Abstract
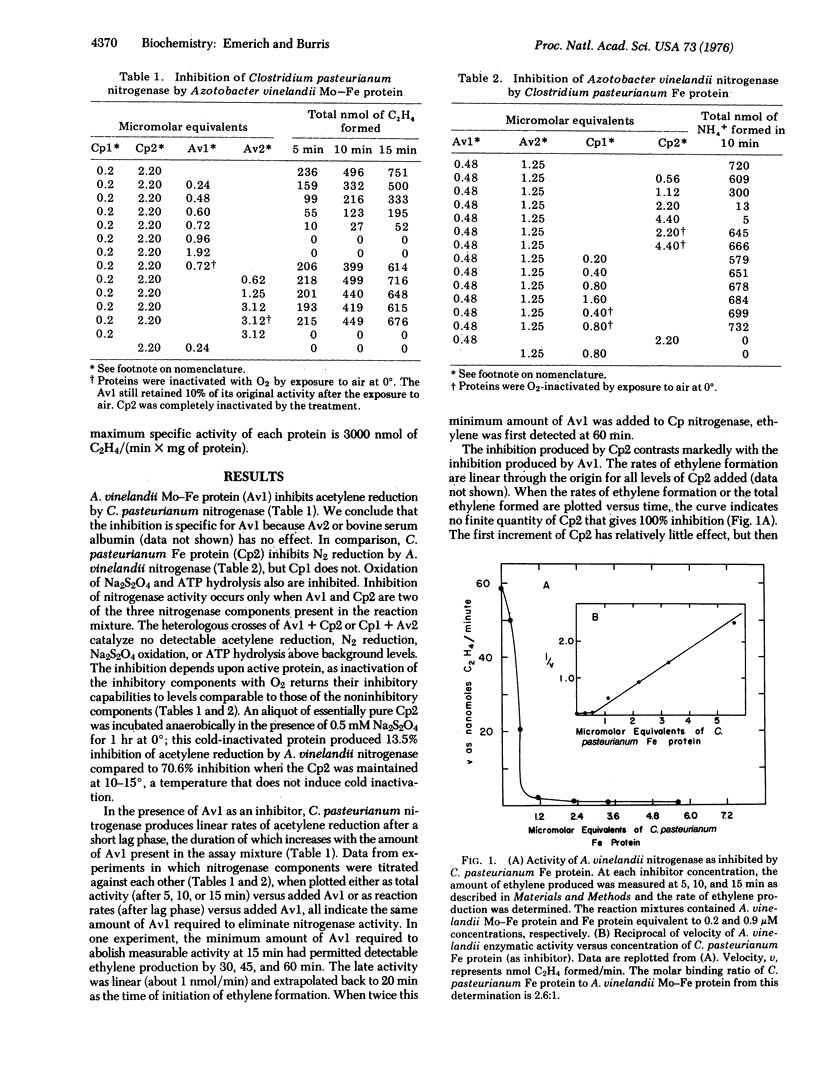
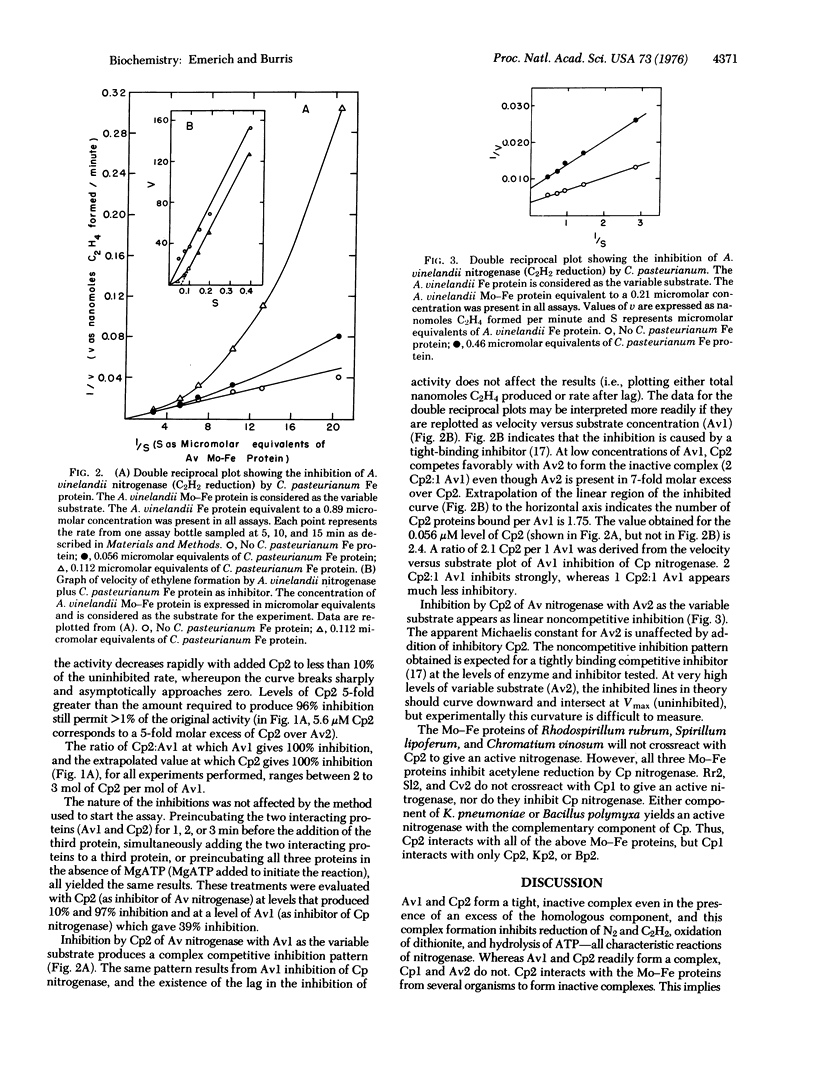
A unique method is described for inhibiting nitrogenase. When Clostridium pasteurianum nitrogenase is assayed in the presence of the Mo-Fe protein of Azotobacter vinelandii, all the characteristic activities of nitrogenase are inhibited. C. pasteurianum nitrogenase is unaffected by the Fe protein of A. vinelandii. The Fe protein, but not the Mo-Fe protein of C. pasteurianum, inhibits A. vinelandii nitrogenase. Both inhibitions described result from the formation of an inactive complex of A. vinelandii Mo-Fe protein and C. pasteurianum Fe protein. Complex formation requires active components, as oxygen-denatured proteins are ineffective. The results for titration of components of the complex against each other and kinetic data each indicate that the inactive complex consists of two molecules of C. pasteurianum Fe protein per molecule of A. vinelandii Mo-Fe protein. The results of kinetic experiments suggest that the Fe protein from each organism competes for the same site(s) on the A. vinelandii Mo-Fe protein. The Fe protein of C. pasteurianum will form an active or an inactive complex with the Mo-Fe proteins from six different organisms. Inhibition by nitrogenase components that form inactive complexes provides numeroius ways to study the mechanism of nitrogenase action.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergersen F. J., Turner G. L. Kinetic studies of nitrogenase from soya-bean root-nodule bacteroids. Biochem J. 1973 Jan;131(1):61–75. doi: 10.1042/bj1310061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins D. R., Kelly M. Interaction of nitrogenase from Klebsiella pneumoniae with ATP or cyanide. Biochim Biophys Acta. 1970;205(2):288–299. doi: 10.1016/0005-2728(70)90258-6. [DOI] [PubMed] [Google Scholar]
- Chaykin S. Assay of nicotinamide deamidase. Determination of ammonia by the indophenol reaction. Anal Biochem. 1969 Oct 1;31(1):375–382. doi: 10.1016/0003-2697(69)90278-4. [DOI] [PubMed] [Google Scholar]
- Eady R. R. Nitrogenase of Klebsiella pneumoniae. Interaction of the component proteins studied by ultracentrifugation. Biochem J. 1973 Nov;135(3):531–535. doi: 10.1042/bj1350531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- Kleiner D., Chen C. H. Physical and chemical properties of the nitrogenase proteins form Azotobacter vinelandii. Arch Mikrobiol. 1974 Jun 7;98(1):93–100. doi: 10.1007/BF00425272. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ljones T. Nitrogenase from Clostridium pasteurianum. Changes in optical absorption spectra during electron transfer and effects of ATP, inhibitors and alternative substrates. Biochim Biophys Acta. 1973 Sep 15;321(1):103–113. doi: 10.1016/0005-2744(73)90064-8. [DOI] [PubMed] [Google Scholar]
- Morrison J. F. Kinetics of the reversible inhibition of enzyme-catalysed reactions by tight-binding inhibitors. Biochim Biophys Acta. 1969;185(2):269–286. doi: 10.1016/0005-2744(69)90420-3. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Nitrogenase. IV. Simple method of purification to homogeneity of nitrogenase components from Azotobacter vinelandii. Biochim Biophys Acta. 1973 May 30;305(2):445–454. doi: 10.1016/0005-2728(73)90190-4. [DOI] [PubMed] [Google Scholar]
- Thorneley R. N., Eady R. R. Nitrogenase of Klebsiella pneumoniae: evidence for an adenosine triphosphate-induced association of the iron-sulphur protein. Biochem J. 1973 Jun;133(2):405–408. doi: 10.1042/bj1330405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N., Eady R. R., Yates M. G. Nitrogenases of Klebsiella pneumoniae and Azotobacter chroococum. Complex formation between the component proteins. Biochim Biophys Acta. 1975 Oct 22;403(2):269–284. doi: 10.1016/0005-2744(75)90057-1. [DOI] [PubMed] [Google Scholar]
- Thorneley R. N. Nitrogenase of Klebsiella pneumoniae. A stopped-flow study of magnesium-adenosine triphosphate-induce electron transfer between the compeonent proteins. Biochem J. 1975 Feb;145(2):391–396. doi: 10.1042/bj1450391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso M. Y., Ljones T., Burris R. H. Purification of the nitrogenase proteins from Clostridium pasteurianum. Biochim Biophys Acta. 1972 Jun 23;267(3):600–604. doi: 10.1016/0005-2728(72)90193-4. [DOI] [PubMed] [Google Scholar]
- Tso M. Y. Some properties of the nitrogenase proteins from Clostridium pasteurianum. Molecular weight, subunit structure, isoelectric point and EPR spectra. Arch Microbiol. 1974;99(1):71–80. doi: 10.1007/BF00696223. [DOI] [PubMed] [Google Scholar]
- Vandecasteele J. P., Burris R. H. Purification and properties of the constituents of the nitrogenase complex from Clostridium pasteurianum. J Bacteriol. 1970 Mar;101(3):794–801. doi: 10.1128/jb.101.3.794-801.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H. C., Burris R. H. Nitrogenase. Annu Rev Biochem. 1976;45:409–426. doi: 10.1146/annurev.bi.45.070176.002205. [DOI] [PubMed] [Google Scholar]


