67. doi: 10.1038/sj.ijo.0802606. [DOI] [PubMed] [Google Scholar]
- 51.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79(3):379–84. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 52.Mendez MA, Monteiro CA, Popkin BM. Overweight exceeds underweight among women in most developing countries. Am J Clin Nutr. 2005;81(3):714–21. doi: 10.1093/ajcn/81.3.714. [DOI] [PubMed] [Google Scholar]
- 53.Neuman M, Kawachi I, Gortmaker S, Subramanian SV. Urban-rural differences in BMI in low- and middle-income countries: the role of socioeconomic status. Am J Clin Nutr. 2013;97(2):428–36. doi: 10.3945/ajcn.112.045997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ebrahim S, Kinra S, Bowen L, et al. The effect of rural-to-urban migration on obesity and diabetes in India: a cross-sectional study. PLoS medicine. 2010;7(4):e1000268. doi: 10.1371/journal.pmed.1000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abdullah A, Wolfe R, Stoelwinder JU, et al. The number of years lived with obesity and the risk of all-cause and cause-specific mortality. Int J Epidemiol. 2011;40(4):985–96. doi: 10.1093/ije/dyr018. [DOI] [PubMed] [Google Scholar]
- 56.Stolzenberg-Solomon RZ, Schairer C, Moore S, Hollenbeck A, Silverman DT. Lifetime adiposity and risk of pancreatic cancer in the NIH-AARP Diet and Health Study cohort. Am J Clin Nutr. 2013;98(4):1057–65. doi: 10.3945/ajcn.113.058123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reis JP, Loria CM, Lewis CE, et al. Association between duration of overall and abdominal obesity beginning in young adulthood and coronary artery calcification in middle age. JAMA. 2013;310(3):280–8. doi: 10.1001/jama.2013.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88(1):15–9. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 60.Amadou A, Ferrari P, Muwonge R, et al. Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: a systematic review and dose-response meta-analysis. Obes Rev. 2013;14(8):665–78. doi: 10.1111/obr.12028. [DOI] [PubMed] [Google Scholar]
- 61.Larsson SC, Wolk A. Body mass index and risk of non-Hodgkin's and Hodgkin's lymphoma: a meta-analysis of prospective studies. Eur J Cancer. 2011;47(16):2422–30. doi: 10.1016/j.ejca.2011.06.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.