Abstract
BACKGROUND:
Palliative care, integrated early, may reduce symptom burden in patients with idiopathic pulmonary fibrosis (IPF). However, limited information exists on timing and clinical practice. The purpose of this study was to describe the time course of events prior to death in patients with IPF managed at a specialty center with a focus on location of death and timing of referral for palliative care.
METHODS:
Data were retrospectively extracted from the health system’s data repository and obituary listings. The sample included all decedents, excluding lung transplant recipients, who had their first visit to the center between 2000 and 2012.
RESULTS:
Median survival for 404 decedents was 3 years from diagnosis and 1 year from first center visit. Of 277 decedents whose location of death could be determined, > 50% died in the hospital (57%). Only 38 (13.7%) had a formal palliative care referral and the majority (71%) was referred within 1 month of their death. Decedents who died in the academic medical center ICU were significantly younger than those who died in a community hospital ward (P = .04) or hospice (P = .001).
CONCLUSIONS:
The majority of patients with IPF died in a hospital setting and only a minority received a formal palliative care referral. Referral to palliative care occurred late in the disease. These findings indicate the need to study adequacy of end-of-life management in IPF and promote earlier discussion and referral to palliative care.
Idiopathic pulmonary fibrosis (IPF) is a fatal, progressive, scarring lung disease with a variable course.1 Mean survival from diagnosis ranges from 2 to 3 years, with some patients dying within the first year following diagnosis.2,3 Management of patients with IPF is particularly challenging because there is no medical therapy with proven survival benefit. Lung transplantation remains the only option to improve survival.4
IPF is characterized by an unpredictable course with some patients experiencing prolonged periods of slow, progressive decline and others succumbing to acute exacerbations.5,6 Consequently, clinicians, patients, and caregivers often fail to perceive disease course variability and experience high levels of stress and anxiety as the disease relentlessly progresses.7‐9 For these reasons, patients with IPF represent a distinct minority group eligible for early referral to palliative care. How early and how often such referrals occur is unknown. This information is important because improved understanding of referral patterns may lead to improved patient outcomes.10
Regardless of disease course, patients with IPF eventually reach the stage where death is imminent. To have a meaningful effect, palliative care services should be provided early in the course of disease.11 The goals of palliative care are to provide symptom management, prevent and relieve suffering, and support the best possible quality of life, regardless of stage of the disease or need for other therapies.12 In patients with newly diagnosed metastatic lung cancer, referral to palliative care within 12 weeks of diagnosis led to improvement in quality of life and mood compared with patients receiving standard care. Those patients receiving palliative care also had less aggressive care at the end of life, but longer survival.13 For patients with IPF who are not approved for lung transplant, there is an increasing consensus that palliative care should occur early following diagnosis, considering the lack of effective medical interventions.7,8,14,15 However, we were unable to identify specific recommendations in the literature regarding timing. Our clinical impression was that referral to palliative care more commonly occurred late, a concern given the unpredictable course of this disease.
The purpose of this study was to describe the time course of events prior to death in patients with IPF who did not receive a lung transplant and were managed at a specialized center, with a focus on location of death and timing of referral to palliative care. Lung transplant recipients were excluded because, once transplanted, disease management differs.
Materials and Methods
Setting
Data were extracted retrospectively for decedents with IPF who had their first visit to the University of Pittsburgh Dorothy P. and Richard P. Simmons Center for Interstitial Lung Disease (ILD) between 2000 and 2012. The Simmons Center, a large specialty ILD program affiliated with the University of Pittsburgh Medical Center, annually evaluates approximately 200 new patients with IPF referred from national and international locations. The study was approved by the University of Pittsburgh Committee for Oversight of Research and Clinical Training Involving Decedents (CORID No. 411).
Procedure
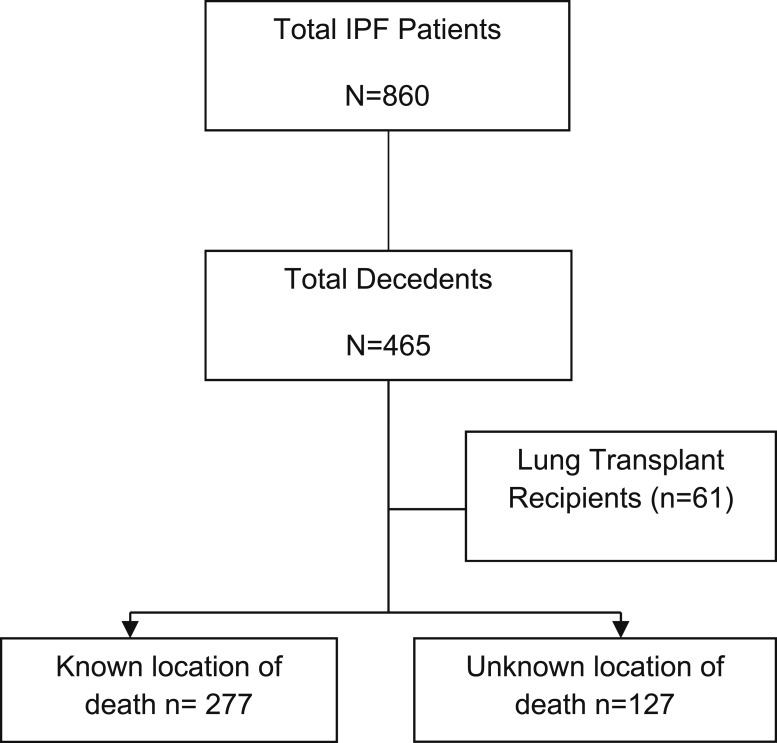
The Simmons Center maintains a clinical database of all clinic visits and tracks outcomes for each patient during their disease course. During the study interval (2000-2012), 860 patients with a confirmed diagnosis of IPF were seen at the center. Using the center’s database, we identified 465 patients with a confirmed diagnosis of IPF who died between 2000 and 2012. To determine location of death, two sources were used: the health system’s data repository to capture in-hospital mortality in the 18 hospitals belonging to our health system network16 and an Internet search for published death notices. Obituary information was crosschecked against the Simmons Center clinical database to confirm patient identity.
Using this search strategy, we were able to identify date of the first center visit and date of death for the 465 decedents. After excluding lung transplant recipients (n = 61), location of death was determined for 277 decedents and could not be identified for 127 decedents (Fig 1). Those under evaluation or listed for lung transplantation were included in this analysis. We identified 41 patients with a palliative care consultation through a search of dictated palliative-care consultation notes in the data repository. Of those patients, 38 had a known location of death and were included in the data analysis.
Figure 1 –
A total of 465 decedents were referred for evaluation between 2000 and 2012. After excluding lung transplant recipients and those with an unknown location of death, 277 decedents remained for analysis. IPF = idiopathic pulmonary fibrosis.
Measurements
Sociodemographic and disease characteristics obtained from the ILD database and confirmed from the health-system data repository included age at death, sex, race, date of diagnosis, date of first center visit, date at death, FVC % predicted, and diffusing capacity of the lung for carbon monoxide (Dlco) % predicted. Survival from first center visit and survival from diagnosis were computed by determining the interval (years) between diagnosis and the first center visit and death.
Location of death was categorized as academic medical center ICU, defined as the medical ICU at our main academic hospital, academic medical center floor, community hospital ICU, community hospital floor, or hospice. Some, but not all, community hospitals were affiliated with the University of Pittsburgh Medical Center. Location of death was further categorized into two groups (hospital or hospice) for predictor analysis.
Palliative care referral was defined as a formal request for consultation documented in the health system repository. Time from palliative care referral to death was computed by determining the interval (months) between these events.
Data Analysis
Data analysis was conducted using SPSS, version 21 (IBM). The association between time from first center visit to death and time from first center visit to formal palliative care referral was analyzed using the Pearson correlation coefficient. To explore associations between palliative care referral and location of death, the χ2 test was used. One-way analysis of variance was used to compare differences in age at death and survival from diagnosis for location of death. Post hoc analysis was used to determine differences between groups. Survival was obtained using Kaplan-Meier survival curves. Logistic regression was performed to assess the impact of a number of factors on the likelihood that decedents would die in a hospital or nonhospital setting.
Results
Demographic and Clinical Characteristics
The 404 decedents were predominately men (65.6%) and white (97.3%). Mean age at death was 71.5 years (SD ± 9.2 years). Mean FVC % predicted and Dlco % predicted at the first center visit were 60.5% ± 18.1% and 41.2% ± 16.7%, respectively (Table 1). Those whose location of death could not be identified were older (P = .04) and more likely to be women (P = .06).
TABLE 1 ] .
Comparison of Demographic Characteristics and Pulmonary Function Data for Decedents in the Total Cohort With a Known and Unknown Location of Death and Those Who Received a Formal Palliative Care Referral
| Characteristic | Total Cohort (N = 404) | Known Location of Death (n = 277) | Unknown Location of Death (n = 127) | Formal PC Referral (n = 38) |
| Age at death, mean ± SD (range), y | 71.5 ± 9.2 (39-95) | 70.9 ± 9.4a (39-95) | 72.9 ± 8.7a (39-90) | 71.8 ± 9.7 (52-95) |
| Male sex, No. (%) | 263 (65.6) | 194 (70)b | 71 (55.9)b | 25 (65.8) |
| White race, No. (%) | 393 (97.3) | 268 (96.8) | 125 (98.4) | 35 (92.1) |
| FVC % predicted at first center visitc, (range); No. | 60.5 ± 18.1 (17-117); 376 | 59.7 ± 18.3 (18-117); 260 | 62.3 ± 17.7 (26-116); 116 | 57.8 ± 19.3 (18-117); 37 |
| Dlco % predicted at first center visitc, mean ± SD (range); No. | 41.2 ± 16.7 (6-107); 305 | 41.5 ± 17.0 (6-107); 207 | 40.6 ± 17.7 (6-98); 98 | 44.1 ± 14.6 (23-80); 25 |
Dlco = diffusing capacity of the lung for carbon monoxide; PC = palliative care.
P = .04 for known vs unknown location of death.
P = .06 for known vs unknown location of death.
Some data are missing.
Time From Diagnosis and First Center Visit to Death
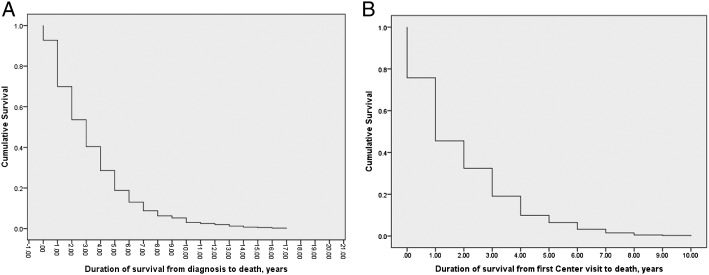
Median survival from diagnosis was 3 years (interquartile range, 1-5 years). Median survival from first center visit was 1 year (interquartile range, 1-3 years). Thus, on average, there was a 2-year delay from diagnosis to referral (Fig 2, Table 2).
Figure 2 –
A, Duration of survival from diagnosis to death for decedents who had idiopathic pulmonary fibrosis and who did not receive a lung transplant (n = 404). B, Duration of survival from first center visit to death for decedents who had idiopathic pulmonary fibrosis and who did not receive a lung transplant (n = 404).
TABLE 2 ] .
Duration of Survival From Diagnosis to Death and From First Center Visit to Death
| Period Measured | Patients Surviving to End of First Year, No. | Patients Surviving to End of Fifth Year, No. | Patients Surviving to End of 10th Year, No. | Patients Surviving to End of 15th Year, No. | Patients Surviving to End of 20th Year, No. |
| Diagnosis to death | 370 | 75 | 12 | 2 | 0 |
| First center visit to death | 307 | 26 | 0 | 0 | 0 |
Time to Palliative Care Referral
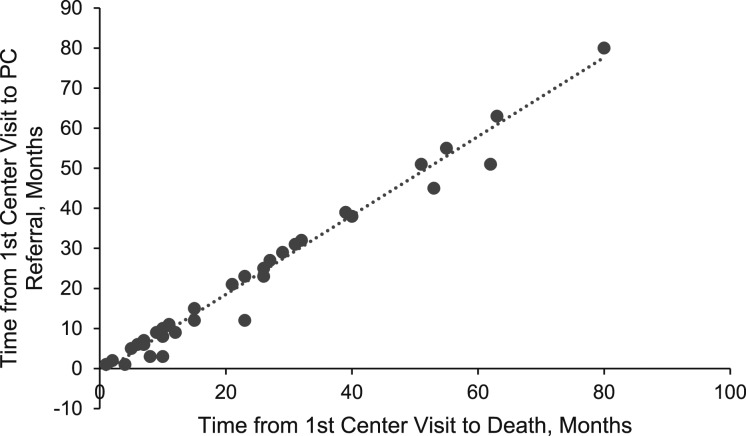
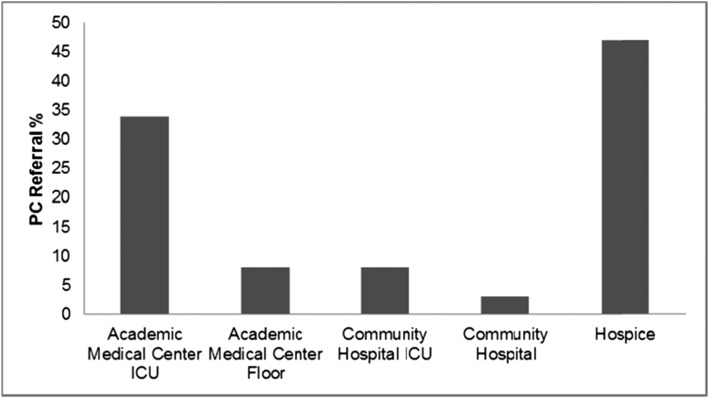
More than one-half of the 277 decedents died in the hospital (57%). Thirty-eight decedents (13.7%) received a formal palliative care consultation. Time of the referral was highly correlated with the time of death (r = 0.99; P < .001) (Fig 3). Formal palliative care referral occurred within 1 month of death for 71% of decedents, within 6 months for 18%, and within 12 months for 11%. There were significant differences in the number of decedents who received a palliative care referral in regard to the location of death (P = .048). Those decedents whose death occurred in an academic medical center ICU (34.2%; n = 13) or hospice (47.4%; n = 18) were more likely to receive a referral compared with those who died on a clinical unit (18.4%; n = 7) (Fig 4). There was a significant difference (P = .004) in the number of total center visits for those who received a formal palliative care referral (mean, 8.0 visits [SD ± 6.1]) compared with those did not (mean, 5.2 visits [SD ± 5.4]).
Figure 3 –
Scatterplot of months from time of formal PC referral to death (n = 38). The time of PC referral occurred very closely to the time of death, indicating limited time between the two events. PC = palliative care.
Figure 4 –
Percentage of decedents who received a formal PC referral by location of death. Decedents who received a formal PC referral were more likely to die in an academic medical center ICU or hospice. See Figure 3 legend for expansion of abbreviation.
Factors Influencing Location of Death
There were significant differences in the mean age at death when analyzed by location of death (P = .003) (Table 3). Decedents who died in the academic medical center ICU were significantly younger than those who died in a community hospital ward (P = .04) or hospice (P = .001). Survival length was not significantly different between the five death locations (P = .21). Age at death, sex, years of survival from diagnosis, last recorded FVC % predicted, and last recorded Dlco % predicted were analyzed to determine if any of these variables predicted location of death. Older patients with IPF were more likely to die in a nonhospital setting (P = .02). There were no other predictors identified.
TABLE 3 ] .
Mean Age at Death and Survival From First Center Visit to Death, by Location of Death (N = 277)
| Location of Death | Patients, No. (%) | Age at Death, Mean ± SD, ya | Survival From First Center Visit to Death, Mean ± SD, mo |
| Academic medical center ICU | 78 (28) | 67.6 ± 10.3 | 18.1 ± 19.7 |
| Academic medical center floor | 12 (4) | 68.8 ± 7.1 | 21.1 ± 25.4 |
| Community hospital ICU | 13 (5) | 70.3 ± 8.0 | 18.5 ± 20.2 |
| Community hospital | 55 (20) | 72.2 ± 8.1 | 23.6 ± 26.8 |
| Hospice | 119 (43) | 72.8 ± 9.2 | 25.81 ± 23.3 |
Overall significance (P = .003) when analyzed by analysis of variance for mean age at death; post hoc between-group analysis: academic medical center ICU vs community hospital, P = .04; academic medical center ICU vs hospice, P = .001.
Discussion
To our knowledge, this is the first relatively large study to describe the time course of events prior to death in patients with IPF, with a focus on referral to palliative care and location of death. The majority died in the hospital and few (13.7%) received formal referral to palliative care. Referral to palliative care typically occurred late in the course of their disease, a finding of significant concern. Optimally, given the typically relentless progression of IPF and inability to predict survival, such discussions should occur early following diagnosis.11,12 Palliative care offers the potential to improve quality of life, decrease symptom burden, and initiate discussion of end-of-life issues. Nevertheless, there were few formal palliative care referrals in our study and the majority of patients were referred late in the course of their illness (within 1 month of death). Most referrals occurred after ICU admission or prior to initiation of hospice.
Prior studies provide support for early referral. Bajwah and colleagues8 interviewed 18 patients with IPF and their family caregivers and reported the profound impact of this disease both physically and psychologically. Swigris and colleagues17 conducted a systematic review identifying a variety of symptoms that impacted quality of life and would benefit from a palliative care referral. Palliative care includes a broad menu of interventions designed to provide symptom relief, improve quality of life, address spiritual needs, and promote shared end-of-life decision-making.12,15,18 Studies have reported positive outcomes among patients with widely differing diseases.18
Other studies have demonstrated that palliative care referral occurred late in the disease. From a study of > 2,400 deaths in patients with chronic illness, Beernaert and colleagues18 reported that patients with COPD (20%), heart failure (34%), and severe dementia (37%) were less likely to receive a palliative care referral than patients diagnosed with cancer (60%). From a study of 45 patients with IPF, Bajwah and colleagues12 reported the majority (76%) died in a hospital setting and a minority (38%) had specialist palliative-care-team involvement. There are a number of reasons for late palliative care referral, including the discomfort of many physicians, caregivers, and family members with discussion about end-of-life care and a fear that the discussion will diminish hope.19,20 In addition, many often equate palliative care, which can be provided at any point in the disease course, with hospice care, which is provided when estimated life expectancy is < 6 months. Decedents who were seen had more clinic more visits and, therefore, were likely to be more familiar to center staff, and were more likely to receive a formal palliative care referral, suggesting a reluctance to suggest this option without an opportunity to establish rapport and evaluate prognosis.
A noteworthy finding in our study was the significant delay between diagnosis and the initial center visit, based on data extracted from the health system data repository; this may also influence the time between palliative care referral and death. This number is in agreement with the delay of 2.2 years from onset of symptoms reported by Lamas and colleagues21 and disappointingly also similar to the delay reported 10 years earlier by King and colleagues.22 While we chose to report time from diagnosis rather than time from onset of symptoms, our findings appear consistent with these and other studies,23,24 given the likely interval between onset of symptoms and diagnosis. Thus, time between onset of symptoms, diagnosis, and evaluation at a specialized care center appears essentially unchanged over the past 12 years, although early evaluation has been recommended.3 Although there is no definitive medical treatment, specialty centers offer multiple benefits, including education, participation in research protocols, timely transplant evaluation and referral, access to integrated outpatient palliative care, and optimum strategies for symptom relief and support group participation.9,12,17 While the reasons for persistence of delay are unclear, their impact on patient outcome, timely evaluation for transplant, or referral to palliative care should be further studied. Patients and caregivers may not be willing to accept early palliative care referral despite the benefits, viewing referral as “giving up hope.” Our experience also suggests that the term palliative care, itself, can lead to patient and family reluctance to accept referral. Other terminology (eg, supportive care, quality of life care) may be more acceptable.25 In addition, future studies should evaluate the benefits of early integrated palliative care in patients with IPF.
There are a number of limitations in our study. The study used a retrospective design, which limited data to that included in the health system data repository and an Internet search of obituary notices. Decedents whose location of death could not be identified may have differed from those included in our study. We elected to limit our definition of palliative care to documentation of a formal consultation in the health system data repository. It is possible that health-care providers provided similar information and support, absent a formal referral. Thus, the provision of palliative care services might be underreported. Finally, our database limited detailed information on patient characteristics (such as tobacco history, BMI, and comorbidities) and our study involved one specialty center. Outcomes of patients managed at other centers or who are not referred may differ from our findings. In some geographic areas, specialized palliative care programs may not be available, leaving pulmonary and other physicians to provide such care.
Conclusions
The consistent delay in referrals, significant percentage of patients dying in the hospital, and small number of formal referrals to palliative care suggest that significant effort is needed to establish new guidelines, with specific attention to timely referral to transplant and early discussion about palliative care. In critical care settings, concerns about timing have been addressed by initiating early referral for all patients, regardless of acuity.26 Because the disease course of IPF is unpredictable, early introduction of palliative care should be considered as a standard of care to maximize benefits and improve quality of life. Our results suggest that research into reasons for delay in referral is required, as are initiatives aimed to improve early access to palliative care consultations.
Acknowledgments
Author contributions: K. O. L. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. K. O. L. contributed to the study concept and served as principal author. K. O. L., Z. L., L. A. H., K. F. G., and N. K., contributed to the study design. K. O. L. and Z. L. contributed to data collection. K. O. L., Z. L., and L. A. H. contributed to data analysis. K. O. L., Z. L., L. A. H., and M. Q. R. contributed to the literature search and drafting the manuscript. K. O. L., Z. L., L. A. H., M. Q. R., M. I. S., J. M. P., K. F. G., and N. K. contributed to critical review of the manuscript. M. I. S. extracted data from electronic health records. K. F. G. and N. K. contributed to data interpretation.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Pilewski has contracts with Vertex Pharmaceuticals Inc, N30 Pharmaceuticals LLC, Constellation Pharmaceuticals, and, previously, with KalaBios Pharmaceuticals Inc. Dr Gibson is a consultant for Gilead Sciences Inc. Dr Kaminski is a consultant to InterMune Inc, Sanofi-Aventis LLC, Biogen Idec Inc, Vertex Pharmaceuticals Inc, Takeda Pharmaceutical Co Ltd, Promedior Inc, Moerae Matrix Inc, and Boehringer Ingelheim GmbH; a past recipient of grants from Gilead Sciences Inc and Celgene Corp; and inventor on patent applications on the use of microRNAs in IPF and on peripheral blood biomarkers in IPF. The remaining authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
ABBREVIATIONS
- Dlco
diffusing capacity of the lung for carbon monoxide
- IPF
idiopathic pulmonary fibrosis
- ILD
interstitial lung disease
Footnotes
Drs Gibson and Kaminski are senior authors of this article.
FUNDING/SUPPORT: This study was supported by the Dorothy P. and Richard P. Simmons endowed chair for Interstitial Lung Disease and the National Institutes of Health [Grant UL1TR000005].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Adkins JM, Collard HR. Idiopathic pulmonary fibrosis. Semin Respir Crit Care Med. 2012;33(5):433-439. [DOI] [PubMed] [Google Scholar]
- 2.King TE, Jr, Schwarz MI, Brown K, et al. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164(6):1025-1032. [DOI] [PubMed] [Google Scholar]
- 3.Raghu G, Collard HR, Egan JJ, et al. ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thabut G, Mal H, Castier Y, et al. Survival benefit of lung transplantation for patients with idiopathic pulmonary fibrosis. J Thorac Cardiovasc Surg. 2003;126(2):469-475. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-Pérez ER, Yilmaz M, Jenad H, et al. Ventilator settings and outcome of respiratory failure in chronic interstitial lung disease. Chest. 2008;133(5):1113-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song JW, Hong SB, Lim CM, Koh Y, Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37(2):356-363. [DOI] [PubMed] [Google Scholar]
- 7.Lewis D, Scullion J. Palliative and end-of-life care for patients with idiopathic pulmonary fibrosis: challenges and dilemmas. Int J Palliat Nurs. 2012;18(7):331-337. [DOI] [PubMed] [Google Scholar]
- 8.Bajwah S, Higginson IJ, Ross JR, et al. The palliative care needs for fibrotic interstitial lung disease: a qualitative study of patients, informal caregivers and health professionals. Palliat Med. 2013;27(9):869-876. [DOI] [PubMed] [Google Scholar]
- 9.Lindell KO, Olshansky E, Song MK, et al. Impact of a disease-management program on symptom burden and health-related quality of life in patients with idiopathic pulmonary fibrosis and their care partners. Heart Lung. 2010;39(4):304-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spagnolo PTR, Cocconcelli E, Stefani A, Richeldi L. Idiopathic pulmonary fibrosis: Diagnostic pitfalls and therapeutic challenges. Multidiscip Respir Med. 2012;7(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravaglia C, Tomassetti S, Gurioli C, et al. Palliative medicine and end-of-life care in idiopathic pulmonary fibrosis. J Palliat Med. 2013;16(4):339. [DOI] [PubMed] [Google Scholar]
- 12.Bajwah S, Higginson IJ, Ross JR, et al. Specialist palliative care is more than drugs: a retrospective study of ILD patients. Lung. 2012;190(2):215-220. [DOI] [PubMed] [Google Scholar]
- 13.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742. [DOI] [PubMed] [Google Scholar]
- 14.Danoff SK, Schonhoft EH. Role of support measures and palliative care. Curr Opin Pulm Med. 2013;19(5):480-484. [DOI] [PubMed] [Google Scholar]
- 15.Lee JS, McLaughlin S, Collard HR. Comprehensive care of the patient with idiopathic pulmonary fibrosis. Curr Opin Pulm Med. 2011;17(5):348-354. [DOI] [PubMed] [Google Scholar]
- 16.Yount RJ, Vries JK, Councill CD. The medical archival system: an information retrieval system based on distributed parallel processing. Inf Process Manage. 1991;27(4):379-389. [Google Scholar]
- 17.Swigris JJ, Kuschner WG, Jacobs SS, Wilson SR, Gould MK. Health-related quality of life in patients with idiopathic pulmonary fibrosis: a systematic review. Thorax. 2005;60(7):588-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beernaert K, Cohen J, Deliens L, et al. Referral to palliative care in COPD and other chronic diseases: a population-based study. Respir Med. 2013;107(11):1731-1739. [DOI] [PubMed] [Google Scholar]
- 19.Dellon EPSG, Sawicki GS, Shores MD, Wolfe J, Hanson LC. Physician practices for communicating with patients with cystic fibrosis about the use of noninvasive and invasive mechanical ventilation. Chest. 2012;141(4):1010-1017. [DOI] [PubMed] [Google Scholar]
- 20.Colman RECJ, Curtis JR, Nelson JE, et al. Barriers to optimal palliative care of lung transplant candidates. Chest. 2013;143(3):736-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamas DJ, Kawut SM, Bagiella E, Philip N, Arcasoy SM, Lederer DJ. Delayed access and survival in idiopathic pulmonary fibrosis: a cohort study. Am J Respir Crit Care Med. 2011;184(7):842-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King TE, Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med. 2001;164(7):1171-1181. [DOI] [PubMed] [Google Scholar]
- 23.Collard HRTG, Tino G, Noble PW, et al. Patient experiences with pulmonary fibrosis. Respir Med. 2007;101(6):1350-1354. [DOI] [PubMed] [Google Scholar]
- 24.Schoenheit G, Becattelli I, Cohen AH. Living with idiopathic pulmonary fibrosis: an in-depth qualitative survey of European patients. Chron Respir Dis. 2011;8(4):225-231. [DOI] [PubMed] [Google Scholar]
- 25.Maciasz RM AR, Chu E, Park SY, White DB, Vater LB, Schenker Y. Does it matter what you call it? A randomized trial of language used to describe palliative care services. Support Care Cancer. 2013;21(12):3411-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamba S, Murphy P, McVicker S, Harris Smith J, Mosenthal AC. Changing end-of-life care practice for liver transplant service patients: structured palliative care intervention in the surgical intensive care unit. J Pain Symptom Manage. 2012;44(4):508-519. [DOI] [PubMed] [Google Scholar]