Abstract
The functions of the nervous system can be powerfully modulated by the immune system. Although traditionally considered to be quite separate, neuro-immune interactions are increasingly recognized as critical for both normal and pathological nervous system function in the adult. However, a growing body of information supports a critical role for neuro-immune interactions before birth, particularly in the prenatal programming of later-life neurobehavioral disease risk. This review will focus on maternal obesity, as it represents an environment of pathological immune system function during pregnancy that elevates offspring neurobehavioral disease risk. We will first delineate the normal role of the immune system during pregnancy, including the role of the placenta as both a barrier and relayer of inflammatory information between the maternal and fetal environments. This will be followed by the current exciting findings of how immuno-modulatory molecules may elevate offspring risk of neurobehavioral disease by altering brain development and, consequently, later life function. Finally, by drawing parallels with pregnancy complications other than obesity, we will suggest that aberrant immune activation, irrespective of its origin, may lead to neuro-immune interactions that otherwise would not exist in the developing brain. These interactions could conceivably derail normal brain development and/or later life function, and thereby elevate risk for obesity and other neurobehavioral disorders later in the offspring's life.
Keywords: inflammation, cytokines, in utero, brain development, DOHaD, pregnancy, epigenetics
Introduction
The period of life before birth is the most important time in our lives. During this time, developmental mechanisms put into place the cellular foundations on which our functioning, thinking, and feeling bodies will exist, in sickness and in health, across our lifespan. During the period of embryonic and fetal development, the mother's health appears to be absolutely critical to the later life health of her offspring.
This concept was first appreciated when epidemiological studies examined the long-term health outcomes of individuals who were in utero during the Dutch hunger winter (1944–1945). The offspring of these undernourished mothers had low birth weight and impaired glucose tolerance later in life compared to individuals born in flanking years (Ravelli et al., 1998). Interestingly, the timing of exposure to famine within the gestational period is also critically important. Thus, people exposed to famine in early gestation in the Dutch hunger winter were at greater risk of developing coronary heart disease and obesity in later life, whereas those exposed to famine in mid and late gestation were not similarly affected (Ravelli et al., 1976; Roseboom et al., 2000). Moreover, impaired glucose tolerance in offspring appeared more likely to result from undernutrition in late gestation (Ravelli et al., 1998). These observations inspired the concept that an individual's risk of disease across their lifespan could be shaped by events that occurred much earlier in their lives; indeed during their time in the womb (Barker, 2004). With the recent massive increase in obesity across the western world, much focus has now shifted to understanding the long-term health outcomes of individuals whose mothers were obese during pregnancy. Curiously, the epidemiological data paint a similar picture to undernutrition. Individuals whose mothers were obese during pregnancy show a significantly higher risk for later life obesity, and “the metabolic syndrome” (Law et al., 1992; Gale et al., 2007; Armitage et al., 2008; Crozier et al., 2010; Tamashiro and Moran, 2010; Alfaradhi and Ozanne, 2011; Ornoy, 2011). However, they also show increased risk for a constellation of behavioral and mental health problems including autism, attention deficit/hyperactivity disorder, developmental delay, anxiety, and depression (Herva et al., 2008; Rodriguez, 2010; Van Lieshout and Boyle, 2011a,b; Colman et al., 2012; Halmoy et al., 2012; Krakowiak et al., 2012; Moore et al., 2012; Wojcik et al., 2013).
Although nutrition during gestation and nutrition after birth are key contributors to neurobehavioral disease risk across the lifespan, the mechanisms that link these factors, either alone or together, to health risks and outcomes remain poorly understood. Animal models, typically rodent, sheep, or non-human primate (NHP), have been invaluable in shedding light on the cellular and molecular details occurring behind the scenes. In these models, research has traditionally focused on defining the deregulated function of offspring peripheral organs involved in metabolism, such as the pancreas, liver, adipose depots, and skeletal muscle whose function is impaired in obesity and the metabolic syndrome. However, since body weight and metabolic function are regulated by the brain (Stanley and Leibowitz, 1984; Fan et al., 1997; Cowley et al., 1999; Horvath et al., 1999; Cone et al., 2001; Elmquist et al., 2005), and since maternal nutrition during pregnancy programs an assortment of offspring neurological abnormalities, studies examining changes in the offspring brain have become increasingly prevalent.
In pregnancies complicated by maternal obesity, changes in the regions of the fetal brain that will regulate body weight later in life, including the arcuate nucleus of the hypothalamus (ARC) and the paraventricular nucleus of the hypothalamus (PVN), and brain areas associated with reward and food seeking have been reported. In the body weight regulating areas, the adult offspring of obese mothers have been reported to show: (i) altered expression of the appetite-regulating neuropeptides, including agouti-related peptide (AgRP, reduced) and pro-opiomelanocortin (POMC, reduced), in the ARC; (ii) altered expression of receptors for appetite-regulating neuropeptides, including neuropeptide Y (NPY) receptor Y1 and melanocortin receptor MC4R (both increased), in the PVN; (iii) altered neurogenesis leading to increased numbers of body weight-regulating neurons expressing orexin, melanin concentrating hormone (MCH), galanin, enkephalin, and dynorphin (Chang et al., 2008; Chen et al., 2009); (iv) ARC neuron leptin resistance, as judged by reduced pSTAT3 in response to exogenous leptin administration; (v) and developmental alterations in the neural circuitry that regulates body weight (Bouret et al., 2008; Kirk et al., 2009; Sanders et al., 2014). In the reward pathways, there are reports of altered gene expression in the opioid system (Grissom et al., 2014), changes in the mesolimbic dopamine pathways, including increased dopamine synthesis in the nucleus accumbens (NAc) and ventral tegmental area (VA), and altered dopamine responsiveness in the NAc accompanied by reduced dopamine receptor D2 expression in the VTA (Naef et al., 2008, 2011). In the offspring hippocampus, abnormalities have been observed in neural circuit formation, neurogenesis and cell death both in the late gestation fetus and in the early post-natal period (Niculescu and Lupu, 2009; Tozuka et al., 2009, 2010), increased oxidative stress, and reduced brain derived neurotrophic factor (BDNF) mRNA and protein (Tozuka et al., 2009, 2010). Such offspring also exhibited behavioral changes that are consistent with the associations between maternal obesity and some aspects of offspring mental illness observed in humans. For example, the offspring of obese non-human mothers show defects in spatial learning in the Barnes maze test (Tozuka et al., 2010), elevated anxiety (Sasaki et al., 2014), and altered reward seeking behaviors (Naef et al., 2008, 2011).
Although not comprehensive, the above summary demonstrates that substantial progress has been, and is being, made in defining the spectrum of changes that eventuate after birth when an individual has undergone gestation in an obese mother. What remains to be discovered is what happens during gestation, what molecular factors are important, and how they act on the developing organism to affect the changes that increase disease risk much later in life. Although many factors could be at work, the potential importance of the immune system stands out for two main reasons. Firstly, it has a critical and highly regulated role at the fetal-maternal interface during normal pregnancy. Secondly, pregnancies complicated by obesity, be they human or non-human, are accompanied by elevated cytokine levels in both the maternal (Catalano et al., 2009; Madan et al., 2009; Roberts et al., 2011; Kepczynska et al., 2013) and fetal circulation (Heerwagen et al., 2013; Kim et al., 2014) across gestation (summarized in Table 1). Moreover, if one considers additional complications of pregnancy that also elevate offspring risk for metabolic disease and behavioral abnormalities, one feature that they all have in common is abnormal immune system activation and elevated cytokine levels during gestation.
Table 1.
Summary of inflammatory response in maternal obesity across different species.
| Species | Source | Elevated cytokines | Developmental stage |
|---|---|---|---|
| Human | Maternal circulation | Leptin (Stewart et al., 2007; Challier et al., 2008; Catalano et al., 2009; Madan et al., 2009), CRP (Stewart et al., 2007; Challier et al., 2008; Catalano et al., 2009; Madan et al., 2009), IL6 (Challier et al., 2008; Catalano et al., 2009; Roberts et al., 2011), MCP-1 (Madan et al., 2009) | Second trimester (Madan et al., 2009), first to third trimester (Stewart et al., 2007), Pre-labor (Challier et al., 2008; Catalano et al., 2009), Term (Roberts et al., 2011) |
| Placental sample (isolated placental macrophages) | IL6 (Challier et al., 2008), IL1 (Challier et al., 2008; Roberts et al., 2011), IL8 (Roberts et al., 2011), TNF (Challier et al., 2008), MCP1 (Challier et al., 2008) | Term (Challier et al., 2008; Roberts et al., 2011) | |
| Fetus (umbilical cord blood) | Leptin (Catalano et al., 2009), IL6 (Catalano et al., 2009) | Term (Catalano et al., 2009) | |
| Non-human primate | Maternal circulation | Leptin (Frias et al., 2011) | Day 130 (of 170–180 d gestation)(Frias et al., 2011) |
| Placental sample | MCP1 (Frias et al., 2011), IL1B (Frias et al., 2011) | Day 130 (of 170–180 d gestation)(Frias et al., 2011) | |
| Sheep | Placental sample | TNF (Zhu et al., 2010), IL6 (Zhu et al., 2010), IL8 (Zhu et al., 2010), IL18 (Zhu et al., 2010) | Day 75 (of 135 d gestation)(Zhu et al., 2010) |
| Mouse | Maternal circulation | IL1B (Heerwagen et al., 2013; Kim et al., 2014), IL6 (Heerwagen et al., 2013; Ingvorsen et al., 2014; Kim et al., 2014), IL10 (Kim et al., 2014), IFNG (Heerwagen et al., 2013; Kim et al., 2014), TNF (Heerwagen et al., 2013; Ingvorsen et al., 2014; Kim et al., 2014), CXCL1 (Heerwagen et al., 2013), MIP2a (Heerwagen et al., 2013), CCL25 (Heerwagen et al., 2013), GM-CSF (Heerwagen et al., 2013), IL2 (Heerwagen et al., 2013), IL3 (Heerwagen et al., 2013), IL9 (Heerwagen et al., 2013) | GD15.5 (Kim et al., 2014), GD17.5 (Kim et al., 2014), GD18.5 (Heerwagen et al., 2013; Ingvorsen et al., 2014) |
| Placental sample | IL6 (Kim et al., 2014), IL1b (Heerwagen et al., 2013; Kim et al., 2014), TNF (Kim et al., 2014), IL10 (Kim et al., 2014), iNOS (Heerwagen et al., 2013) | GD17.5 (Kim et al., 2014), GD18.5 (Heerwagen et al., 2013) | |
| Fetal circulation | IL6 (Kim et al., 2014), IL17A (Kim et al., 2014), IFNG (Kim et al., 2014) | GD17.5 (Kim et al., 2014) |
The placenta and immune system during pregnancy
There are multiple changes occurring in a mother's body during pregnancy, including increased adiposity and low-grade inflammation (Mor et al., 2011; Zhang et al., 2011). Such dynamic pregnancy-specific changes are believed, at least in part, to be responsible for the maternal adaptation to the presence of the developing fetus. Indeed, the fetus, despite being immunologically foreign, is not rejected by the maternal immune system. In mammals, this protection is partly provided by the placenta, a transient organ that forms at the interface between the mother and fetus. The placenta is composed of both maternal and fetal cells and develops upon implantation in humans and early gestation in rodents (Watson and Cross, 2005). Despite the variability in placentation across species, placental function is strikingly similar among most placental mammals (Enders and Blankenship, 1999; Malassine et al., 2003).
Normal function of the placenta
One of the most critical functions of the placenta is to act as an immunological barrier, which protects the fetus from the maternal immune system (Kanellopoulos-Langevin et al., 2003; Mor et al., 2011). Although the full spectrum of functions that the placenta performs in its role as an immunological barrier are not well-understood, current understanding indicates that placental and decidual immune cells, such as macrophages, have an immunosuppressive function, and thus dampen the maternal immune response toward the immunologically distinct fetus (Chang et al., 1993; Lin et al., 1993; Heikkinen et al., 2003; Gustafsson et al., 2008; Houser et al., 2011; Arck and Hecher, 2013). The placenta can also respond to cytokines (Jones et al., 2009; Hsiao and Patterson, 2011); and it has been argued that this serves as a mechanism for relaying information from the maternal environment to the fetus. The ability of maternal cytokines to cross the placental barrier and directly affect the fetus is still debatable. Previous studies have shown that some cytokines, when injected into pregnant rodent dams, are able to cross the placenta and enter the fetal circulation. Some cytokines have also been shown to cross the human placenta ex vivo (Zaretsky et al., 2004; Dahlgren et al., 2006). This is contrasted, however, by other studies in humans and rodents, which have demonstrated a lack of cytokine transport from maternal to fetal circulation (Carbo et al., 1998; Aaltonen et al., 2005). Reconciling these datasets will require improved understanding of developmental timing in order to interweave better our knowledge of the development of cytokine-specific transport mechanisms in the placenta, changing cytokine production across gestation (Steinborn et al., 1995, 1998; Mark et al., 2013), and how both cytokine-specific and non-specific placental permeability change across gestation (Atkinson et al., 1991; Kent et al., 1994; Dahlgren et al., 2006). Nevertheless, what is clear is that the normal placenta, and its resident immune cells, performs a critical balancing act in order to be both the barrier and communicator between the mother and fetus.
Altered placental function in maternal obesity may mean fetal exposure to cytokines and other immune system modulators
Human and animal studies have shown that maternal obesity alters placental morphology and vascularization (Hayes et al., 2012; Hayward et al., 2013; Kim et al., 2014). In rodent models of maternal obesity, decreased layer thickness, reduced trophoblast proliferation, reduced vascular supply and resultant hypoxia in the labyrinth were described (Hayes et al., 2012; Kim et al., 2014). In humans, maternal obesity caused reduced chorionic plate artery function, which may reduce blood supply to the fetus (Hayward et al., 2013). In addition, maternal obesity led to placental inflammation and altered cytokine production in human, rodent, and ovine models (Challier et al., 2008; Zhu et al., 2010; Roberts et al., 2011; Kim et al., 2014), including elevation in IL-6, IL-1β, and TNF production (all models, see Table 1 for complete list and associated animal model), as well as an increase in infiltrating monocytes and activated macrophages (Challier et al., 2008; Roberts et al., 2011; Kim et al., 2014). Also, since cytokines can alter placental nutrient transport, and maternal obesity can disrupt the placental vascular system (Jones et al., 2009; Hayes et al., 2012; Hayward et al., 2013), it is likely that fetuses developing in obese mothers would be exposed to factors from maternal and placental sources that would not be present in a normal pregnancy.
Evidence for altered brain development and offspring obesity consequent to pathological cytokine exposure: a common link?
The idea that inappropriate exposure of the fetal brain to cytokines can interfere with neural development is an interesting idea, but has yet to gain traction in the field of developmental programming by maternal obesity, with currently only one publication suggesting that inappropriate exposure to inflammatory cytokines specifically may derail brain development (Sanders et al., 2014). Thus, we may be well-served to look to other models of developmental programming for clues to both common and diverse mechanisms leading to offspring neurobehavioral disorders. If activation of the immune system were to be a shared causative agent in the offspring programming of metabolic and mental health disorders, then any pregnancy conditions that lead to these health outcomes should also exhibit immune system activation. Below we briefly review several instances where the inflammatory environment is changed during gestation, and where adverse neurobehavioral consequences to the offspring are observed (summarized in Table 2).
Table 2.
Summary of different pregnancy states and their metabolic or behavioral sequelae in the offspring.
| Maternal state | Offspring phenotype | Animal models | Human data |
|---|---|---|---|
| Maternal infection | Neurobehavioral disorders | Maternal immune activated (MIA) rodents produce offspring with schizophrenia and ASD – like phenotypes (Smith et al., 2007; Meyer et al., 2008; Hsiao et al., 2012; Malkova et al., 2012) Offspring of IL-6 injected rats display a subset of the MIA phenotype (Smith et al., 2007) | Maternal influenza, rubella, and respiratory tract infections during pregnancy increases offspring risk for Schizophrenia and ASDs (Williams and Mackenzie, 1977; Irving et al., 2000; Smith et al., 2007; Meyer et al., 2008; Hsiao et al., 2012; Malkova et al., 2012) |
| Metabolic disease | Offspring of MIA and IL-6 injected rodents display metabolic symptoms (Dahlgren et al., 2006; Pacheco-López et al., 2011) IL6 affects fetal hypothalamic circuitry development (Sanders et al., 2014) | Schizophrenic patients more likely to develop metabolic disease (Ryan et al., 2003; Thakore, 2004) | |
| Maternal autoimmune disease | Neurobehavioral disorders | N/A | Maternal autoimmune diseases associated with increased risk of offspring neurodevelopmental disorders such as learning disabilities and ASD (McAllister et al., 1997; Ross et al., 2003; Neri et al., 2004) |
| Metabolic disease | N/A | Maternal systemic lupus erythematosus associated with low birthweight – a risk factor for offspring obesity (Baer et al., 2011) | |
| Maternal smoking | Neurobehavioral disorders | Prenatal cigarette exposure mouse model results in offspring displaying hyperactive behaviors with disrupted memory (Balsevich et al., 2014) | Maternal smoking a risk factor for offspring aggressive behavior, inattention and conduct disorder, and attention deficit disorder (Fergusson et al., 1993, 1998; Orlebeke et al., 1999; Linnet et al., 2003) |
| Metabolic disease | Mice exposed to cigarette smoke during pregnancy have offspring with increased body weight and plasma leptin (Chen et al., 2011) | Maternal smoking predisposes offspring to obesity in adolescence (Power and Jefferis, 2002; Al Mamun et al., 2006) | |
| Air pollution exposure | Neurobehavioral disorders | Offspring of diesel exhaust exposed mice display decreased locomotion, changes to dopamine levels, and neurodevelopmental changes (Hougaard et al., 2010; Suzuki et al., 2010; Jackson et al., 2011) | Children exposed in utero to pollutants from coal fired power plant had motor, language and social developmental delays (Tang et al., 2008) |
| Metabolic disease | Offspring of diesel exhaust exposed mice display increased bodyweight, insulin resistance particularly on high fat diet (Bolton et al., 2012) | N/A |
Maternal immune activation
Since the late 1980s it has been acknowledged that maternal infection may play a major role in the development of offspring mental illness. Many studies have reported an association between influenza infection (as well as rubella and respiratory tract infections) during pregnancy and an increased risk for schizophrenia and autism spectrum disorders (ASD) in the offspring (Mednick et al., 1988; McGrath et al., 1994; Izumoto et al., 1999; Brown et al., 2001, 2004; Brown, 2006, 2012). However, none of these epidemiological studies in humans have been able to prove causation or suggest a mechanism beyond pointing to the link between maternal immune system activation and adverse offspring outcomes.
Because very different pathogens seem to cause the same offspring phenotype, and since it is unlikely they are able to directly infect the fetus (Williams and Mackenzie, 1977; Irving et al., 2000; Shi et al., 2005), the maternal immune response has been implicated. Maternal immune activation (MIA) rodent models have been particularly helpful to examine in more detail the in vivo effects of the activated maternal immune system on the developing fetal brain. These models predominantly administer either bacterial lipopolysaccharide (LPS) or double-stranded viral RNA mimic polyinosinic:polycytidylic acid (poly I:C) to pregnant rats or mice. This induces an immune response, and increases circulating levels of multiple cytokines without the confounding effects of the introduction of a viral or bacterial pathogen itself. Offspring of these MIA rodents have been reported to show a “schizophrenic-like” phenotype, including deficits in prepulse inhibition (PPI), latent inhibition (LI), anxiety, locomotion and social interaction (Smith et al., 2007; Meyer et al., 2008), as well as behaviors reminiscent of ASD (Hsiao et al., 2012; Malkova et al., 2012).
Efforts to tease apart the mechanism have found that the inflammatory cytokine IL-6 appears to play a significant role in MIA's effects on offspring behavioral programming. Inducing MIA while simultaneously blocking IL-6 actions, via IL-6 receptor deletion or IL-6 function blocking antibody, was able to prevent the ability of MIA to induce a schizophrenic-like phenotype in offspring (Smith et al., 2007). Additionally, injection of IL-6, but not the other MIA-induced cytokines IL-1α, tumor necrosis factor α (TNFα), or interferon γ (IFNγ), during pregnancy was enough to cause PPI deficits, a hallmark of schizophrenia, in offspring (Smith et al., 2007).
There is a curious link between MIA and metabolic disease, raising the possibility of some shared mechanistic underpinnings. Firstly, humans with schizophrenia appear to be more likely to develop metabolic disease (Ryan et al., 2003; Thakore, 2004; Meyer and Stahl, 2009). In addition, there is some evidence from MIA rodents that metabolic symptoms may also result from prenatal immune challenge (Pacheco-López et al., 2011). Also, injection of IL-6 into pregnant rats can produce offspring with increased adipose tissue and body weight (Dahlgren et al., 2006). Finally, IL-6 appears to affect fetal hypothalamic neural circuit development in maternal obesity (Sanders et al., 2014), and has been reported to have many neurotrophic roles (Spooren et al., 2011). Given these links, it seems plausible that inflammation, perhaps IL-6 in particular, irrespective of how it becomes elevated, impinges on fetal brain development. The big remaining questions relate to whether cytokines have their effects directly or indirectly on the brain, and/or whether they cooperate with other cytokines or abnormally elevated factors to stimulate downstream events that impinge on brain development.
Maternal autoimmune disease
Curiously, the recent increase in obesity prevalence across the world has been mirrored by a similar rise in autoimmune disease prevalence. The literature suggests that obesity is a major contributing risk factor for the development of autoimmune disease in adults. Maternal autoimmune disease represents a spectrum of maternal disorders in which the fetus is exposed to a proinflammatory environment, and where neurobehavioral abnormalities in the offspring have been identified. For example, children whose mothers had systemic lupus erythematosus (SLE), had normal heath and intelligence scores, but a higher rate of learning disabilities such as dyslexia (McAllister et al., 1997; Ross et al., 2003; Neri et al., 2004). Additionally, primary antiphospholipid syndrome, is associated with increased risk of ASD and learning disabilities in the offspring (Nacinovich et al., 2008; Abisror et al., 2013). Although there is not yet a retrospective study examining body weight and metabolic parameters in the offspring of mothers with SLE, maternal SLE is associated with babies who are small for gestational age (Baer et al., 2011), itself a risk factor for developing obesity later in life (Barker, 2006; Gluckman et al., 2008).
Maternal smoking
Maternal cigarette smoking, in addition to its direct effects on maternal and fetal health, predisposes offspring to increased risk of several later life adverse neurobehavioral outcomes, including aggressive behavior (Orlebeke et al., 1999), inattention and conduct disorder (Fergusson et al., 1993, 1998), and attention deficit disorder (Linnet et al., 2003). Animal models of prenatal cigarette smoke exposure have reported behavioral changes in adult offspring which parallel these human disorders (Balsevich et al., 2014). Further to this, maternal smoking is also associated with increased risk of obesity and the metabolic syndrome in the offspring, which show increased plasma leptin and impaired glucose tolerance in both humans and animal models (Power and Jefferis, 2002; Al Mamun et al., 2006; Chen et al., 2011). Although it can be difficult to tease apart the various effects of smoking during pregnancy, and exposure during early postnatal life, observations currently suggest a critical prenatal window during the first trimester of human pregnancy as the time when smoking is most likely to confer adverse health outcomes on the offspring (Toschke et al., 2003; Oken et al., 2005; Al Mamun et al., 2006).
Here, as with other complications of pregnancy that lead to developmental programming of neurobehavioral and metabolic disorders in the offspring, there appears to be chronic inflammation with elevated cytokines and other markers of immune system activation during gestation (Bermudez et al., 2002; Yanbaeva et al., 2007; Gosker et al., 2009).
Air pollution
Human studies have found that children exposed in utero to pollutants from a nearby coal fired power plant had motor, language and social developmental delays (Tang et al., 2008). Animal models have shown that the air pollution present in modern day cities could cause a proinflammatory state during pregnancy, and increase the risk of metabolic and psychological disorders in the offspring. Pregnant mice exposed to common nanoparticle pollutants or diesel exhaust, showed elevated proinflammatory cytokines including IL-1β and IL-6, some of which were detected in the fetal brain (Hougaard et al., 2010; Bolton et al., 2012; Jackson et al., 2012). The offspring of mice exposed to diesel exhaust displayed decreased locomotion, changes in the levels of the neurotransmitter dopamine, and neurobehavioral changes, including a tendency to avoid the central zone in an open field test and enhanced prepulse inhibition (Hougaard et al., 2010; Suzuki et al., 2010; Jackson et al., 2011). In addition, these offspring showed increased body weight and insulin resistance, classic features of the metabolic syndrome, particularly when placed on a high-fat diet (Bolton et al., 2012).
Taking these events or exposures together with their effects on cytokine abundance and offspring outcomes, it is tempting to speculate that abnormal exposure of the fetus to immuno-modulatory molecules is the critical element linking adverse pregnancy experiences with developmental programming of obesity and neurobehavioral risks for the offspring.
How might immuno-modulatory molecules elevate offspring risk of neurobehavioral disease?
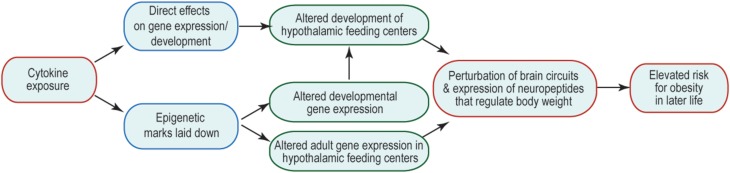
Many of the changes to offspring phenotype that result from prenatal cytokine exposure are likely to be underpinned by changes in brain function, including cellular physiology, neural connectivity, and gene expression. The consequences are that the offspring's neural control of body weight and behavior are perturbed and/or are less robust to environmental challenge. How prenatal exposure affects later-life function is an area of much interest, but currently very few mechanisms have been revealed to explain this curious relationship. One possibility is that prenatal brain development is altered by abnormal exposure to cytokines, leading to structural changes in the brain that might underpin suboptimal function. Another is that abnormal cytokine exposure during fetal life alters the epigenetic regulation of genes whose expression and regulation later in life is critical to appropriate body weight regulation and behavior. These options and their potential consequences to the later life ability of the brain to regulate body weight are summarized in Figure 1. Similar mechanisms might be invoked to account for other abnormal offspring behaviors, such as socialization and learning, consequent to maternal obesity; and in these cases brain areas outside hypothalamic feeding centers (e.g., those that regulate the behaviors in question) are likely to be affected.
Figure 1.
Mechanisms by which inappropriate cytokine exposure may alter later life brain function. Schematic indicates that cytokine exposure could result in immediate non-genomic (e.g., via kinase cascades) or genomic (e.g., change in transcription factor activity) effects on the developing brain. Cytokine exposure may also initiate activity of enzymes that catalyze epigenetic modifications, which could have immediate or long-term consequences on gene expression. The output of such cytokine exposure would be disrupted adult function, such that neural mechanisms regulating body weight do not function optimally, and thus give rise to a risk of obesity.
Access of cytokines to the fetal brain
During normal development, the embryonic and fetal brain are believed to have limited exposure to cytokines because either their circulating levels are low (early pregnancy), or because entry into the brain is regulated (mid-late pregnancy). The blood-brain barrier (BBB) is believed to become functional between GD15 and GD17 in the mouse (Ben-Zvi et al., 2014). This time is theorized to relate approximately to late second/early third trimester in the human, but the actual time of BBB formation in humans is not well-characterized. Thus, prior to BBB formation, if there were elevated cytokines in the fetal circulation they might simply diffuse into the developing brain. Later in gestation, early life and into adulthood, when the BBB is intact, the main route into the brain for blood-borne molecules is via specific transporters that move molecules, including leptin, insulin, and growth factors, from the blood into the brain (Pohl et al., 2013; Banks, 2015; Koch et al., 2014). Some molecules may also enter the brain via fenestrated capillaries in circumventricular organs or the choroid plexus. How molecules that pass through the choroid plexus endothelium and into the ventricles then pass into the brain parenchyma are not well-characterized, but transporters as well as transcellular signaling have been implicated (Balland et al., 2014). The time in development at which the genes encoding these transporters begin to be expressed is currently unknown, but abnormally elevated cytokines might gain access to the fetal brain through these normal transport mechanisms. Perturbation of either BBB formation or transporter function/gene expression in the brains of offspring developing in obese mothers are interesting further possible mechanisms that could account for abnormal exposure of the fetal brain to elevated cytokines. Another possible source of cytokines in the developing brain are microglia. These brain-resident immune cells of the monocyte lineage, are generated in the yolk sac around GD10, and can be found in the fetal brain several days later (Saijo and Glass, 2011). These seem likely candidates for producing cytokines within the brain proper, but they would need to become activated before they could do so. In support of this, recent evidence from the non-human primate suggests that microglia become abnormally activated in the prenatal hypothalamus of fetuses developing in high-fat diet-fed mothers (Grayson et al., 2010). These same fetuses also showed elevated cytokine gene expression in the hypothalamus, indicating that resident cells, such as activated microglia, provided an endogenous source of cytokines (Grayson et al., 2010).
Direct effects of cytokines on the developing brain
Although there is no apparent role for immune system-derived cytokines in normal brain development, the activation of cytokine signal transduction pathways, including JAK-STAT and MEK/ERK, are well-known to modulate brain development, including neurogenesis, cell type specification, migration, and axon growth (Schwarting et al., 2006; Markham et al., 2007; Qin and Zhang, 2012; Lee et al., 2013; Urayama et al., 2013). Thus, should cytokines gain access to the fetal brain inappropriately, they have the potential for substantial interference with normal neural development (Figure 1). Should such a mechanism be occurring, it is predicted that individuals who were exposed to cytokines in utero, would show structural changes in their brains at birth. In both humans and animal models, there are numerous reports of offspring brain changes that are evident beyond the neonatal period and in adulthood. However, few studies report structural or other alterations in the brain that are evident at birth, and which are related either to pregnancy complications or later-life outcomes. On the day of birth in a mouse model of gestational obesity, the neural network regulating body weight is malformed in the offspring's brain, with fewer neural projections from the ARC reaching their targets (Sanders et al., 2014). Further exploration of the underlying mechanism revealed a role for IL-6, which altered axon growth and underlying developmental gene expression (Sanders et al., 2014). Also evident on the day of birth in a rat model of maternal obesity, were: (i) increases in the expression of orexigenic peptides, orexin and melanin concentrating hormone, in the lateral hypothalamic area; and (ii) increased galanin, enkephalin, and dynorphin expression in the PVN (Chang et al., 2008). Further exploration of the underlying mechanism revealed altered neurogenesis, leading to an increase in the generation of these cell types in the brains of fetuses developing in obese mothers (Chang et al., 2008). Also in a rat model of maternal obesity, CD11b and TLR4, both markers of activated microglia, were elevated on the day of birth (Bilbo and Tsang, 2010). This further suggests the possibility of an inflammatory state during the prenatal period. Better defining brain abnormalities as having their origins during prenatal or postnatal development (or some elements of each) will be critical to understanding underlying mechanisms, and ongoing research is beginning to provide this key information.
Effects of cytokines on epigenetic regulators
Another possibility is that genes whose expression is critical for later life neural function are epigenetically modified at the time of cytokine exposure. Such genes may not necessarily be expressed during gestation, but the epigenetic marks laid down before birth would be there to affect gene expression whenever such genes were needed for normal neural function later in life; should such changes alter normal neural function, disease risk would be elevated. There is no need for the two mechanisms to be mutually exclusive (or exclusive of other possibilities), so it is likely that multiple mechanisms are at play when a fetus develops in an environment complicated by inappropriate cytokine exposure (Figure 1).
In many cases where life experience is associated with phenotypic change, alterations in DNA methylation, histone modifications, regulatory RNAs or some combination thereof have been observed in multiple offspring tissues. What we currently do not understand in relation to maternal obesity is: which environmental factors are important modulators of the molecules that execute epigenetic changes; how particular genes become targeted for epigenetic change by certain factors; and how a constellation of epigenetic modifications occurring across the genome affect gene function at discrete loci and at particular times during development and adult life.
Should cytokines be causative agents in developmental programming via an epigenetic mechanism, then they should be able to modulate the activity of the proteins that enact epigenetic change, but how they might do so is less clear. It is known from cancer studies that inflammation-induced DNA methylation and histone changes are associated with polycomb-group target genes, which are significant here because their regulation relies on chromatin remodeling (Hahn et al., 2008). Also in cancer cell lines, IL-6 is able to affect both the expression and nuclear translocation of DNA methyltransferase 1 (DNMT1), consequently changing its activity within the cell (Hodge et al., 2001, 2007; Foran et al., 2010), and potentially targeting methylation to specific gene promoter regions (Li et al., 2012). As many epigenetic modifying enzymes are regulated by phosphorylation cascades (Jeltsch and Jurkowska, 2014), and since cytokines are known to stimulate multiple kinase cascades, including ERK, Jnk, PI3 kinase/Akt (Hirano et al., 1997; Mak and Yeh, 2002; Pestka et al., 2004a,b), the mechanistic links seem apparent, and available for further testing. In addition, transcription factors play a key role in directing epigenetic changes to particular genomic regions (Ding et al., 2008; Hashimoto et al., 2010; Feldmann et al., 2013). Cytokines also notoriously signal through the JAK/STAT pathway, which leads to DNA binding and transcriptional modulation by the activated STAT proteins (Murray, 2007). STAT proteins already have been described for their ability to recruit DNA binding and transcriptional regulatory molecules to specific DNA sequences, so this represents another signaling pathway through which cytokine exposure could lead to epigenetic modifications across the genome (Vahedi et al., 2012; Hedrich et al., 2014; Li et al., 2014).
Can anti-inflammation treatment protect the offspring brain?
If abnormally elevated inflammatory mediators play a causative role in the acquisition of offspring phenotype, then ameliorating them should protect normal offspring physiology. This line of reasoning has been used to test the efficacy of a number of pharmacological and physiological strategies that reduce inflammation. The literature here is growing rapidly, and this section is not intended to be exhaustive, but rather to give a glimpse into where the field is headed, and to bolster the hypothesis that increased exposure of the developing brain to inflammatory mediators is a key player in the developmental programming of offspring neurobehavioral disorders, including deregulated body weight control.
Transgenic elevation of the anti-inflammatory n-3 polyunsaturated fatty acids (PUFA) relative to the pro-inflammatory n-6-PUFAs in a mouse model of maternal obesity, reduced the metabolic phenotype of offspring, including normalizing liver triglycerides and insulin sensitivity, and reducing body weight (Heerwagen et al., 2013). Unfortunately, inflammatory markers were not examined in the brain. As the transgene would presumably have been active during development, then it is likely that an anti-inflammatory milieu during gestation played a key role in determining the offspring phenotype, however, this was not examined directly. Several other agents with broad effects including anti-inflammatory, such as resveratrol (Roberts et al., 2014) and taurine (Li et al., 2013), have also been trialed in non-human primate or rat models of maternal obesity, respectively. Both reported mixed effects and confounding details that limit their practical utility, and neither examined inflammation in the brain.
Manipulation of diet and exercise also have been used to target inflammation as a means to combat offspring consequences of maternal obesity. Reversing maternal diet from high fat to control during the lactation period and after weaning in a mouse model of maternal obesity, was able to reverse social deficits and brain inflammation in offspring (Kang et al., 2014). This suggests that the neural circuits regulating social behavior rely on a constant exposure, particularly in the neonatal period, to inflammatory mediators in order to form and/or function improperly later in life. At least some of the neural circuits governing social behaviors show a great deal of plasticity in the early postnatal period, suggesting that once inflammation is reduced, these brain areas have the ability to form/reform normally. Other brain areas, governing other behaviors, will likely have different schedules of development and plasticity. Identifying these opportune times for plasticity will be critical to applying anti-inflammatory interventions to reduce offspring metabolic disease.
Exercise is another intervention that has been used to reverse the effects of maternal obesity on offspring health. Although exercise has been used to combat a variety of health disorders, the mechanisms underlying its effects are not well-understood. In a rat model of maternal obesity, several studies have observed that exercise, in either the early postnatal period or adulthood, was able to improve the offspring metabolic dysfunction caused by maternal obesity (Bahari et al., 2013; Rajia et al., 2013). The effects of early life voluntary exercise have not examined inflammatory marker expression in the brain (Rajia et al., 2013). Exercise in the adult offspring of obese dams who received either control or high fat diet after weaning showed a trend toward reduced IL-6 mRNA levels in the ARC and IL-1β in the hippocampus following exercise compared to animals with similar diet history, but no exercise (Bahari et al., 2013).
Conclusions
Although the causative factors that link pregnancy complications or exposures to offspring health and disease risks later in life have yet to be fully cataloged, cytokines, classically known for their roles in immune system modulation, are surfacing as key players. Doubtless there are many more factors remaining to be identified. In addition, the mechanisms by which such factors impinge on the developing fetal brain and other organs remain very poorly explored at present. Identifying these mechanisms and linking them causally with later life disease susceptibility will be a monumental challenge. Such understanding, however, is critical to developing therapeutic approaches to protect the developing brain in the face of adverse pregnancy complications. Of important further consideration, is the role of factors, including those from the immune system, in the postnatal developmental period, where the brain's extensive plasticity could be harnessed. The body of literature in this area also is growing at a rapid pace, and the interplay among prenatal and postnatal factors, immune activation, and brain development and function later in life are well on their way to being more clearly defined. The next great challenges will be to translate this mechanistic understanding into preventative and directed interventions aimed at reducing the burden of disease risk that stems from pregnancy complications and exposures that are not currently easily preventable.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was funded by the University of Otago, the Maurice and Phyllis Paykel Trust New Zealand, and Gravida: New Zealand National Centre for Growth and Development.
References
- Aaltonen R., Heikkinen T., Hakala K., Laine K., Alanen A. (2005). Transfer of proinflammatory cytokines across term placenta. Obstet. Gynecol. 106, 802–807. 10.1097/01.AOG.0000178750.84837.ed [DOI] [PubMed] [Google Scholar]
- Abisror N., Mekinian A., Lachassinne E., Nicaise-Roland P., De Pontual L., Chollet-Martin S., et al. (2013). Autism spectrum disorders in babies born to mothers with antiphospholipid syndrome. Semin. Arthritis Rheum. 43, 348–351. 10.1016/j.semarthrit.2013.07.001 [DOI] [PubMed] [Google Scholar]
- Alfaradhi M. Z., Ozanne S. E. (2011). Developmental programming in response to maternal overnutrition. Front. Genet. 2:27. 10.3389/fgene.2011.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Mamun A., Lawlor D. A., Alati R., O'Callaghan M. J., Williams G. M., Najman J. M. (2006). Does maternal smoking during pregnancy have a direct effect on future offspring obesity? evidence from a prospective birth cohort study. Am. J. Epidemiol. 164, 317–325. 10.1093/aje/kwj209 [DOI] [PubMed] [Google Scholar]
- Arck P. C., Hecher K. (2013). Fetomaternal immune cross-talk and its consequences for maternal and offspring's health. Nat. Med. 19, 548–556. 10.1038/nm.3160 [DOI] [PubMed] [Google Scholar]
- Armitage J. A., Poston L., Taylor P. D. (2008). Developmental origins of obesity and the metabolic syndrome: the role of maternal obesity. Front. Horm. Res. 36, 73–84. 10.1159/000115355 [DOI] [PubMed] [Google Scholar]
- Atkinson D. E., Robinson N. R., Sibley C. P. (1991). Development of passive permeability characteristics of rat placenta during the last third of gestation. Am. J. Physiol. 261(6 Pt 2), R1461–R1464. [DOI] [PubMed] [Google Scholar]
- Baer A. N., Witter F. R., Petri M. (2011). Lupus and pregnancy. Obstet. Gynecol. Surv. 66, 639–653. 10.1097/OGX.0b013e318239e1ee [DOI] [PubMed] [Google Scholar]
- Bahari H., Caruso V., Morris M. J. (2013). Late-onset exercise in female rat offspring ameliorates the detrimental metabolic impact of maternal obesity. Endocrinology 154, 3610–3621. 10.1210/en.2013-1059 [DOI] [PubMed] [Google Scholar]
- Balland E., Dam J., Langlet F., Caron E., Steculorum S., Messina A., et al. (2014). Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab. 19, 293–301. 10.1016/j.cmet.2013.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsevich G., Poon A., Goldowitz D., Wilking J. A. (2014). The effects of pre- and post-natal nicotine exposure and genetic background on the striatum and behavioral phenotypes in the mouse. Behav. Brain Res. 266, 7–18. 10.1016/j.bbr.2014.02.038 [DOI] [PubMed] [Google Scholar]
- Banks W. A. (2015). The blood-brain barrier in neuroimmunology: tales of separation and assimilation. Brain Behav. Immun. 44C, 1–8. 10.1016/j.bbi.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. J. (2004). The developmental origins of chronic adult disease. Acta Paediatr. Suppl. 93, 26–33. 10.1111/j.1651-2227.2004.tb00236.x [DOI] [PubMed] [Google Scholar]
- Barker D. J. (2006). Adult consequences of fetal growth restriction. Clin. Obstet. Gynecol. 49, 270–283. 10.1097/00003081-200606000-00009 [DOI] [PubMed] [Google Scholar]
- Ben-Zvi A., Lacoste B., Kur E., Andreone B. J., Mayshar Y., Yan H., et al. (2014). Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 509, 507–511. 10.1038/nature13324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez E. A., Rifai N., Buring J. E., Manson J. E., Ridker P. M. (2002). Relation between markers of systemic vascular inflammation and smoking in women. Am. J. Cardiol. 89, 1117–1119. 10.1016/S0002-9149(02)02284-1 [DOI] [PubMed] [Google Scholar]
- Bilbo S. D., Tsang V. (2010). Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 10.1096/fj.09-144014 [DOI] [PubMed] [Google Scholar]
- Bolton J. L., Smith S. H., Huff N. C., Gilmour M. I., Foster W. M., Auten R. L., et al. (2012). Prenatal air pollution exposure induces neuroinflammation and predisposes offspring to weight gain in adulthood in a sex-specific manner. FASEB J. 26, 4743–4754. 10.1096/fj.12-210989 [DOI] [PubMed] [Google Scholar]
- Bouret S. G., Gorski J. N., Patterson C. M., Chen S., Levin B. E., Simerly R. B. (2008). Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab. 7, 179–185. 10.1016/j.cmet.2007.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. S. (2006). Prenatal infection as a risk factor for schizophrenia. Schizophr. Bull. 32, 200–202. 10.1093/schbul/sbj052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. S. (2012). Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev. Neurobiol. 72, 1272–1276. 10.1002/dneu.22024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. S., Begg M. D., Gravenstein S., Schaefer C. A., Wyatt R. J., Bresnahan M., et al. (2004). Serologic-evidence of prenatal influenza in the etiology of schizophrenia. Arch. Gen. Psychiatry 61, 774–780. 10.1001/archpsyc.61.8.774 [DOI] [PubMed] [Google Scholar]
- Brown A. S., Cohen P., Harkavy-Friedman J., Babulas V., Malaspina D., Gorman J. M., et al. (2001). Prenatal rubella, premorbid abnormalities, and adult schizophrenia. Biol. Psychiatry 49, 473–486. 10.1016/S0006-3223(01)01068-X [DOI] [PubMed] [Google Scholar]
- Carbo N., Lopez-Soriano F. J., Argiles J. M. (1998). Tumour necrosis factor-alpha does not cross the rat placenta. Cancer Lett. 128, 101–104. 10.1016/S0304-3835(98)00057-3 [DOI] [PubMed] [Google Scholar]
- Catalano P. M., Presley L., Minium J., Hauguel-De Mouzon S. (2009). Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 32, 1076–1080. 10.2337/dc08-2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challier J. C., Basu S., Bintein T., Minium J., Hotmire K., Catalano P. M., et al. (2008). Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 29, 274–281. 10.1016/j.placenta.2007.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G. Q., Gaysinskaya V., Karatayev O., Leibowitz S. F. (2008). Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J. Neurosci. 28, 12107–12119. 10.1523/JNEUROSCI.2642-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M. D., Pollard J. W., Khalili H., Goyert S. M., Diamond B. (1993). Mouse placental macrophages have a decreased ability to present antigen. Proc. Natl. Acad. Sci. U.S.A. 90, 462–466. 10.1073/pnas.90.2.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Iglesias M. A., Caruso V., Morris M. J. (2011). Maternal cigarette smoke exposure contributes to glucose intolerance and decreased brain insulin action in mice offspring independent of maternal diet. PLoS ONE 6:e27260. 10.1371/journal.pone.0027260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Simar D., Morris M. J. (2009). Hypothalamic neuroendocrine circuitry is programmed by maternal obesity: interaction with postnatal nutritional environment. PLoS ONE 4:e6259. 10.1371/journal.pone.0006259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman I., Ataullahjan A., Naicker K., Van Lieshout R. J. (2012). Birth weight, stress, and symptoms of depression in adolescence: evidence of fetal programming in a national Canadian cohort. Can. J. Psychiatry 57, 422–428. [DOI] [PubMed] [Google Scholar]
- Cone R. D., Cowley M. A., Butler A. A., Fan W., Marks D. L., Low M. J. (2001). The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int. J. Obes. Relat. Metab. Disord. 25Suppl. 5, S63–S67. 10.1038/sj.ijo.0801913 [DOI] [PubMed] [Google Scholar]
- Cowley M. A., Pronchuk N., Fan W., Dinulescu D. M., Colmers W. F., Cone R. D. (1999). Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 24, 155–163. 10.1016/S0896-6273(00)80829-6 [DOI] [PubMed] [Google Scholar]
- Crozier S. R., Inskip H. M., Godfrey K. M., Cooper C., Harvey N. C., Cole Z. A., et al. (2010). Southampton Women's Survey Study Group. Weight gain in pregnancy and childhood body composition: findings from the Southampton Women's Survey. Am. J. Clin. Nutr. 91, 1745–1751. 10.3945/ajcn.2009.29128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren J., Samuelsson A.-M., Jansson T., Holmang A. (2006). Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr. Res. 60, 147–151. 10.1203/01.pdr.0000230026.74139.18 [DOI] [PubMed] [Google Scholar]
- Ding N., Zhou H., Esteve P. O., Chin H. G., Kim S., Xu X., et al. (2008). Mediator links epigenetic silencing of neuronal gene expression with x-linked mental retardation. Mol. Cell 31, 347–359. 10.1016/j.molcel.2008.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist J. K., Coppari R., Balthasar N., Ichinose M., Lowell B. B. (2005). Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J. Comp. Neurol. 493, 63–71. 10.1002/cne.20786 [DOI] [PubMed] [Google Scholar]
- Enders A. C., Blankenship T. N. (1999). Comparative placental structure. Adv. Drug Deliv. Rev. 38, 3–15. 10.1016/S0169-409X(99)00003-4 [DOI] [PubMed] [Google Scholar]
- Fan W., Boston B. A., Kesterson R. A., Hruby V. J., Cone R. D. (1997). Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385, 165–168. 10.1038/385165a0 [DOI] [PubMed] [Google Scholar]
- Feldmann A., Ivanek R., Murr R., Gaidatzis D., Burger L., Schubeler D. (2013). Transcription factor occupancy can mediate active turnover of DNA methylation at regulatory regions. PLoS Genet. 9:e1003994. 10.1371/journal.pgen.1003994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson D. M., Horwood L. J., Lynskey M. T. (1993). Maternal smoking before and after pregnancy: effects on behavioral outcomes in middle childhood. Pediatrics 92, 815. [PubMed] [Google Scholar]
- Fergusson D. M., Woodward L. J., Horwood L. (1998). Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Arch. Gen. Psychiatry 55, 721–727. 10.1001/archpsyc.55.8.721 [DOI] [PubMed] [Google Scholar]
- Foran E., Garrity-Park M. M., Mureau C., Newell J., Smyrk T. C., Limburg P. J., et al. (2010). Upregulation of DNA methyltransferase–mediated gene silencing, anchorage-independent growth, and migration of colon cancer cells by interleukin-6. Mol. Cancer Res. 8, 471–481. 10.1158/1541-7786.MCR-09-0496 [DOI] [PubMed] [Google Scholar]
- Frias A. E., Morgan T. K., Evans A. E., Rasanen J., Oh K. Y., Thornburg K. L., et al. (2011). Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology 152, 2456–2464. 10.1210/en.2010-1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale C. R., Javaid M. K., Robinson S. M., Law C. M., Godfrey K. M., Cooper C. (2007). Maternal size in pregnancy and body composition in children. J. Clin. Endocrinol. Metab. 92, 3904–3911. 10.1210/jc.2007-0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P. D., Hanson M. A., Cooper C., Thornburg K. L. (2008). Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 359, 61–73. 10.1056/NEJMra0708473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosker H. R., Langen R. C. J., Bracke K. R., Joos G. F., Brusselle G. G., Steele C., et al. (2009). Extrapulmonary manifestations of chronic obstructive pulmonary disease in a mouse model of chronic cigarette smoke exposure. Am. J. Respir. Cell Mol. Biol. 40, 710–716. 10.1165/rcmb.2008-0312OC [DOI] [PubMed] [Google Scholar]
- Grayson B. E., Levasseur P. R., Williams S. M., Smith M. S., Marks D. L., Grove K. L. (2010). Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology 151, 1622–1632. 10.1210/en.2009-1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom N. M., Lyde R., Christ L., Sasson I. E., Carlin J., Vitins A. P., et al. (2014). Obesity at conception programs the opioid system in the offspring brain. Neuropsychopharmacology 39, 801–810. 10.1038/npp.2013.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson C., Mjosberg J., Matussek A., Geffers R., Matthiesen L., Berg G., et al. (2008). Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS ONE 3:e2078. 10.1371/journal.pone.0002078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M. A., Hahn T., Lee D. H., Esworthy R. S., Kim B. W., Riggs A. D., et al. (2008). Methylation of polycomb target genes in intestinal cancer is mediated by inflammation. Cancer Res. 68, 10280–10289. 10.1158/0008-5472.CAN-08-1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halmoy A., Klungsoyr K., Skjaerven R., Haavik J. (2012). Pre- and perinatal risk factors in adults with attention-deficit/hyperactivity disorder. Biol. Psychiatry 71, 474–481. 10.1016/j.biopsych.2011.11.013 [DOI] [PubMed] [Google Scholar]
- Hashimoto H., Vertino P. M., Cheng X. (2010). Molecular coupling of DNA methylation and histone methylation. Epigenomics 2, 657–669. 10.2217/epi.10.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes E. K., Lechowicz A., Petrik J. J., Storozhuk Y., Paez-Parent S., Dai Q., et al. (2012). Adverse fetal and neonatal outcomes associated with a life-long high fat diet: role of altered development of the placental vasculature. PLoS ONE 7:e33370. 10.1371/journal.pone.0033370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward C. E., Higgins L., Cowley E. J., Greenwood S. L., Mills T. A., Sibley C. P., et al. (2013). Chorionic plate arterial function is altered in maternal obesity. Placenta 34, 281–287. 10.1016/j.placenta.2013.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich C. M., Rauen T., Apostolidis S. A., Grammatikos A. P., Rodriguez Rodriguez N., Ioannidis C., et al. (2014). Stat3 promotes IL-10 expression in lupus T cells through trans-activation and chromatin remodeling. Proc. Natl. Acad. Sci. U.S.A. 111, 13457–13462. 10.1073/pnas.1408023111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerwagen M. J., Stewart M. S., De La Houssaye B. A., Janssen R. C., Friedman J. E. (2013). Transgenic increase in N-3/n-6 Fatty Acid ratio reduces maternal obesity-associated inflammation and limits adverse developmental programming in mice. PLoS ONE 8:e67791. 10.1371/journal.pone.0067791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen J., Mottonen M., Komi J., Alanen A., Lassila O. (2003). Phenotypic characterization of human decidual macrophages. Clin. Exp. Immunol. 131, 498–505. 10.1046/j.1365-2249.2003.02092.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herva A., Pouta A., Hakko H., Laksy K., Joukamaa M., Veijola J. (2008). Birth measures and depression at age 31 years: the Northern Finland 1966 Birth Cohort Study. Psychiatry Res. 160, 263–270. 10.1016/j.psychres.2007.07.020 [DOI] [PubMed] [Google Scholar]
- Hirano T., Nakajima K., Hibi M. (1997). Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev. 8, 241–252. 10.1016/S1359-6101(98)80005-1 [DOI] [PubMed] [Google Scholar]
- Hodge D. R., Cho E., Copeland T. D., Guszczynski T., Yang E., Seth A. K., et al. (2007). IL-6 enhances the nuclear translocation of DNA cytosine-5-methyltransferase 1 (DNMT1) via phosphorylation of the nuclear localization sequence by the AKT kinase. Cancer Genomics Proteomics 4, 387–398. [PubMed] [Google Scholar]
- Hodge D. R., Xiao W., Clausen P. A., Heidecker G., Szyf M., Farrar W. L. (2001). Interleukin-6 regulation of the human DNA methyltransferase (HDNMT) gene in human erythroleukemia cells. J. Biol. Chem. 276, 39508–39511. 10.1074/jbc.C100343200 [DOI] [PubMed] [Google Scholar]
- Horvath T. L., Diano S., Van Den Pol A. N. (1999). Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J. Neurosci. 19, 1072–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougaard K. S., Jackson P., Jensen K. A., Sloth J. J., Löschner K., Larsen E. H., et al. (2010). Effects of prenatal exposure to surface-coated nanosized titanium dioxide (UV-Titan). A study in mice. Part. Fibre Toxicol. 7:16. 10.1186/1743-8977-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser B. L., Tilburgs T., Hill J., Nicotra M. L., Strominger J. L. (2011). Two unique human decidual macrophage populations. J. Immunol. 186, 2633–2642. 10.4049/jimmunol.1003153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao E. Y., McBride S. W., Chow J., Mazmanian S. K., Patterson P. H. (2012). Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc. Natl. Acad. Sci. U.S.A. 109, 12776–12781. 10.1073/pnas.1202556109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao E. Y., Patterson P. H. (2011). Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav. Immun. 25, 604–615. 10.1016/j.bbi.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvorsen C., Thysen A. H., Fernandez-Twinn D., Nordby P., Nielsen K. F., Ozanne S. E., et al. (2014). Effects of pregnancy on obesity-induced inflammation in a mouse model of fetal programming. Int. J. Obes. (Lond.). 38, 1282–1289. 10.1038/ijo.2014.69 [DOI] [PubMed] [Google Scholar]
- Irving W. L., James D. K., Stephenson T., Laing P., Jameson C., Oxford J. S., et al. (2000). Influenza virus infection in the second and third trimesters of pregnancy: a clinical and seroepidemiological study. Br. J. Obstet. Gynaecol. 107, 1282–1289. 10.1111/j.1471-0528.2000.tb11621.x [DOI] [PubMed] [Google Scholar]
- Izumoto Y., Inoue S., Yasuda N. (1999). Schizophrenia and the influenza epidemics of 1957 in Japan. Biol. Psychiatry 46, 119–124. 10.1016/S0006-3223(98)00359-X [DOI] [PubMed] [Google Scholar]
- Jackson P., Hougaard K. S., Vogel U., Wu D., Casavant L., Williams A., et al. (2012). Exposure of pregnant mice to carbon black by intratracheal instillation: Toxicogenomic effects in dams and offspring. Mutat. Res. 745, 73–83. 10.1016/j.mrgentox.2011.09.018 [DOI] [PubMed] [Google Scholar]
- Jackson P., Vogel U., Wallin H., Hougaard K. S. (2011). Prenatal exposure to carbon black (Printex 90): Effects on sexual development and neurofunction. Basic Clin. Pharmacol. Toxicol. 109, 434–437. 10.1111/j.1742-7843.2011.00745.x [DOI] [PubMed] [Google Scholar]
- Jeltsch A., Jurkowska R. Z. (2014). New concepts in DNA methylation. Trends Biochem. Sci. 39, 310–318. 10.1016/j.tibs.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Jones H. N., Jansson T., Powell T. L. (2009). IL-6 stimulates system A amino acid transporter activity in trophoblast cells through STAT3 and increased expression of SNAT2. Am. J. Physiol. Cell Physiol. 297, C1228–C1235. 10.1152/ajpcell.00195.2009 [DOI] [PubMed] [Google Scholar]
- Kanellopoulos-Langevin C., Caucheteux S. M., Verbeke P., Ojcius D. M. (2003). Tolerance of the fetus by the maternal immune system: role of inflammatory mediators at the feto-maternal interface. Reprod. Biol. Endocrinol. 1:121. 10.1186/1477-7827-1-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. S., Kurti A., Fair D. A., Fryer J. D. (2014). Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring. J. Neuroinflammation 11, 156. 10.1186/s12974-014-0156-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent A. S., Sullivan M. H., Elder M. G. (1994). Transfer of cytokines through human fetal membranes. J. Reprod. Fertil. 100, 81–84. 10.1530/jrf.0.1000081 [DOI] [PubMed] [Google Scholar]
- Kepczynska M. A., Wargent E. T., Cawthorne M. A., Arch J. R. S., O'Dowd J. F., Stocker C. J. (2013). Circulating levels of the cytokines IL10, IFNγ and resistin in an obese mouse model of developmental programming. J. Dev. Orig. Health Dis. 4, 491–498. 10.1017/S2040174413000263 [DOI] [PubMed] [Google Scholar]
- Kim D. W., Young S. L., Grattan D. R., Jasoni C. L. (2014). Obesity during pregnancy disrupts placental morphology, cell proliferation, and inflammation in a sex-specific manner across gestation in the mouse. Biol. Reprod. 90, 130. 10.1095/biolreprod.113.117259 [DOI] [PubMed] [Google Scholar]
- Kirk S. L., Samuelsson A. M., Argenton M., Dhonye H., Kalamatianos T., Poston L., et al. (2009). Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PLoS ONE 4:e5870. 10.1371/journal.pone.0005870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. E., Lowe C., Pretz D., Steger J., Williams L. M., Tups A. (2014). High-fat diet induces leptin resistance in leptin-deficient mice. J. Neuroendocrinol. 26, 58–67. 10.1111/jne.12131 [DOI] [PubMed] [Google Scholar]
- Krakowiak P., Walker C. K., Bremer A. A., Baker A. S., Ozonoff S., Hansen R. L., et al. (2012). Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 129, e1121–e1128. 10.1542/peds.2011-2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law C. M., Barker D. J., Osmond C., Fall C. H., Simmonds S. J. (1992). Early growth and abdominal fatness in adult life. J. Epidemiol. Community Health 46, 184–186. 10.1136/jech.46.3.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Shen R., Cho H. H., Kwon R. J., Seo S. Y., Lee J. W., et al. (2013). STAT3 promotes motor neuron differentiation by collaborating with motor neuron-specific LIM complex. Proc. Natl. Acad. Sci. U.S.A. 110, 11445–11450. 10.1073/pnas.1302676110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Reynolds C. M., Sloboda D. M., Gray C., Vickers M. H. (2013). Effects of taurine supplementation on hepatic markers of inflammation and lipid metabolism in mothers and offspring in the setting of maternal obesity. PLoS ONE 8:e76961. 10.1371/journal.pone.0076961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Spolski R., Liao W., Leonard W. J. (2014). Complex interactions of transcription factors in mediating cytokine biology in T cells. Immunol. Rev. 261, 141–156. 10.1111/imr.12199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Deuring J., Peppelenbosch M. P., Kuipers E. J., De Haar C., Van Der Woude C. J. (2012). IL-6-induced DNMT1 activity mediates SOCS3 promoter hypermethylation in ulcerative colitis-related colorectal cancer. Carcinogenesis 33, 1889–1896. 10.1093/carcin/bgs214 [DOI] [PubMed] [Google Scholar]
- Lin H., Mosmann T. R., Guilbert L., Tuntipopipat S., Wegmann T. G. (1993). Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J. Immunol. 151, 4562–4573. [PubMed] [Google Scholar]
- Linnet K. M., Dalsgaard S., Obel C., Wisborg K., Henriksen T. B., Rodriguez A., et al. (2003). Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am. J. Psychiatry 160, 1028–1040. 10.1176/appi.ajp.160.6.1028 [DOI] [PubMed] [Google Scholar]
- Madan J. C., Davis J. M., Craig W. Y., Collins M., Allan W., Quinn R., et al. (2009). Maternal obesity and markers of inflammation in pregnancy. Cytokine 47, 61–64. 10.1016/j.cyto.2009.05.004 [DOI] [PubMed] [Google Scholar]
- Mak T. W., Yeh W. C. (2002). Signaling for survival and apoptosis in the immune system. Arthritis Res. 4Suppl 3, S243–S252. 10.1186/ar569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malassine A., Frendo J. L., Evain-Brion D. (2003). A comparison of placental development and endocrine functions between the human and mouse model. Hum. Reprod. Update 9, 531–539. 10.1093/humupd/dmg043 [DOI] [PubMed] [Google Scholar]
- Malkova N. V., Yu C. Z., Hsiao E. Y., Moore M. J., Patterson P. H. (2012). Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav. Immun. 26, 607–616. 10.1016/j.bbi.2012.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark P. J., Lewis J. L., Jones M. L., Keelan J. A., Waddell B. J. (2013). The inflammatory state of the rat placenta increases in late gestation and is further enhanced by glucocorticoids in the labyrinth zone. Placenta 34, 559–566. 10.1016/j.placenta.2013.04.006 [DOI] [PubMed] [Google Scholar]
- Markham K., Schuurmans C., Weiss S. (2007). STAT5A/B activity is required in the developing forebrain and spinal cord. Mol. Cell. Neurosci. 35, 272–282. 10.1016/j.mcn.2007.03.001 [DOI] [PubMed] [Google Scholar]
- McAllister D. L., Kaplan B. J., Edworthy S. M., Martin L., Crawford S. G., Ramsey-Goldman R., et al. (1997). The influence of systemic lupus erythematosus on fetal development: cognitive, behavioral, and health trends. J. Int. Neuropsychol. Soc. 3, 370–376. [PubMed] [Google Scholar]
- McGrath J. J., Pemberton M. R., Welham J. L., Murray R. M. (1994). Schizophrenia and the influenza epidemics of 1954, 1957 and 1959 – a southern hemisphere study. Schizophr. Res. 14, 1–8. 10.1016/0920-9964(94)90002-7 [DOI] [PubMed] [Google Scholar]
- Mednick S. A., Machon R. A., Huttunen M. O., Bonett D. (1988). Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch. Gen. Psychiatry 45, 189–192. 10.1001/archpsyc.1988.01800260109013 [DOI] [PubMed] [Google Scholar]
- Meyer J. M., Stahl S. M. (2009). The metabolic syndrome and schizophrenia. Acta Psychiatr. Scand. 119, 4–14. 10.1111/j.1600-0447.2008.01317.x [DOI] [PubMed] [Google Scholar]
- Meyer U., Nyffeler M., Yee B. K., Knuesel I., Feldon J. (2008). Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav. Immun. 22, 469–486. 10.1016/j.bbi.2007.09.012 [DOI] [PubMed] [Google Scholar]
- Moore G. S., Kneitel A. W., Walker C. K., Gilbert W. M., Xing G. (2012). Autism risk in small- and large-for-gestational-age infants. Am. J. Obstet. Gynecol. 206, 314.e1-9. 10.1016/j.ajog.2012.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G., Cardenas I., Abrahams V., Guller S. (2011). Inflammation and pregnancy: the role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 1221, 80–87. 10.1111/j.1749-6632.2010.05938.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. J. (2007). The JAK-STAT signaling pathway: input and output integration. J. Immunol. 178, 2623–2629. 10.4049/jimmunol.178.5.2623 [DOI] [PubMed] [Google Scholar]
- Nacinovich R., Galli J., Bomba M., Filippini E., Parrinello G., Nuzzo M., et al. (2008). Neuropsychological development of children born to patients with antiphospholipid syndrome. Arthritis Care Res. 59, 345–351. 10.1002/art.23311 [DOI] [PubMed] [Google Scholar]
- Naef L., Moquin L., Dal Bo G., Giros B., Gratton A., Walker C. D. (2011). Maternal high-fat intake alters presynaptic regulation of dopamine in the nucleus accumbens and increases motivation for fat rewards in the offspring. Neuroscience 176, 225–236. 10.1016/j.neuroscience.2010.12.037 [DOI] [PubMed] [Google Scholar]
- Naef L., Srivastava L., Gratton A., Hendrickson H., Owens S. M., Walker C. D. (2008). Maternal high fat diet during the perinatal period alters mesocorticolimbic dopamine in the adult rat offspring: reduction in the behavioral responses to repeated amphetamine administration. Psychopharmacology 197, 83–94. 10.1007/s00213-007-1008-4 [DOI] [PubMed] [Google Scholar]
- Neri F., Chimini L., Bonomi F., Filippini E., Motta M., Faden D., et al. (2004). Neuropsychological development of children born to patients with systemic lupus erythematosus. Lupus 13, 805–811. 10.1191/0961203304lu2018oa [DOI] [PubMed] [Google Scholar]
- Niculescu M. D., Lupu D. S. (2009). High fat diet-induced maternal obesity alters fetal hippocampal development. Int. J. Dev. Neurosci. 27, 627–633. 10.1016/j.ijdevneu.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E., Huh S. Y., Taveras E. M., Rich-edwards J. W., Gillman M. W. (2005). Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes. Res. 13, 2021–2028. 10.1038/oby.2005.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlebeke J. F., Knol D. L., Verhulst F. C. (1999). Child behavior problems increased by maternal smoking during pregnancy. Arch. Environ. Health 54, 15–19. 10.1080/00039899909602231 [DOI] [PubMed] [Google Scholar]
- Ornoy A. (2011). Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod. Toxicol. 32, 205–212. 10.1016/j.reprotox.2011.05.002 [DOI] [PubMed] [Google Scholar]
- Pacheco-López G., Giovanoli S., Langhans W., Meyer U. (2011). Priming of metabolic dysfunctions by prenatal immune activation in mice: relevance to schizophrenia. Schizophr. Bull. 39, 319–329. 10.1093/schbul/sbr178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S., Krause C. D., Sarkar D., Walter M. R., Shi Y., Fisher P. B. (2004a). Interleukin-10 and related cytokines and receptors. Annu. Rev. Immunol. 22, 929–979. 10.1146/annurev.immunol.22.012703.104622 [DOI] [PubMed] [Google Scholar]
- Pestka S., Krause C. D., Walter M. R. (2004b). Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 202, 8–32. 10.1111/j.0105-2896.2004.00204.x [DOI] [PubMed] [Google Scholar]
- Pohl J., Luheshi G. N., Woodside B. (2013). Effect of obesity on the acute inflammatory response in pregnant and cycling female rats. J. Neuroendocrinol. 25, 433–445. 10.1111/jne.12023 [DOI] [PubMed] [Google Scholar]
- Power C., Jefferis B. J. (2002). Fetal environment and subsequent obesity: a study of maternal smoking. Int. J. Epidemiol. 31, 413–419. 10.1093/ije/31.2.413 [DOI] [PubMed] [Google Scholar]
- Qin S., Zhang C. L. (2012). Role of Kruppel-like factor 4 in neurogenesis and radial neuronal migration in the developing cerebral cortex. Mol. Cell. Biol. 32, 4297–4305. 10.1128/MCB.00838-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajia S., Chen H., Morris M. J. (2013). Voluntary post weaning exercise restores metabolic homeostasis in offspring of obese rats. Nutr. Metab. Cardiovasc. Dis. 23, 574–581. 10.1016/j.numecd.2011.12.009 [DOI] [PubMed] [Google Scholar]
- Ravelli A. C., Van Der Meulen J. H., Michels R. P., Osmond C., Barker D. J., Hales C. N., et al. (1998). Glucose tolerance in adults after prenatal exposure to famine. Lancet 351, 173–177. 10.1016/S0140-6736(97)07244-9 [DOI] [PubMed] [Google Scholar]
- Ravelli G. P., Stein Z. A., Susser M. W. (1976). Obesity in young men after famine exposure in utero and early infancy. N. Engl. J. Med. 295, 349–353. 10.1056/NEJM197608122950701 [DOI] [PubMed] [Google Scholar]
- Roberts K. A., Riley S. C., Reynolds R. M., Barr S., Evans M., Statham A., et al. (2011). Placental structure and inflammation in pregnancies associated with obesity. Placenta 32, 247–254. 10.1016/j.placenta.2010.12.023 [DOI] [PubMed] [Google Scholar]
- Roberts V. H., Pound L. D., Thorn S. R., Gillingham M. B., Thornburg K. L., Friedman J. E., et al. (2014). Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. FASEB J. 28, 2466–2477. 10.1096/fj.13-245472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A. (2010). Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. J. Child Psychol. Psychiatry 51, 134–143. 10.1111/j.1469-7610.2009.02133.x [DOI] [PubMed] [Google Scholar]
- Roseboom T. J., Van Der Meulen J. H., Osmond C., Barker D. J., Ravelli A. C., Schroeder-Tanka J. M., et al. (2000). Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart 84, 595–598. 10.1136/heart.84.6.595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G., Sammaritano L., Nass R., Lockshin M. (2003). Effects of mothers' autoimmune disease during pregnancy on learning disabilities and hand preference in their children. Arch. Pediatr. Adolesc. Med. 157, 397–402. 10.1001/archpedi.157.4.397 [DOI] [PubMed] [Google Scholar]
- Ryan M. C., Collins P., Thakore J. H. (2003). Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am. J. Psychiatry 160, 284–289. 10.1176/appi.ajp.160.2.284 [DOI] [PubMed] [Google Scholar]
- Saijo K., Glass C. K. (2011). Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 11, 775–787. 10.1038/nri3086 [DOI] [PubMed] [Google Scholar]
- Sanders T. R., Kim D. W., Glendining K. A., Jasoni C. L. (2014). Maternal obesity and IL-6 lead to aberrant developmental gene expression and deregulated neurite growth in the fetal arcuate nucleus. Endocrinology 155, 2566–2577. 10.1210/en.2013-1968 [DOI] [PubMed] [Google Scholar]
- Sasaki A., De Vega W., Sivanathan S., St-Cyr S., McGowan P. (2014). Maternal high-fat diet alters anxiety behavior and glucocorticoid signaling in adolescent offspring. Neuroscience 272C, 92–101. 10.1016/j.neuroscience.2014.04.012 [DOI] [PubMed] [Google Scholar]
- Schwarting G. A., Henion T. R., Nugent J. D., Caplan B., Tobet S. (2006). Stromal cell-derived factor-1 (chemokine C-X-C motif ligand 12) and chemokine C-X-C motif receptor 4 are required for migration of gonadotropin-releasing hormone neurons to the forebrain. J. Neurosci. 26, 6834–6840. 10.1523/JNEUROSCI.1728-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L. M., Tu N., Patterson P. H. (2005). Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. Int. J. Dev. Neurosci. 23, 299–305. 10.1016/j.ijdevneu.2004.05.005 [DOI] [PubMed] [Google Scholar]
- Smith S. E., Li J., Garbett K., Mirnics K., Patterson P. H. (2007). Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 27, 10695–10702. 10.1523/JNEUROSCI.2178-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooren A., Kolmus K., Laureys G., Clinckers R., De Keyser J., Haegeman G., et al. (2011). Interleukin-6, a mental cytokine. Brain Res. Rev. 67, 157–183. 10.1016/j.brainresrev.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Stanley B. G., Leibowitz S. F. (1984). Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life sciences 35, 2635–2642. 10.1016/0024-3205(84)90032-8 [DOI] [PubMed] [Google Scholar]
- Steinborn A., Geisse M., Kaufmann M. (1998). Expression of cytokine receptors in the placenta in term and preterm labour. Placenta 19, 165–170. 10.1016/S0143-4004(98)90005-4 [DOI] [PubMed] [Google Scholar]
- Steinborn A., Gunes H., Halberstadt E. (1995). Signal for term parturition is of trophoblast and therefore of fetal origin. Prostaglandins 50, 237–252. 10.1016/0090-6980(95)00138-7 [DOI] [PubMed] [Google Scholar]
- Stewart F. M., Freeman D. J., Ramsay J. E., Greer I. A., Caslake M., Ferrell W. R. (2007). Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J. Clin. Endocrinol. Metab. 92, 969–975. 10.1210/jc.2006-2083 [DOI] [PubMed] [Google Scholar]
- Suzuki T., Oshio S., Iwata M., Saburi H., Odagiri T., Udagawa T., et al. (2010). In utero exposure to a low concentration of diesel exhaust affects spontaneous locomotor activity and monoaminergic system in male mice. Part. Fibre Toxicol. 7:7. 10.1186/1743-8977-7-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro K. L., Moran T. H. (2010). Perinatal environment and its influences on metabolic programming of offspring. Physiol. Behav. 100, 560–566. 10.1016/j.physbeh.2010.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D., Li T. Y., Liu J. J., Zhou Z. J., Yuan T., Chen Y. H., et al. (2008). Effects of prenatal exposure to coal-burning pollutants on children's development in China. Environ. Health Perspect. 116, 674–679. 10.1289/ehp.10471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakore J. H. (2004). Metabolic disturbance in first-episode schizophrenia. Br. J. Psychiatry 184, s76–s79. 10.1192/bjp.184.47.s76 [DOI] [PubMed] [Google Scholar]
- Toschke A. M., Montgomery S. M., Pfeiffer U., Von Kries R. (2003). Early intrauterine exposure to tobacco-inhaled products and obesity. Am. J. Epidemiol. 158, 1068–1074. 10.1093/aje/kwg258 [DOI] [PubMed] [Google Scholar]
- Tozuka Y., Kumon M., Wada E., Onodera M., Mochizuki H., Wada K. (2010). Maternal obesity impairs hippocampal BDNF production and spatial learning performance in young mouse offspring. Neurochem. Int. 57, 235–247. 10.1016/j.neuint.2010.05.015 [DOI] [PubMed] [Google Scholar]
- Tozuka Y., Wada E., Wada K. (2009). Diet-induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. FASEB J. 23, 1920–1934. 10.1096/fj.08-124784 [DOI] [PubMed] [Google Scholar]
- Urayama S., Semi K., Sanosaka T., Hori Y., Namihira M., Kohyama J., et al. (2013). Chromatin accessibility at a STAT3 target site is altered prior to astrocyte differentiation. Cell Struct. Funct. 38, 55–66. 10.1247/csf.12034 [DOI] [PubMed] [Google Scholar]
- Vahedi G., Takahashi H., Nakayamada S., Sun H. W., Sartorelli V., Kanno Y., et al. (2012). STATs shape the active enhancer landscape of T cell populations. Cell 151, 981–993. 10.1016/j.cell.2012.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lieshout R. J., Boyle M. H. (2011a). Canadian youth born large or small for gestational age and externalizing and internalizing problems. Can. J. psychiatry 56, 227–234. [DOI] [PubMed] [Google Scholar]
- Van Lieshout R. J., Boyle M. H. (2011b). Is bigger better? macrosomia and psychopathology later in life. Obes. Rev. 12, e405–e411. 10.1111/j.1467-789X.2010.00816.x [DOI] [PubMed] [Google Scholar]
- Watson E. D., Cross J. C. (2005). Development of structures and transport functions in the mouse placenta. Physiology 20, 180–193. 10.1152/physiol.00001.2005 [DOI] [PubMed] [Google Scholar]
- Williams K., Mackenzie J. S. (1977). Influenza infections during pregnancy in the mouse. J. Hyg. 79, 249–257. 10.1017/S0022172400053067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik W., Lee W., Colman I., Hardy R., Hotopf M. (2013). Foetal origins of depression? A systematic review and meta-analysis of low birth weight and later depression. Psychol. Med. 43, 1–12. 10.1017/S0033291712000682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanbaeva D. G., Dentener M. A., Creutzberg E. C., Wesseling G., Wouters E. F. (2007). Systemic effects of smoking. Chest J. 131, 1557–1566. 10.1378/chest.06-2179 [DOI] [PubMed] [Google Scholar]
- Zaretsky M. V., Alexander J. M., Byrd W., Bawdon R. E. (2004). Transfer of inflammatory cytokines across the placenta. Obstet. Gynecol. 103, 546–550. 10.1097/01.AOG.0000114980.40445.83 [DOI] [PubMed] [Google Scholar]
- Zhang L., Sugiyama T., Murabayashi N., Umekawa T., Ma N., Kamimoto Y., et al. (2011). The inflammatory changes of adipose tissue in late pregnant mice. J. Mol. Endocrinol. 47, 157–165. 10.1530/JME-11-0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M. J., Du M., Nathanielsz P. W., Ford S. P. (2010). Maternal obesity up-regulates inflammatory signaling pathways and enhances cytokine expression in the mid-gestation sheep placenta. Placenta 31, 387–391. 10.1016/j.placenta.2010.02.002 [DOI] [PubMed] [Google Scholar]