Abstract
BACKGROUND:
Few studies have prospectively reported outcomes in patients with pulmonary arterial hypertension (PAH) treated with epoprostenol in the modern-day era of oral therapy and combination treatments. The Registry to Prospectively Describe Use of Epoprostenol for Injection (Veletri, prolonged room temperature stable-epoprostenol [RTS-Epo]) in Patients with Pulmonary Arterial Hypertension (PROSPECT) was established to prospectively describe the course of PAH in patients prescribed RTS-Epo.
METHODS:
PROSPECT is a multicenter, US-based drug registry of primarily group 1 patients with PAH treated with RTS-Epo who were parenteral-naive or parenteral-transitioned at enrollment. Patients were followed until discontinuation of RTS-Epo, withdrawal, loss to follow-up, death, or end of study (maximum 1 year). One-year freedom from hospitalization (FH) and survival estimates were summarized by prostacyclin history (parenteral-naive or parenteral-transitioned), sex, and chronic renal insufficiency (CRI).
RESULTS:
A total of 336 patients were included. The overall 1-year FH estimate was 51.0% ± 2.8% and was lower in parenteral-naive patients than parenteral-transitioned patients (42.8% ± 4.3% vs 57.1% ± 3.7%, respectively; P = .002). FH estimates were lower in male patients than female patients (38.3% ± 5.9% vs 54.6% ± 3.2%, respectively; P < .015) and in patients with CRI than patients without CRI (17.0% ± 8.4% vs 53.7% ± 2.9%, respectively; P < .001). The overall 1-year survival estimate was 84.0% ± 2.1%. Survival was poorer in parenteral-naive patients, male patients, and patients with CRI.
CONCLUSIONS:
Risk of hospitalization and mortality remain high in patients with PAH. In particular, patients who are parenteral-naive at initiation of RTS-Epo therapy, male patients, and patients with CRI require close monitoring and aggressive clinical management.
Pulmonary arterial hypertension (PAH) is a rare, progressive disease characterized by increasing pulmonary vascular resistance and pressures, resulting in right-side heart failure, dyspnea, and decreasing functional status.1 Untreated, the median survival time in idiopathic pulmonary arterial hypertension (IPAH) is 2.8 years, with estimated survival rates of 68% at 1 year, 48% at 3 years, and 34% at 5 years.2 While outcomes have improved with the advent of new therapies, further progress is needed. In The Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL Registry), the 1-year, 3-year, 5-year, and 7-year survival rates from time of diagnostic right-sided heart catheterization in a more diverse group of patients with PAH were 85%, 68%, 57%, and 49%, respectively.3
Hospitalization has been suggested as an indicator of poor survival; death, lung transplantation, and hospitalization have been identified as clinical efficacy measures in PAH clinical research.4 Additionally, in a study evaluating definitions of clinical worsening as a predictor of proximate (within 1 year) risk for subsequent major events (ie, death, transplantation, or atrial septostomy), hospitalization was independently associated with poor survival: the 1-year survival rate in patients hospitalized was only 71.4% ± 1.5%.5
Epoprostenol is an IV synthetic prostacyclin shown to improve exercise capacity and World Health Organization (WHO) functional class, while prolonging survival.1,6‐9 Epoprostenol remains the recommended treatment of the most advanced cases of PAH.10 There are limited data documenting the long-term rates of hospitalization and survival beyond 12 weeks with epoprostenol. Prior studies of survival in patients on epoprostenol have been limited to patients with primary pulmonary hypertension (PPH, now known as IPAH) in an era where prostacyclins were the only available therapy, and are limited to single-center cohort studies.1,8,9,11
Epoprostenol for injection (Veletri, prolonged room temperature stable-epoprostenol [RTS-Epo]) is a US Food and Drug Administration (FDA)-approved formulation of epoprostenol that has improved stability over the standard formulation of epoprostenol at room temperature.12 The Registry to Prospectively Describe the Use of RTS-Epo in Patients with Pulmonary Arterial Hypertension (PROSPECT) is a multicenter, prospective, observational registry of patients who were prescribed RTS-Epo. The registry longitudinally describes the modern-day course of patients with PAH treated with epoprostenol (RTS-Epo), by evaluating a broad range of clinical and patient-reported outcomes for 1 year after enrollment. The objective of this analysis is to assess the baseline characteristics and the risk of hospitalization and mortality in this cohort.
Materials and Methods
Registry Design and Participants
Participants were enrolled between September 2010 and January 2012 at 50 PAH centers across the United States, all of which received institutional review board approval. Patients were eligible if they had been diagnosed with PAH (group 1 pulmonary hypertension) and were parenteral-naive or parenteral-transitioned from another formulation of epoprostenol or treprostinil therapy to RTS-Epo. Patients were followed for a maximum of 1 year or until discontinuation of RTS-Epo, withdrawal of consent, loss to follow-up, death, or end of study.
Data Collection
Patient data were collected at enrollment and quarterly intervals (months 3, 6, 9, and 12) (e-Table 1 (98.2KB, pdf) ). No treatments or assessments were mandated by the protocol. Clinical characteristics, medical history, and comorbid conditions, including pulmonary function tests, echocardiogram, New York Heart Association functional class (NYHA FC) evaluation, and 6-min walk distance (6MWD) were collected using the most recent clinical visits, hospitalization, and/or telephone contacts. The treating physician determined renal insufficiency by selecting a checkbox in the electronic case report form. Among those patients who had a creatinine recorded, estimated glomerular filtration rate was computed. Dosing regimen and titration schedule for RTS-Epo and concomitant PAH medications were also collected quarterly.
Outcomes
Clinical outcomes from PROSPECT included demographics and disease characteristics of patients receiving RTS-Epo. Safety outcomes included all-cause deaths and hospitalizations and blood stream infections (BSIs). All outcomes were stratified by patient demographics and disease characteristics. Hospitalizations were assigned by the treating physician as related to the use of RTS-Epo, due to PAH, both, or neither. Reports of BSIs leading to hospitalization or death were also collected. Hospitalizations for initiation of RTS-Epo were not collected. Hospitalizations were coded using the MedDRA coding dictionary (version 14.0).
Deaths were collected during the course of the study. Patients who discontinued the study for any reason were censored on the date of discontinuation.
Statistical Analysis
Baseline demographic and clinical characteristics were summarized using percentages for categorical variables and means ± SD for continuous variables. The REVEAL Risk Score calculator was included, which incorporates 19 variables that predict 1-year survival in patients with PAH.13 Patient characteristics were summarized by hospitalization status (hospitalized vs not hospitalized), survival status (survived vs died), and sex. Rates of on-study hospitalizations (defined as those with a date of hospital admission after the date of enrollment) per 1,000 patient-days were calculated for all patients and by prostacyclin history (parenteral-naive or parenteral-transitioned).
Survival over the 1 year of follow-up was estimated based on Kaplan-Meier curves. For patients who died during follow-up, length of survival was calculated from the date of enrollment to the date of death reported. Patients who did not die were censored at the earlier of their date of discontinuation from the study or the date of the last data collection. Freedom from hospitalization, survival estimates, and SEs were summarized for all patients, and by prostacyclin history, sex, and chronic renal insufficiency (CRI). Risk-adjusted hazard ratios were computed from a multivariable Cox regression model.
Results
Patient Demographic and Clinical Characteristics
A total of 354 patients enrolled in PROSPECT; 18 did not have PAH. Thus, the analytic cohort included the remaining 336 patients. Patient demographics and comorbid conditions at enrollment are summarized in Table 1. The clinical characteristics of all patients, and by prostacyclin history at enrollment, are summarized in Table 2. Patient characteristics at baseline by hospitalization status, survival status (over 1 year of follow-up), and sex are summarized in Table 3. The most common reason for initiation to RTS-Epo was PAH progression in the parenteral-naive group (57.1%) and other in the parenteral-transitioned group (42.3%) (e-Table 2 (98.2KB, pdf) ). Among the 18 non-group 1 patients, chronic thromboembolic pulmonary hypertension and sarcoidosis each represented approximately one-third (Table 4). Concomitant PAH medications at start of RTS-Epo in the non-group 1 patients are summarized in Table 2. A summary of concomitant medications at enrollment for the patients in the non-WHO group 1 are summarized in Table 4.
TABLE 1 ] .
Patient Demographics and Comorbid Conditions at Enrollment (N = 336)
| Demographics and Conditions | Value |
| Age, y | 50.0 ± 14.6 |
| Age groups, y | |
| < 18 | 6 (1.8) |
| 18-34 | 52 (15.5) |
| 35-44 | 56 (16.7) |
| 45-55 | 89 (26.5) |
| 56-64 | 75 (22.3) |
| ≥65 | 58 (17.3) |
| Female sex | 258 (76.8) |
| Race | |
| White | 251 (74.7) |
| Black | 31 (9.2) |
| Hispanic | 29 (8.6) |
| Asian/Pacific Islander | 15 (4.5) |
| Unknown | 7 (2.1) |
| Other | 3 (0.9) |
| Height in cm | 164.2 ± 10.2 |
| Weight in kg | 74.4 ± 20.5 |
| BMI, kg/m2 | 27.4 ± 6.4 |
| Comorbid conditions, 10 most frequent | |
| Hypertension | 112 (33.3) |
| Obesity | 82 (24.4) |
| Hypothyroidism | 66 (19.6) |
| Scleroderma | 58 (17.3) |
| Diabetes | 54 (16.1) |
| Clinical depression | 44 (13.1) |
| Asthma | 36 (10.7) |
| COPD | 32 (9.5) |
| Renal insufficiency | 28 (8.3) |
| None | 24 (7.1) |
Data are given as mean ± SD or No. (%).
TABLE 2 ] .
Clinical Characteristics at Enrollment for All Patients and by Prostacyclin History
| Characteristics | All Patients (N = 336) | Parenteral-Naive (n = 147)a | Parenteral-Transitioned (n = 189)b |
| Specialty of diagnosing physician | |||
| Pulmonologist | 222 (66.1) | 101 (68.7) | 121 (64.0) |
| Cardiologist | 96 (28.6) | 37 (25.2) | 59 (31.2) |
| Other | 18 (5.4) | 9 (6.1) | 9 (4.8) |
| PAH duration in y | 4.7 ± 4.9 | 3.1 ± 4.5 | 5.9 ± 4.9 |
| NYHA functional class at enrollment | |||
| Class I | 14 (4.2) | 2 (1.4) | 12 (6.3) |
| Class II | 85 (25.3) | 24 (16.3) | 61 (32.3) |
| Class III | 169 (50.3) | 93 (63.3) | 76 (40.2) |
| Class IV | 38 (11.3) | 23 (15.6) | 15 (7.9) |
| Unknown | 30 (8.9) | 5 (3.4) | 25 (13.2) |
| 6MWD, m, mean ± SD | 334.6 ± 128.0 | 308.2 ± 113.4 | 350.5 ± 133.8 |
| Hemodynamic parameters | |||
| RAP, mm Hg | n = 259 | n = 112 | n = 147 |
| 11.9 ± 7.0 | 13.6 ± 7.0 | 10.7 ± 6.7 | |
| mPAP, mm Hg | n = 275 | n = 123 | n = 152 |
| 52.9 ± 14.3 | 54.8 ± 13.1 | 51.4 ± 15.1 | |
| PCWP, mm Hg | n = 266 | n = 116 | n = 150 |
| 11.5 ± 5.6 | 12.0 ± 6.2 | 11.1 ± 5.2 | |
| Cardiac index, L/min/m2 | n = 242 | n = 105 | n = 137 |
| 2.5 ± 1.0 | 2.3 ± 0.8 | 2.7 ± 1.1 | |
| Svo2, % | n = 59 | n = 24 | n = 35 |
| 61.8 ± 11.8 | 60.9 ± 11.3 | 62.5 ± 12.2 | |
| PVRI, Wood units × m2 | n = 225 | n = 99 | n = 126 |
| 19.7 ± 12.3 | 21.7 ± 12.0 | 18.0 ± 12.4 | |
| Concomitant PAH medications, at RTS-Epo start | |||
| PDE5i without ERA | 82 (24.4) | 32 (21.8) | 50 (26.5) |
| ERA without PDE5i | 49 (14.6) | 21 (14.3) | 28 (14.8) |
| PDE5i + ERA | 81 (24.1) | 37 (25.2) | 44 (23.3) |
| Neither | 124 (36.9) | 57 (38.8) | 67 (35.4) |
| Initial dose of RTS-Epoc | n = 316 | n = 127 | n = 189 |
| Median | 16.0 | 2.0 | 29.0 |
Data are given as mean ± SD or No. (%). 6MWD = 6-min walk distance; ERA = endothelial receptor antagonist; mPAP = mean pulmonary arterial pressure; NYHA = New York Heart Association; PDE5i = type 5 phosphodiesterase inhibitor; PAH = pulmonary arterial hypertension; PCWP = pulmonary capillary wedge pressure; PVRI = pulmonary vascular resistance index (Wood units × m2); RAP = right arterial pressure; RTS-Epo = room temperature stable-epoprostenol; Svo2 = venous oxygen saturation.
Includes n = 119 patients who were prostacyclin-naive, n = 26 patients who transitioned from an inhaled prostacyclin, and n = 2 patients who transitioned from an oral prostacyclin at study entry.
Includes n = 180 patients who transitioned from epoprostenol and n = 9 patients who transitioned from treprostinil at study entry.
The initial dose at time of initiation of prolonged RTS-Epo, which may not be at enrollment.
TABLE 3 ] .
Baseline Demographic and Clinical Characteristics Categorized by Hospitalization Status, Survival Status (> 1 Y of Follow-up), and Sex
| Characteristics | All Patients | Hospitalized | Survived | Male | Female | ||
| Yes | No | Yes | No | ||||
| Patients, No. | 336 | 157 | 179 | 285 | 51 | 78 | 258 |
| Age, y | 50.0 ± 14.6 | 50.7 ± 14.6 | 49.4 ± 14.7 | 49.5 ± 14.7 | 52.8 ± 14.3 | 49.0 ± 14.7 | 50.3 ± 14.6 |
| Sex | |||||||
| Male | 78 (23.2) | 44 (28.0) | 34 (19.0) | 56 (19.6) | 22 (43.1) | 78 (100) | 0 (0) |
| Female | 258 (76.8) | 113 (72.0) | 145 (81.0) | 229 (80.4) | 29 (56.9) | 0 (0) | 258 (100) |
| Etiology | |||||||
| IPAH | 172 (51.2) | 78 (49.7) | 94 (52.5) | 149 (52.3) | 23 (45.1) | 42 (53.8) | 130 (50.4) |
| HPAH | 14 (4.2) | 7 (4.5) | 7 (3.9) | 12 (4.2) | 2 (3.9) | 6 (7.7) | 8 (3.1) |
| Drugs/toxins | 18 (5.4) | 8 (5.1) | 10 (5.6) | 14 (4.9) | 4 (7.8) | 4 (5.1) | 14 (5.4) |
| APAH-CTD | 91 (27.1) | 47 (29.9) | 44 (24.6) | 75 (26.3) | 16 (31.4) | 8 (10.3) | 83 (32.2) |
| APAH-CHD | 22 (6.5) | 9 (5.7) | 13 (7.3) | 19 (6.7) | 3 (5.9) | 7 (9.0) | 15 (5.8) |
| APAH-PoPH | 16 (4.8) | 8 (5.1) | 8 (4.5) | 14 (4.9) | 2 (3.9) | 8 (10.3) | 8 (3.1) |
| APAH-HIV | 2 (0.6) | 0 (0.0) | 2 (1.1) | 1 (0.4) | 1 (2.0) | 2 (2.6) | 0 (0.0) |
| PPHN | 1 (0.3) | 0 (0.0) | 1 (0.6) | 1 (0.4) | 0 (0.0) | 1 (1.3) | 0 (0.0) |
| NYHA FC | |||||||
| Class I | 14 (4.6) | 7 (4.9) | 7 (4.3) | 14 (5.4) | 0 (0.0) | 2 (2.7) | 12 (5.2) |
| Class II | 85 (27.8) | 27 (18.8) | 58 (35.8) | 76 (29.5) | 9 (18.8) | 23 (30.7) | 62 (26.8) |
| Class III | 169 (55.2) | 88 (61.1) | 81 (50.0) | 145 (56.2) | 24 (50.0) | 39 (52.0) | 130 (56.3) |
| Class IV | 38 (12.4) | 22 (15.3) | 16 (9.9) | 23 (8.9) | 15 (31.3) | 11 (14.7) | 27 (11.7) |
| 6MWD, m | 334.6 ± 128.0 | 314.5 ± 117.1 | 352.8 ± 135.0 | 347.9 ± 122.2 | 254.7 ± 134.2 | 354.6 ± 129.6 | 329.2 ± 127.3 |
| REVEAL Registry Risk Score | 8.4 ± 2.4 | 8.9 ± 2.3 | 8.0 ± 2.4 | 8.1 ± 2.3 | 10.0 ± 2.0 | 8.8 ± 2.4 | 8.3 ± 2.4 |
| Parenteral-naive | 147 (43.8) | 79 (50.3) | 68 (38.0) | 118 (41.4) | 29 (56.9) | 48 (61.5) | 99 (38.4) |
| Parenteral-transitioned | 189 (56.3) | 78 (49.7) | 111 (62.0) | 167 (58.6) | 22 (43.1) | 30 (38.5) | 159 (61.6) |
Data are given as mean ± SD or No. (%). APAH = associated pulmonary hypertension; CHD = congenital heart disease; CTD = connective tissue disease; HPAH = heritable pulmonary arterial hypertension; IPAH = idiopathic pulmonary arterial hypertension; PoPH = portopulmonary hypertension; PPHN = persistent pulmonary hypertension of the newborn; REVEAL Registry = Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension Disease Management. See Table 2 legend for expansion of abbreviations.
TABLE 4 ] .
Concomitant Medication History at Enrollment for Non-WHO Group 1 Patients
| Characteristics | All Patients Non-Group 1 (N = 18)a | Parenteral-Naive (n = 8) | Parenteral-Transitioned (n = 10) |
| Concomitant PAH medications, at RTS-Epo start | |||
| PDE5i without ERA | 6 ( 33.3) | 4 ( 50.0) | 2 ( 20.0) |
| ERA without PDE5i | 2 ( 11.1) | 0 ( 0.0) | 2 ( 20.0) |
| PDE5i + ERA | 4 ( 22.2) | 2 ( 25.0) | 2 ( 20.0) |
| Neither | 6 ( 33.3) | 2 ( 25.0) | 4 ( 40.0) |
Data are given as No. (%). WHO = World Health Organization. See Table 2 legend for expansion of other abbreviations.
Chronic thromboembolic pulmonary hypertension (5), sarcoidosis (6), carcinoid syndrome (1), pulmonary veno-occlusive disease/pulmonary capillary hemangiomatosis (1), lung disease (1), pulmonary hypoplasia, giant omphalocele (1), left-sided heart disease/morbid obesity (1), post-liver transplant pulmonary hypertension (1), and pulmonary hypertension due to underlying infl ammatory myopathy (1).
Patients requiring hospitalization in the first year were more likely to be men, have PAH associated with connective tissue disease (CTD), be NYHA FC III or IV, have a lower mean 6MWD, have a higher mean REVEAL Registry Risk Score, and were more likely to be parenteral prostanoid-naive compared with patients who were not hospitalized.
Patients who died during the study were older, were more likely to have PAH associated with CTD, be NYHA FC IV, have a lower mean 6MWD, have a higher mean REVEAL Registry Risk Score, and were more likely to be parenteral prostanoid-naive compared with patients who survived.
Men comprised about 20% of the surviving patients in PROSPECT; 43.1% of the deaths occurred in men. Men were more likely than women to have familial PAH, congenital PAH, or PAH associated with portopulmonary hypertension. A higher mean REVEAL Registry Risk Score was observed in men, who were also more likely to be parenteral prostanoid-naive at enrollment compared with women.
Hospitalizations
A total of 296 on-study all-cause hospitalizations occurred in 157 patients (150 were parenteral-naive patients and 146 were parenteral-transitioned patients). The median length of stay was longer for parenteral-naive patients than for parenteral-transitioned patients (median 5.0, interquartile range: 3.0, 9.0 vs median 4.0, interquartile range: 2.0, 7.0, respectively).
Of the hospitalized patients (n = 157), 77 (49.0%) were not rehospitalized, 51 (32.5%) were rehospitalized once, 12 (7.6%) were rehospitalized twice, and 17 (10.8%) were rehospitalized three or more times during the 1-year follow-up. There were no differences in frequencies of rehospitalization between parenteral-naive and parenteral-transitioned groups.
Rates and All Causes of Hospitalizations
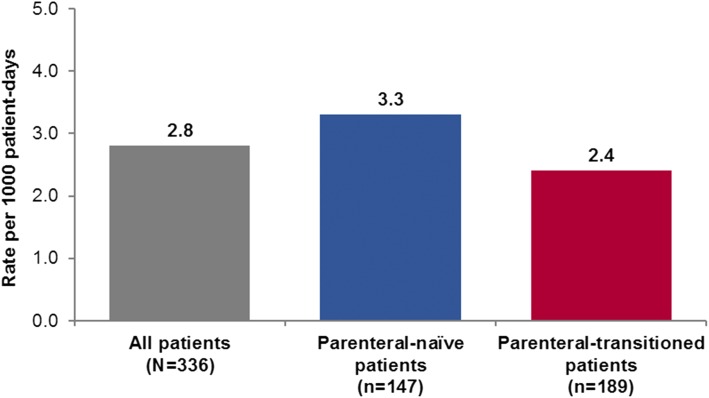
The overall rate of all-cause hospitalizations in group 1 patients with PAH was 2.8 per 1,000 patient-days (3.3 per 1,000 patient-days in parenteral-naive patients and 2.4 per 1,000 patient-days in parenteral-transitioned patients [Fig 1]). Hospitalization rates for the non-group 1 patients was 2.0 per 1,000 patient-days.
Figure 1 –
Rates of on-study hospitalizations* (per 1,000 patient-d) in the PROSPECT cohort by prostacyclin history. *On-study hospitalization defined as those with a date of hospital admission after the date of enrollment into PROSPECT.
All causes of hospitalization with at least five occurrences are shown in Table 5. The most common causes of hospitalizations were infections (51) and heart failures (48). Thirty-seven percent of hospitalizations were categorized as being related to PAH (44% in the parenteral-naive group and 31% in the parenteral-transitioned group).
TABLE 5 ] .
All Causes of Hospitalizationsa (With at Least Five Occurrences) in Patients During 1 Y of Follow-up in PROSPECT
| Cause | Total Count, Absolute No. of Hospitalizations |
| Infections-pathogen unspecifiedb | 51 |
| Heart failures | 48 |
| Respiratory disorders (NEC) | 17 |
| Pulmonary vascular disorders | 15 |
| Electrolyte and fluid balance conditions | 14 |
| Gastrointestinal signs and symptoms | 12 |
| Cardiac arrhythmias | 11 |
| Bacterial infectious disorders | 10 |
| GI hemorrhages (NEC) | 10 |
| Anemias nonhemolytic and marrow depression | 8 |
| Complications associated with device | 8 |
| General system disorders (NEC) | 8 |
| Renal disorders (excluding nephropathies) | 8 |
| Decreased and nonspecific blood pressure disorders and shock | 5 |
| Neurologic disorders (NEC) | 5 |
| Other causesc | 73 |
| Totald | 296 |
HLGT = high-level group term; HLT = high-level term; NEC = not elsewhere classified. See Table 3 legend for expansion of other abbreviations.
Hospitalizations were coded using the MedDRA coding dictionary based on HLGT, with additional detail provided from the HLT for the most common HLGTs.
Includes 19 hospitalizations due to blood stream infections.
Other causes include: cardiac therapeutic procedures (4); device issues (4); respiratory tract therapeutic procedures (4); bone and joint injuries (3); bronchial disorders (excluding neoplasms) (3); diabetic complications (3); investigations, imaging, and histopathology procedures (NEC) (3); pericardial disorders (3); viral infectious disorders (3); anal and rectal conditions (NEC) (2); body temperature conditions (2); cardiac disorder signs and symptoms (2); endocrine gland therapeutic procedures (2); exocrine pancreas conditions (2); hepatic and hepatobiliary disorders (2); joint disorders (2); medication errors (2); therapeutic procedures and supportive care (NEC) (2); cardiac and vascular investigations (excluding enzyme tests) (1); central nervous system vascular disorders (1); chemical injury and poisoning (1); CTDs (excluding congenital) (1); coronary artery disorders (1); epidermal and dermal conditions (1); GI conditions (NEC) (1); GI inflammatory conditions (1); GI motility and defecation conditions (1); GI neoplasms, malignant and unspecified (1); headaches (1); hepatobiliary investigations (1); hepatobiliary therapeutic procedures (1); lymphomas, non-Hodgkin’s unspecified histology (1); musculoskeletal and CTDs (NEC) (1); obstetric and gynecologic therapeutic procedures (1); parathyroid gland disorders (1); peritoneal and retroperitoneal conditions (1); platelet disorders (1); pleural disorders (1); Rickettsial infectious disorders (1); skin and subcutaneous tissue disorders (NEC) (1); therapeutic and nontherapeutic effects (excluding toxicity) (1); thyroid gland disorders (1); upper respiratory tract disorders (excluding infections) (1).
Seven patients with a hospitalization admission date on the same date as enrollment were excluded from the outcomes analysis.
A total of 21 BSIs (0.20 per 1,000 patient-days on study) developed in 16 patients, 19 of which resulted in hospitalizations (10 in parenteral-naive patients [0.24 per 1,000 patient-days] and nine in parenteral-transitioned patients [0.16 per 1,000 patient-days]). Of the 21 BSIs, 15 were gram-positive (71.4%), three were gram-negative (14.3%), and three were of unknown organism(s) (14.3%). There were no hospitalizations related to BSIs in the non-group 1 patients.
Freedom From Hospitalization
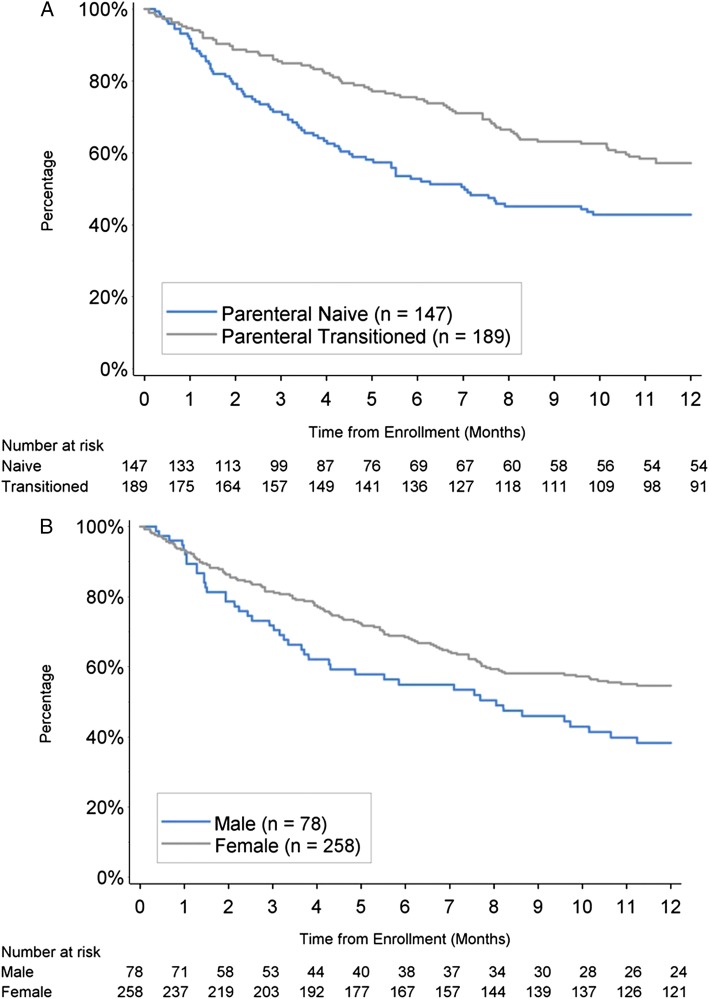
The 1-year freedom from hospitalization estimate (± SE) for the n = 336 group 1 patients was 51.0% ± 2.8%. The freedom from hospitalization estimate for patients who were parenteral-naive at enrollment was 42.8% ± 4.3% compared with 57.1% ± 3.7% for parenteral-transitioned patients (P = .002) (Fig 2A). The freedom from hospitalization estimate for men was 38.3% ± 5.9%; for women, it was 54.6% ± 3.2% (P < .015) (Fig 2B). Patients with CRI had a lower freedom from hospitalization compared with patients without CRI (17.0% ± 8.4% vs 53.7% ± 2.9%; P < .001).
Figure 2 –
A, B, Freedom from hospitalization at 1 y by prostacyclin history (A) and sex (B).
Survival Estimates
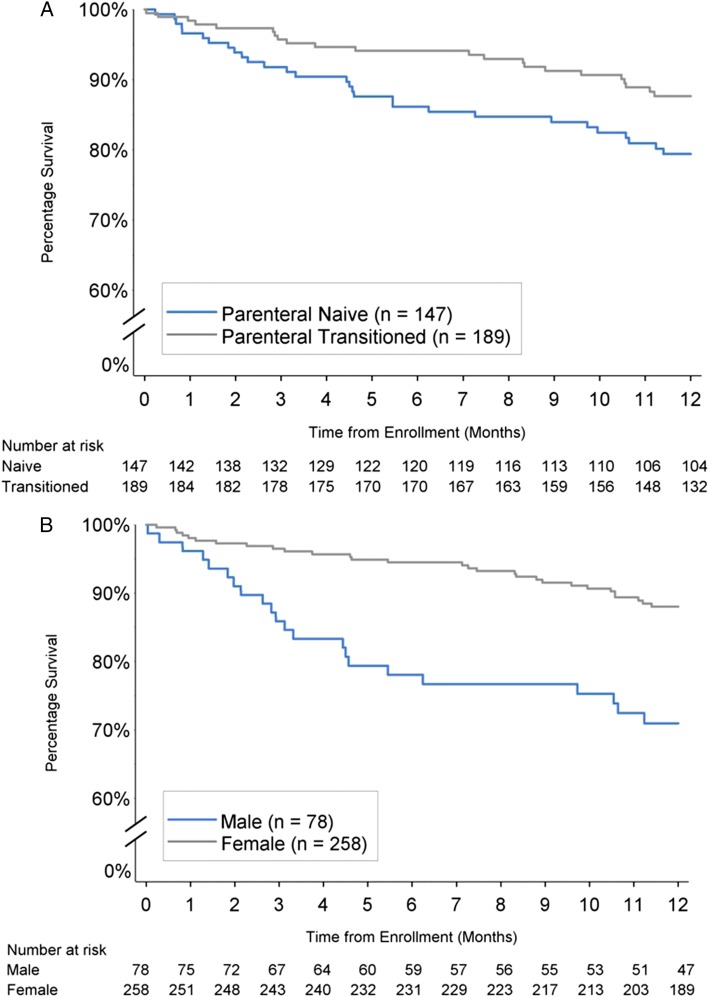
The 1-year survival estimate (± SE) for all patients in PROSPECT was 84.0% ± 2.1%. The 1-year survival estimate for patients who were classified as parenteral-naive at baseline was lower than the survival estimate for patients who were parenteral-transitioned (79.4% ± 3.4% vs 87.7% ± 2.5%; P = .038) (Fig 3A). The 1-year survival estimate in men was lower than in women (71.0% ± 5.2% vs 88.0% ± 2.1%; P < .001) (Fig 3B). A sensitivity analysis using an estimated glomerular filtration rate cutpoint rather than the qualitative definition of CRI produced similarly significant results. A multivariate Cox regression model of group 1 patients confirmed that sex and CRI were independent risk factors, whereas there was no differences between naive and transition patients after adjusting for differences in risk profile. (Table 6). The 1-year survival estimate was lower than in patients without CRI (63.4% ± 9.3% vs 86.0% ± 2.0%; P < .001). The 1-year survival estimate in the non-group 1 patients was 88.9% ± 7.4%.
Figure 3 –
A, B, One-y survival by prostacyclin history (A) and sex (B).
TABLE 6 ] .
Multivariable Predictors of Mortality
| Predictors | Hazard Ratio | 95% CI | P Valuea |
| NYHA/WHO FC IV | 2.88 | (1.53, 5.42) | .001 |
| Renal insufficiency | 2.64 | (1.31, 5.32) | .007 |
| Male | 3.03 | (1.68, 5.50) | < .001 |
| High BNP, > 180 pg/mL or NT > 1,500 pg/mL | 2.04 | (1.16, 3.59) | .014 |
| Low 6MWD, < 165 m | 2.29 | (1.01, 5.16) | .047 |
| Prostacyclin-naive | 1.21 | (0.68, 2.14) | .52 |
Discussion
PROSPECT is the first modern-day registry, to our knowledge, to assess outcomes in a large series of patients with PAH taking epoprostenol and examine outcomes by prostacyclin history, risk of hospitalization, and mortality in patients in a real-world setting. The strengths of the study include the size of the cohort, broad inclusion criteria, and 1-year follow-up.
This analysis shows a high burden of illness in these patients, with an overall 1-year probability of survival of 84% ± 2.1% and only about 50% remaining free of hospitalization at 1 year. Specific subgroups within PROSPECT were at higher risk of both hospitalization and mortality compared with their counterparts, in particular, patients who were parenteral-naive at enrollment, male patients, and patients with CRI. Mean REVEAL Registry Risk Scores were higher in these subgroups, reconfirming the applicability of the score to this patient population.13 An interesting finding is the large proportion of hospitalizations due to various comorbid conditions which could be explained by patients with PAH living longer and developing unrelated or unrecognized complications of PAH.
Survival in patients with PAH in the modern treatment era has improved compared with previously observed survival. However, as outcomes among PAH subpopulations vary substantially,14 continuing rigorous disease management of PAH is important. Patient survival appears to be related to the ability of the right ventricle to adapt to the chronically elevated pulmonary artery pressure.8 Hospitalizations have been recognized as not only an important outcome measure when assessing clinical efficacy in PAH trials, but also an important prognostic factor in patients with PAH.4,5 While PROSPECT confirms that use of RTS-Epo is associated with greater survival rates than that historically expected, rates of hospitalization are still high for patients with the disease. Overall survival of patients taking RTS-Epo was comparable to other studies of epoprostenol.1,8,9 However, these older studies were limited single-site studies of patients with PPH and did not include higher-risk patients from associated PAH subgroups.
Survival in patients with PPH treated with epoprostenol depends on disease severity.9 In PROSPECT, patients who were parenteral-naive at baseline had worse outcomes compared with patients who were parenteral-transitioned. These results support findings that earlier initiation of prostacyclin therapy or more aggressive therapy should be considered.15 A study found that treatment with oral therapy or parenteral prostacyclin as initial strategy was associated with a high survival rate in patients who were WHO functional class III and whose PAH was idiopathic, familial, or anorexigen associated.16 The authors suggest that the more potent prostacyclins be reserved for high-risk patients, such as those with evidence of disease progression or those with treatment failure. It should be noted that findings in this analysis may reflect survivor bias in those already on parenteral therapy or the more critical nature of the disease in patients who are parenteral-naive.
Sex differences observed in this analysis are consistent with other studies. Although the incidence of PAH is higher in women, survival is lower in men. The 5-year survival rate from diagnosis in the REVEAL Registry was 52% in men compared with 62% in women.17 It is unclear why women have a better survival rate than men: some have suggested that women respond better to treatment options or that female sex hormones are mediating protective effects.18 Male sex has been shown to be an independent predictor of mortality.17,19‐21 A recent study suggests that differences in the right ventricular ejection fraction response to initiation of therapy in IPAH explain a significant portion of the poorer survival observed in men.22
BSIs are a critical concern and major contributor to hospitalizations in patients with PAH receiving parenteral therapy. The rate of BSIs observed in PROSPECT was 0.20 per 1,000 patient-days, which is consistent with prior studies of BSIs in epoprostenol-treated patients.23,24 In a retrospective cohort study from the Centers for Disease Control, the rate of BSIs was 0.42 BSIs per 1,000 treatment-days for patients on IV epoprostenol.23 In the REVEAL Registry, the rate of BSIs for IV epoprostenol was 0.12 per 1,000 treatment-days.24
PROSPECT is subject to the inherent limitations of uncontrolled, observational studies for data acquisition and interpretation. Other limitations include the collection of hospitalization data, which was based on primary discharge diagnosis, and the broader categorization of causes of hospitalization, which removed some granularity in the data. PROSPECT patients were not at risk for on-study events until enrollment. Although treatment initiation was proximate to enrollment for many patients, some survival bias likely exists.25 Therefore, these results cannot be generalized directly to newly treated or newly diagnosed patients.
Conclusions
Risk of hospitalization and mortality remain high in patients with PAH. Patients who are parenteral-naive at initiation of RTS-Epo therapy, male patients, and patients with CRI require close monitoring and aggressive clinical management.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: All authors had full access to all of the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. R. P. F. contributed to data collection, analysis, and interpretation, drafting and critical review of the manuscript, and approval of the final version of the manuscript and served as principal author; R. J. S., M. M. C., D. B. B., A. E. F, and V. V. M. contributed to data collection, analysis, and interpretation, drafting and critical review of the manuscript, and approval of the final version of the manuscript; R. J. B. contributed to the study design and data collection, analysis, and interpretation; and D. M. R., D. P. M., B. K. H., W. W. B., and H. W. F. contributed to the study design, data collection, analysis, and interpretation, drafting and critical review of the manuscript, and approval of the final version of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Frantz receives grant/research support from United Therapeutics Corporation and is on the advisory board for Actelion Pharmaceuticals US, Inc and United Therapeutics Corporation. Dr Schilz serves as a consultant, is on the speaker’s bureau for, and is on the advisory board for Actelion Pharmaceuticals US, Inc; Bayer AG; Gilead; and United Therapeutics Corporation. Dr Schilz also receives grant/research support from Actelion Pharmaceuticals US, Inc; Bayer AG; Gilead; and United Therapeutics Corporation. Dr Chakinala serves as a consultant for Actelion Pharmaceuticals US, Inc; Gilead; United Therapeutics Corporation; SteadyMed Therapeutics, Inc; and is on the speaker’s bureau for Gilead and United Therapeutics Corporation. Dr Chakinala also receives grant/research support from Actelion Pharmaceuticals US, Inc; Gilead, United Therapeutics Corporation; Medtronic, Inc; Novartis AG; Ikaria, Inc; Aires Pharmaceuticals, Inc (Mast Therapeutics, Inc); Bayer AG; and Reata Pharmaceuticals, Inc. Dr Badesch has received honoraria for service on steering committees or advisory boards (or as a consultant) to the following companies working in the area of pulmonary hypertension: Actelion Pharmaceuticals US, Inc/CoTherix, Inc; Gilead; Pfizer Inc; United Therapeutics Corporation/Lung Rx LLC (a subsidiary of United Therapeutics Corporation); GlaxoSmithKline plc; Eli Lilly and Company/ICOS Corporation; Bayer AG; Ikaria, Inc; and Arena Pharmaceuticals, Inc. He has received grant support for clinical studies from GlaxoSmithKline plc; Actelion Pharmaceuticals US, Inc/CoTherix, Inc; Gilead; Pfizer Inc; United Therapeutics Corporation/Lung Rx LLC (a subsidiary of United Therapeutics Corporation); Eli Lilly and Company/ICOS Corporation; Bayer AG; and Novartis AG. He provided information pertinent to a legal matter for Actelion Pharmaceuticals US, Inc. Dr Frost has received honoraria for service on steering committees or advisory boards (or as a consultant) to the following companies working in the area of pulmonary hypertension: Actelion Pharmaceuticals US, Inc/CoTherix, Inc; Gilead; Pfizer Inc; United Therapeutics Corporation/Lung Rx LLC (a subsidiary of United Therapeutics Corporation); GlaxoSmithKline plc; Eli Lilly and Company/ICOS Corporation; Bayer AG; Ikaria, Inc; and Arena Pharmaceuticals, Inc. She has received grant support for clinical studies from VentriPoint, Inc; GlaxoSmithKline plc; Actelion Pharmaceuticals US, Inc; Gilead; Pfizer Inc; United Therapeutics Corporation/Lung Rx LLC (a subsidiary of United Therapeutics Corporation); InterMune; Stromedix (Biogen Idec); Bayer AG; and Novartis AG. Dr McLaughlin has acted as a consultant and/or speaker for Actelion Pharmaceuticals US, Inc; Bayer AG; Gilead; and United Therapeutics Corporation and has received research grants from Actelion Pharmaceuticals US, Inc; Bayer AG; Novartis AG; and United Therapeutics Corporation. Dr Barst served as a consultant for and received honoraria from Actelion Pharmaceuticals US, Inc; Bayer AG; Eli Lilly and Company; Gilead; GlaxoSmithKline plc; Ikaria, Inc; the National Institutes of Health/National Heart, Lung, and Blood Institute (NIH/NHLBI); Novartis AG; Pfizer Inc; and VentriPoint, Inc. She also owned shares in VentriPoint, Inc. Dr. Barst provided expert testimony on diet pill litigation for the plaintiffs. Dr Rosenberg is an employee of Actelion Pharmaceuticals US, Inc. Mr Miller is an employee of ICON Clinical Research, the biostatistics Clinical Research Organization for the PROSPECT Registry. Drs Hartline and Benton are employees of Actelion Pharmaceuticals US, Inc. Dr Farber serves as a consultant and is on the speaker’s bureau for Actelion Pharmaceuticals US, Inc; Ikaria, Inc; Bristol-Myers Squibb Company; Novartis AG; United Therapeutics Corporation; and Gilead. Dr Farber also receives grant/research support from Gilead and United Therapeutics Corporation.
Role of sponsors: The sponsor, Actelion Pharmaceuticals US, Inc, provided the study design and statistical analysis plan, managed the study registry, and participated in data analysis, interpretation, and preparation of the manuscript. Assistance in writing the first draft of the manuscript was provided by Tmirah Haselkorn, PhD, of EpiMetrix, Inc and paid by the sponsor.
Other contributions: The authors are saddened by the passing of Robyn J. Barst, MD, in April 2013. She was an esteemed physician, investigator, and colleague, and a distinguished leader in the field of pediatric pulmonary hypertension. Her contributions to the field are invaluable.
Additional information: The e-Tables can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- 6MWD
6-min walk distance
- BSI
blood stream infection
- CRI
chronic renal insufficiency
- CTD
connective tissue disease
- IPAH
idiopathic pulmonary arterial hypertension
- NYHA FC
New York Heart Association functional class
- PAH
pulmonary arterial hypertension
- PPH
primary pulmonary hypertension
- PROSPECT
Registry to Prospectively Describe Use of Epoprostenol for Injection (Veletri, prolonged room temperature stable-epoprostenol [RTS-Epo]) in Patients with Pulmonary Arterial Hypertension
- REVEAL Registry
Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management Registry
- RTS-Epo
room temperature stable-epoprostenol
- WHO
World Health Organization
Footnotes
Drs Frantz and Schilz contributed equally to this manuscript.
FUNDING/SUPPORT: Actelion Pharmaceuticals US, Inc, the sponsor of PROSPECT, provided funding and support for the analysis presented.
References
- 1.Shapiro SM, Oudiz RJ, Cao T, et al. Primary pulmonary hypertension: improved long-term effects and survival with continuous intravenous epoprostenol infusion. J Am Coll Cardiol. 1997;30(2):343-349. [DOI] [PubMed] [Google Scholar]
- 2.Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107(2):216-223. [DOI] [PubMed] [Google Scholar]
- 3.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142(2):448-456. [DOI] [PubMed] [Google Scholar]
- 4.Fleming TR, Powers JH. Biomarkers and surrogate endpoints in clinical trials. Stat Med. 2012;31(25):2973-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frost AE, Badesch DB, Miller DP, Benza RL, Meltzer LA, McGoon MD. Evaluation of the predictive value of a clinical worsening definition using 2-year outcomes in patients with pulmonary arterial hypertension: a REVEAL Registry analysis. Chest. 2013;144(5):1521-1529. [DOI] [PubMed] [Google Scholar]
- 6.Barst RJ, Rubin LJ, Long WA, et al. ; Primary Pulmonary Hypertension Study Group. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334(5):296-301. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin VV, Genthner DE, Panella MM, Rich S. Reduction in pulmonary vascular resistance with long-term epoprostenol (prostacyclin) therapy in primary pulmonary hypertension. N Engl J Med. 1998;338(5):273-277. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation. 2002;106(12):1477-1482. [DOI] [PubMed] [Google Scholar]
- 9.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40(4):780-788. [DOI] [PubMed] [Google Scholar]
- 10.Galiè N, Corris PA, Frost A, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(suppl 25):D60-D72. [DOI] [PubMed] [Google Scholar]
- 11.Taichman DB, Mandel J. Epidemiology of pulmonary arterial hypertension. Clin Chest Med. 2013;34(4):619-637. [DOI] [PubMed] [Google Scholar]
- 12.Lambert O, Bandilla D, Iyer R, Witchey-Lakshmanan L, Palepu N. Stability and microbiological properties of a new formulation of epoprostenol sodium when reconstituted and diluted. Drug Des Devel Ther. 2012;6:61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benza RL, Gomberg-Maitland M, Miller DP, et al. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest. 2012;141(2):354-362. [DOI] [PubMed] [Google Scholar]
- 14.McGoon MD, Benza RL, Escribano-Subias P, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol. 2013;62(suppl 25):D51-D59. [DOI] [PubMed] [Google Scholar]
- 15.Farber HW, Miller DP, Meltzer LA, McGoon MD. Treatment of patients with pulmonary arterial hypertension at the time of death or deterioration to functional class IV: insights from the REVEAL Registry. J Heart Lung Transplant. 2013;32(11):1114-1122. [DOI] [PubMed] [Google Scholar]
- 16.Cornwell WK, McLaughlin VV, Krishnan SM, Rubenfire M. Does the outcome justify an oral-first treatment strategy for management of pulmonary arterial hypertension? Chest. 2011;140(3):697-705. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro S, Traiger GL, Turner M, McGoon MD, Wason P, Barst RJ. Sex differences in the diagnosis, treatment, and outcome of patients with pulmonary arterial hypertension enrolled in the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Chest. 2012;141(2):363-373. [DOI] [PubMed] [Google Scholar]
- 18.Mair KM, Johansen AK, Wright AF, Wallace E, MacLean MR. Pulmonary arterial hypertension: basis of sex differences in incidence and treatment response. Br J Pharmacol. 2014;171(3):567-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122(2):156-163. [DOI] [PubMed] [Google Scholar]
- 20.Humbert M, Sitbon O, Yaïci A, et al. ; French Pulmonary Arterial Hypertension Network. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J. 2010;36(3):549-555. [DOI] [PubMed] [Google Scholar]
- 21.Kane GC, Maradit-Kremers H, Slusser JP, Scott CG, Frantz RP, McGoon MD. Integration of clinical and hemodynamic parameters in the prediction of long-term survival in patients with pulmonary arterial hypertension. Chest. 2011;139(6):1285-1293. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs W, van de Veerdonk MC, Trip P, et al. The right ventricle explains sex differences in survival in idiopathic pulmonary arterial hypertension. Chest. 2014;145(6):1230-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kallen AJ, Lederman E, Balaji A, et al. Bloodstream infections in patients given treatment with intravenous prostanoids. Infect Control Hosp Epidemiol. 2008;29(4):342-349. [DOI] [PubMed] [Google Scholar]
- 24.Kitterman N, Poms A, Miller DP, Lombardi S, Farber HW, Barst RJ. Bloodstream infections in patients with pulmonary arterial hypertension treated with intravenous prostanoids: insights from the REVEAL REGISTRY®. Mayo Clin Proc. 2012;87(9):825-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller DP, Gomberg-Maitland M, Humbert M. Survivor bias and risk assessment. Eur Respir J. 2012;40(3):530-532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement