Abstract
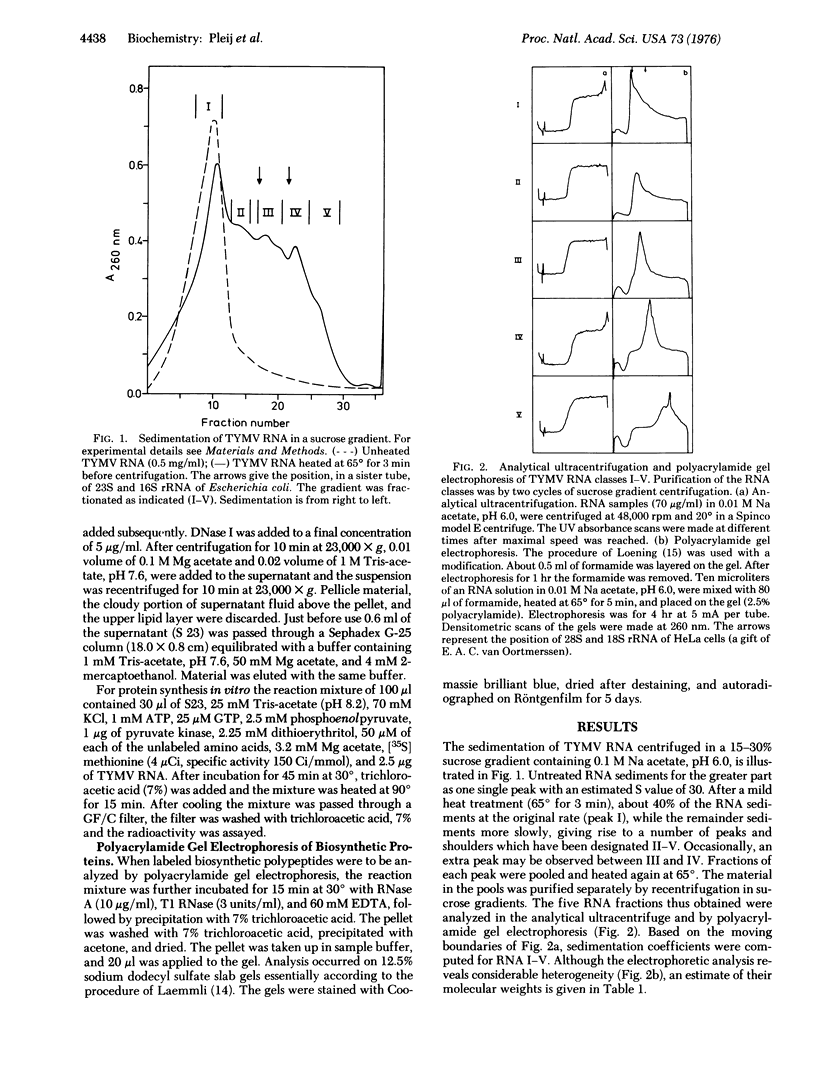
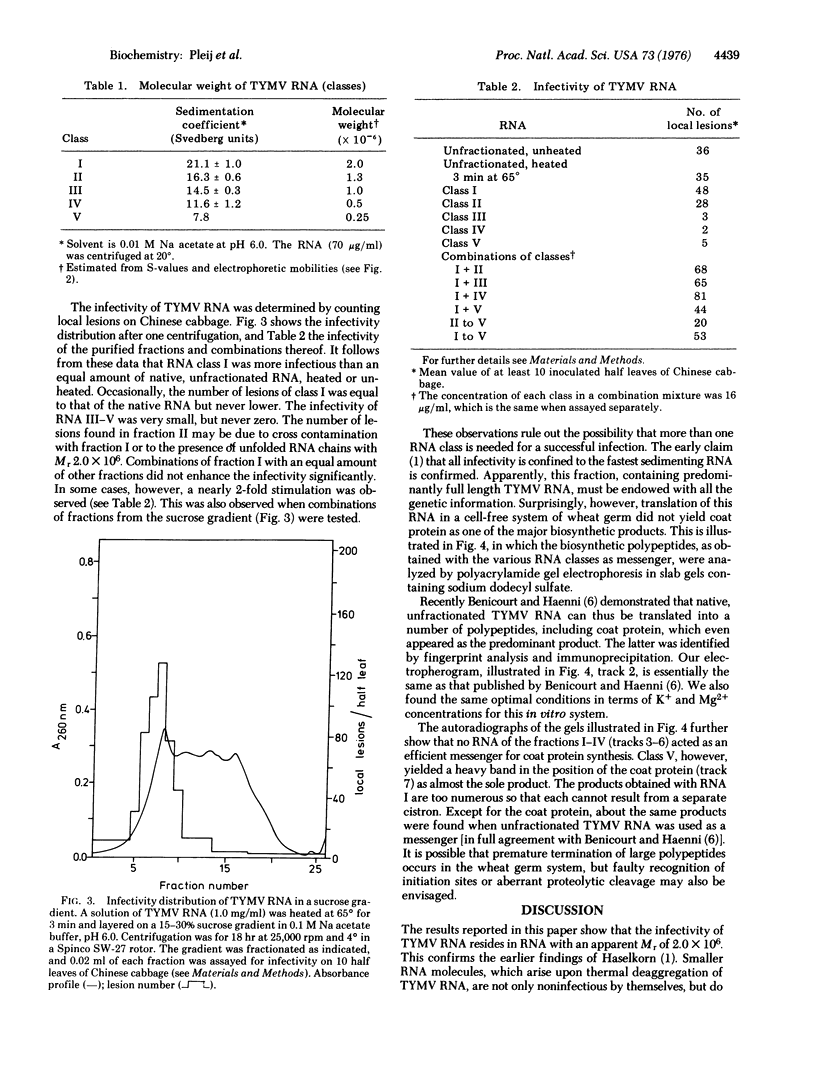
Sucrose gradient centrifugation of heat-denatured RNA of turnip yellow mosaic virus permitted the isolation of five RNA classes with molecular weights ranging from 2.0 to 0.25 X 10(6). The infectivity was shown to be confined to an RNA molecule of molecular weight 2.0 X 10(6). No significant increase in infectivity was obtained by combination of the latter RNA with the RNA classes of smaller size. Translation in vitro of the RNAs of different size classes in a wheat germ cell-free system revealed that the infectious RNA (molecular weight 2.0 X 10(6) does not promote the synthesis of the coat protein of turnip yellow mosaic virus. Efficient production of this coat protein was found exclusively when the smallest RNA class (molecular weight 250,000) was used as a messenger. It is concluded that RNA molecules of turnip yellow mosaic virus of molecular weight 2.0 X 10(6) contain a closed coat protein cistron and that RNA molecules of molecular weight about 2 to 3 X 10(5) with an open coat protein cistron can be isolated from the virions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachy R. N., Zaitlin M. Replication of tobacco mosiac virus, VI Replicative intermediate and TMV-RNA-related RNAs associated with polyribosomes. Virology. 1975 Jan;63(1):84–97. doi: 10.1016/0042-6822(75)90373-6. [DOI] [PubMed] [Google Scholar]
- DUNN D. B., HITCHBORN J. H. THE USE OF BENTONITE IN THE PURIFICATION OF PLANT VIRUSES. Virology. 1965 Feb;25:171–192. doi: 10.1016/0042-6822(65)90198-4. [DOI] [PubMed] [Google Scholar]
- Davies J. W., Kaesberg P. Translation of virus mRNA: synthesis of bacteriophage Q beta proteins in a cell-free extract from wheat embryo. J Virol. 1973 Dec;12(6):1434–1441. doi: 10.1128/jvi.12.6.1434-1441.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faed E. M., Burns D. J., Matthews R. E. Properties of minor nucleoprotein components found in TYMV preparations. Virology. 1972 May;48(2):627–629. doi: 10.1016/0042-6822(72)90079-7. [DOI] [PubMed] [Google Scholar]
- HASELKORN R. Studies on infectious RNA from turnip yellow mosaic virus. J Mol Biol. 1962 May;4:357–367. doi: 10.1016/s0022-2836(62)80016-3. [DOI] [PubMed] [Google Scholar]
- Hunter T. R., Hunt T., Knowland J., Zimmern D. Messenger RNA for the coat protein of tobacco mosaic virus. Nature. 1976 Apr 29;260(5554):759–764. doi: 10.1038/260759a0. [DOI] [PubMed] [Google Scholar]
- JOHNSTON F. B., STERN H. Mass isolation of viable wheat embryos. Nature. 1957 Jan 19;179(4551):160–161. doi: 10.1038/179160b0. [DOI] [PubMed] [Google Scholar]
- Jaspars E. M. Plant viruses with a multipartite genome. Adv Virus Res. 1974;19:37–149. doi: 10.1016/s0065-3527(08)60659-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS R. E. Properties of nucleoprotein fractions isolated from turnip yellow mosaic virus preparations. Virology. 1960 Dec;12:521–539. doi: 10.1016/0042-6822(60)90176-8. [DOI] [PubMed] [Google Scholar]
- Matthews R. F., Ralph R. K. Turnip yellow mosaic virus. Adv Virus Res. 1966;12:273–328. doi: 10.1016/s0065-3527(08)60851-9. [DOI] [PubMed] [Google Scholar]
- Mitra S., Kaesberg P. Biophysical properties of RNA from turnip yellow mosaic virus. J Mol Biol. 1965 Dec;14(2):558–571. doi: 10.1016/s0022-2836(65)80204-2. [DOI] [PubMed] [Google Scholar]
- Peter R., Stehelin D., Reinbolt J., Collot D., Duranton H. Primary structure of turnip yellow mosaic virus coat protein. Virology. 1972 Aug;49(2):615–617. doi: 10.1016/0042-6822(72)90516-8. [DOI] [PubMed] [Google Scholar]
- Pley C. W., Talens J., Bosch L., Mandel M. In situ breakage of turnip yellow mosaic virus RNA and in situ aggregation of the fragments. Analysis of successive stages. Virology. 1969 Jul;38(3):371–379. doi: 10.1016/0042-6822(69)90149-4. [DOI] [PubMed] [Google Scholar]
- Siegel A., Zaitlin M., Duda C. T. Replication of tobacco mosaic virus. IV. Further characterization of viral related RNAs. Virology. 1973 May;53(1):75–83. doi: 10.1016/0042-6822(73)90466-2. [DOI] [PubMed] [Google Scholar]
- Yot P., Pinck M., Haenni A. L., Duranton H. M., Chapeville F. Valine-specific tRNA-like structure in turnip yellow mosaic virus RNA. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1345–1352. doi: 10.1073/pnas.67.3.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]