Abstract
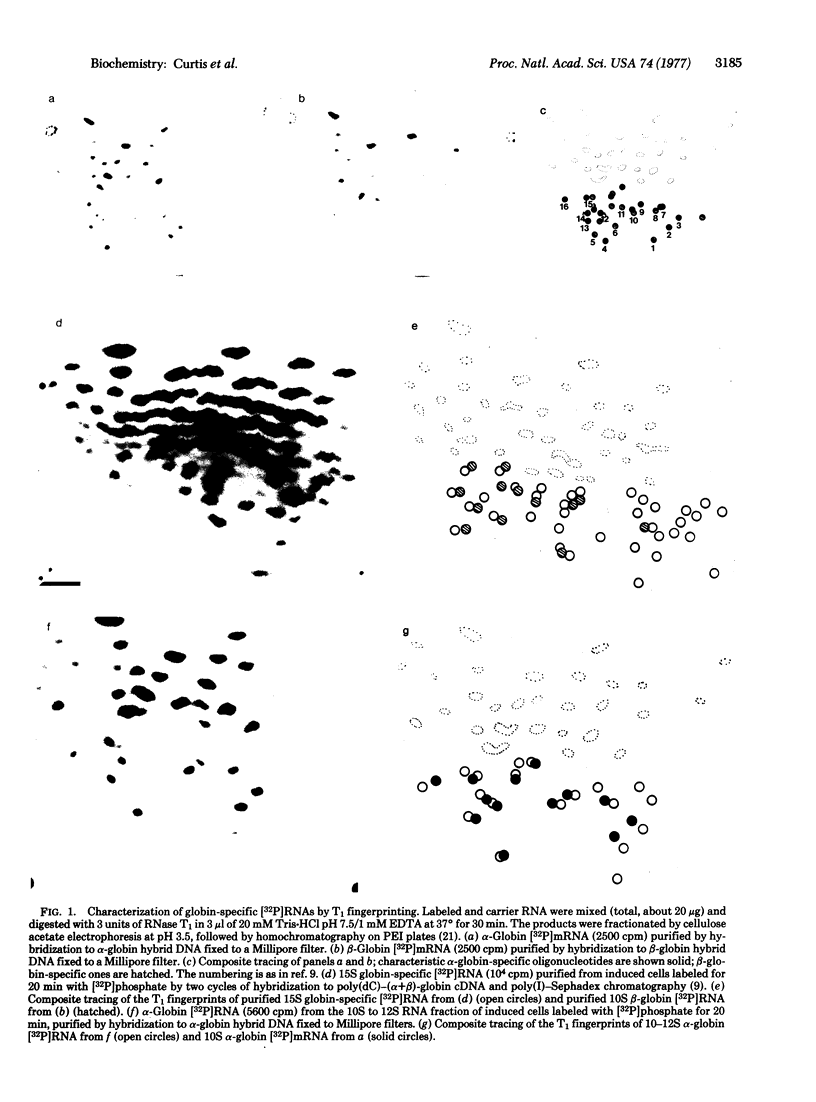
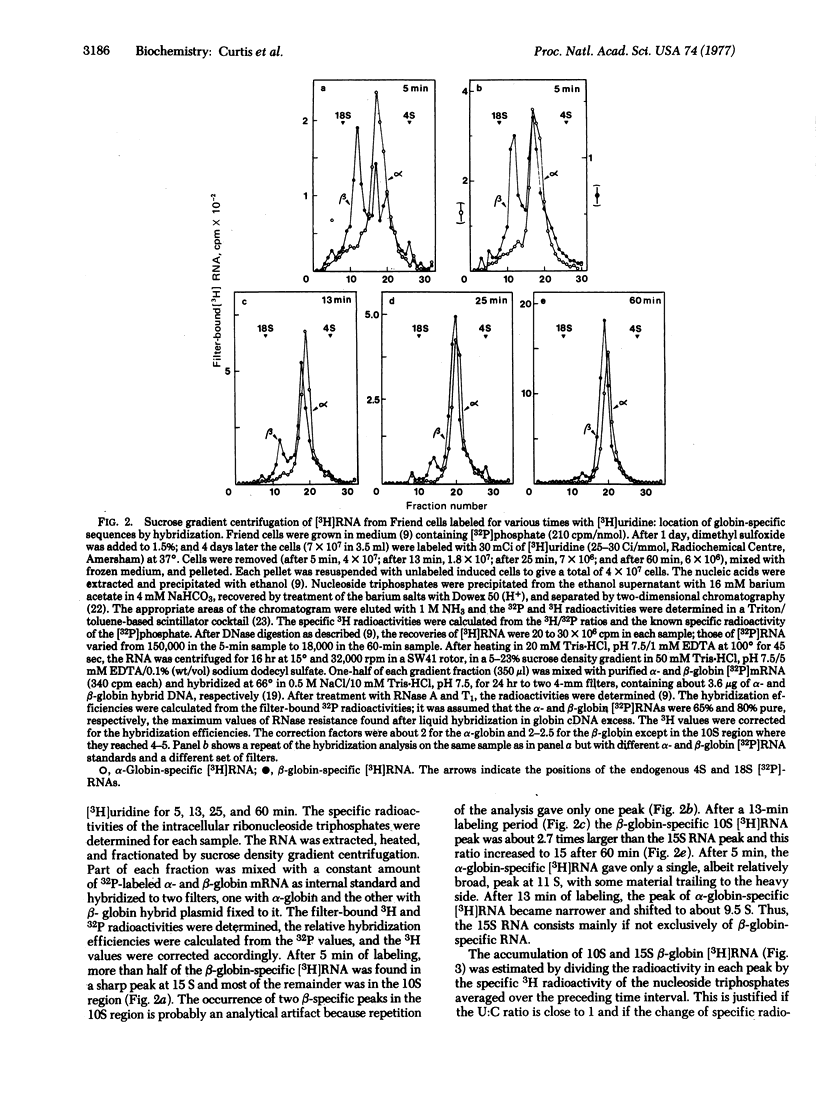
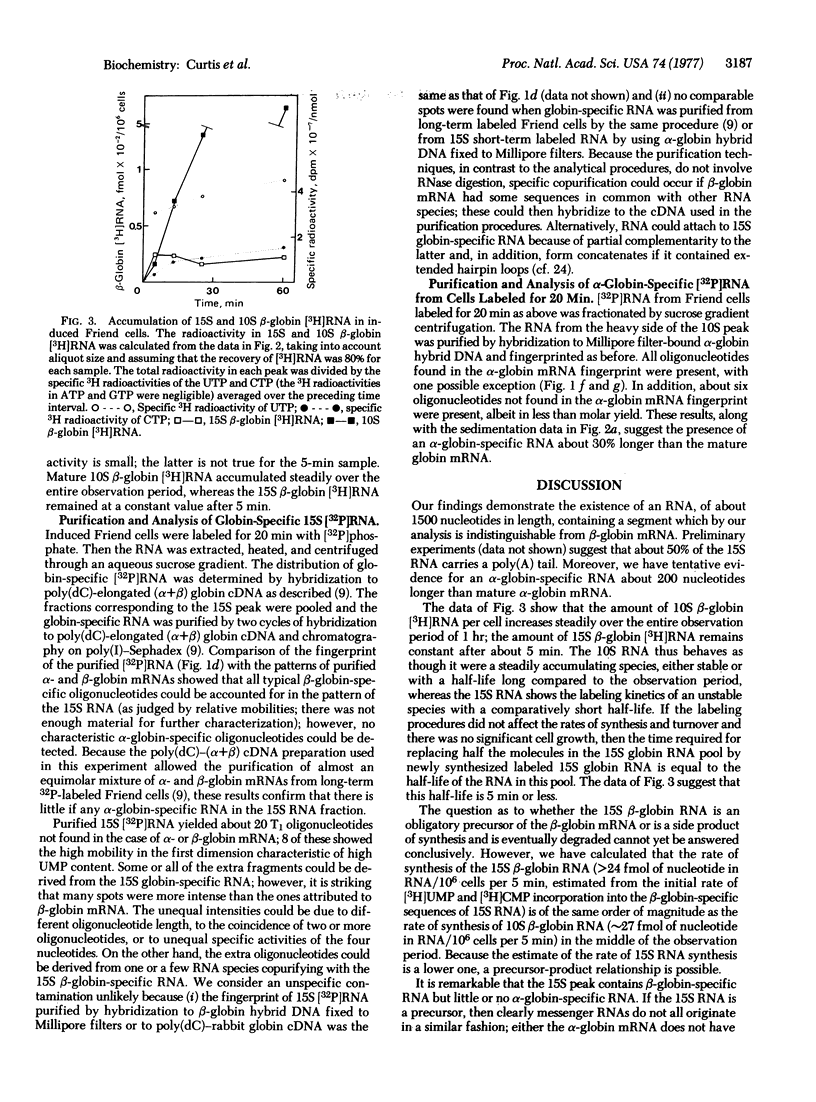
Dimethyl sulfoxide-induced Friend cells were labeled for periods of 5-60 min. The denatured RNA was fractionated by sucrose gradient centrifugation and the distribution of α- and β-globin-specific [3H]RNA was determined by hybridization to hybrid plasmids containing mouse α- and β-globin DNA, respectively. After 5 min of labeling, a 15S peak of β-globin-specific (but not α-globin-specific) [3H]RNA was detected, next to an equal amount of 10S β-globin [3H]RNA. With increasing periods of labeling, the amount of 15S β-globin [3H]RNA remained constant but the amount 10S β-globin [3H]RNA increased steadily. α-Globin-specific [3H]RNA sedimented at 11 S after 5 min of labeling and at 9.5 S after longer labeling periods. Analysis of 15S globin-specific [3H]RNA purified by the poly(dC)-cDNA method [Curtis, P. J. & Weissmann, C. (1976) J. Mol. Biol. 106, 1061-1075] showed oligonucleotides characteristic of β-globin mRNA but not of α-globin mRNA, as well as about 20 new oligonucleotides. Our results suggest that 10S β-globin mRNA arises via a 15S precursor that has a half-life of 5 min or less; 9.5S α-globin mRNA may be derived from an 11S precursor.
Keywords: globin mRNA precursor, mouse globin cDNA plasmid
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coffin J. M., Parsons J. T., Rymo L., Haroz R. K., Weissmann C. A new approach to the isolation of RNA-DNA hybrids and its application to the quantitative determination of labeled tumor virus RNA. J Mol Biol. 1974 Jun 25;86(2):373–396. doi: 10.1016/0022-2836(74)90026-6. [DOI] [PubMed] [Google Scholar]
- Covey C., Richardson D., Carbon J. A method for the deletion of restriction sites in bacterial plasmid deoxyribonucleic acid. Mol Gen Genet. 1976 May 7;145(2):155–158. doi: 10.1007/BF00269587. [DOI] [PubMed] [Google Scholar]
- Curtis P. J., Weissmann C. Purification of globin messenger RNA from dimethylsulfoxide-induced Friend cells and detection of a putative globin messenger RNA precursor. J Mol Biol. 1976 Oct 5;106(4):1067–1075. doi: 10.1016/0022-2836(76)90353-3. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jelinek W. R., Molloy G. R. Biogenesis of mRNA: genetic regulation in mammalian cells. Science. 1973 Sep 28;181(4106):1215–1221. doi: 10.1126/science.181.4106.1215. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenbank R., Guthrie C., Stöffler G., Wittmann H. G., Rosen L., Apirion D. Electrophoretic and immunological studies on ribosomal proteins of 100 Escherichia coli revertants from streptomycin dependence. Mol Gen Genet. 1973 Dec 14;127(1):1–18. doi: 10.1007/BF00267778. [DOI] [PubMed] [Google Scholar]
- Hofstetter H., Schamböck A., Van Den Berg J., Weissmann C. Specific excision of the inserted DNA segment from hybrid plasmids constructed by the poly(dA). poly (dT) method. Biochim Biophys Acta. 1976 Dec 13;454(3):587–591. doi: 10.1016/0005-2787(76)90286-0. [DOI] [PubMed] [Google Scholar]
- Kwan S. P., Wood T. G., Lingrel J. B. Purification of a putative precursor of globin messenger RNA from mouse nucleated erythroid cells. Proc Natl Acad Sci U S A. 1977 Jan;74(1):178–182. doi: 10.1073/pnas.74.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Kee S. G., Efstratiadis A., Kafatos F. C. Amplification and characterization of a beta-globin gene synthesized in vitro. Cell. 1976 Jun;8(2):163–182. doi: 10.1016/0092-8674(76)90001-5. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Schimke R. T. Ovalbumin messenger RNA: evidence that the initial product of transcription is the same size as polysomal ovalbumin messenger. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4327–4331. doi: 10.1073/pnas.71.11.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Coffin J. M., Haroz R. K., Bromley P. A., Weissmann C. Quantitative determination and location of newly synthesized virus-specific ribonucleic acid in chicken cells infected with Rous sarcoma virus. J Virol. 1973 May;11(5):761–774. doi: 10.1128/jvi.11.5.761-774.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. A precursor of globin messenger RNA. J Mol Biol. 1976 Sep 15;106(2):403–420. doi: 10.1016/0022-2836(76)90093-0. [DOI] [PubMed] [Google Scholar]
- Ryskov A. P., Tokarskaya O. V., Georgiev G. P., Coutelle C., Thiele B. Globin mRNA contains a sequence complementary to double-stranded region of nuclear pre-mRNA. Nucleic Acids Res. 1976 Jun;3(6):1487–1498. doi: 10.1093/nar/3.6.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer K., Marcaud L., Zajdela F., Breckenridge B., Gros F. Etude des RNA nucléaires et cytoplasmiques à marquage rapide dans les cellules érythropoiétiques aviaires différenciées. Bull Soc Chim Biol (Paris) 1966;48(10):1037–1075. [PubMed] [Google Scholar]
- Spohr G., Imaizumi T., Scherrer K. Synthesis and processing of nuclear precursor-messenger RNA in avian erythroblasts and HeLa cells. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5009–5013. doi: 10.1073/pnas.71.12.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert G., Jou W. M., Fiers W. Analysis of 32P-labeled bacteriophage MS2 RNA by a mini-fingerprinting procedure. Anal Biochem. 1976 May 7;72:433–446. doi: 10.1016/0003-2697(76)90551-0. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Warnaar S. O., Winocour E. Isolation and characterization of simian virus 40 ribonucleic acid. J Virol. 1972 Aug;10(2):193–201. doi: 10.1128/jvi.10.2.193-201.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann C., Boll W. Reduction of possible hazards in the preparation of recombinant plasmid DNA. Nature. 1976 Jun 3;261(5559):428–429. doi: 10.1038/261428a0. [DOI] [PubMed] [Google Scholar]
- Williamson R., Drewienkiewicz C. E., Paul J. Globin messenger sequences in high molecular weight RNA from embryonic mouse liver. Nat New Biol. 1973 Jan 17;241(107):66–68. doi: 10.1038/newbio241066a0. [DOI] [PubMed] [Google Scholar]