Abstract
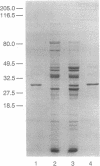
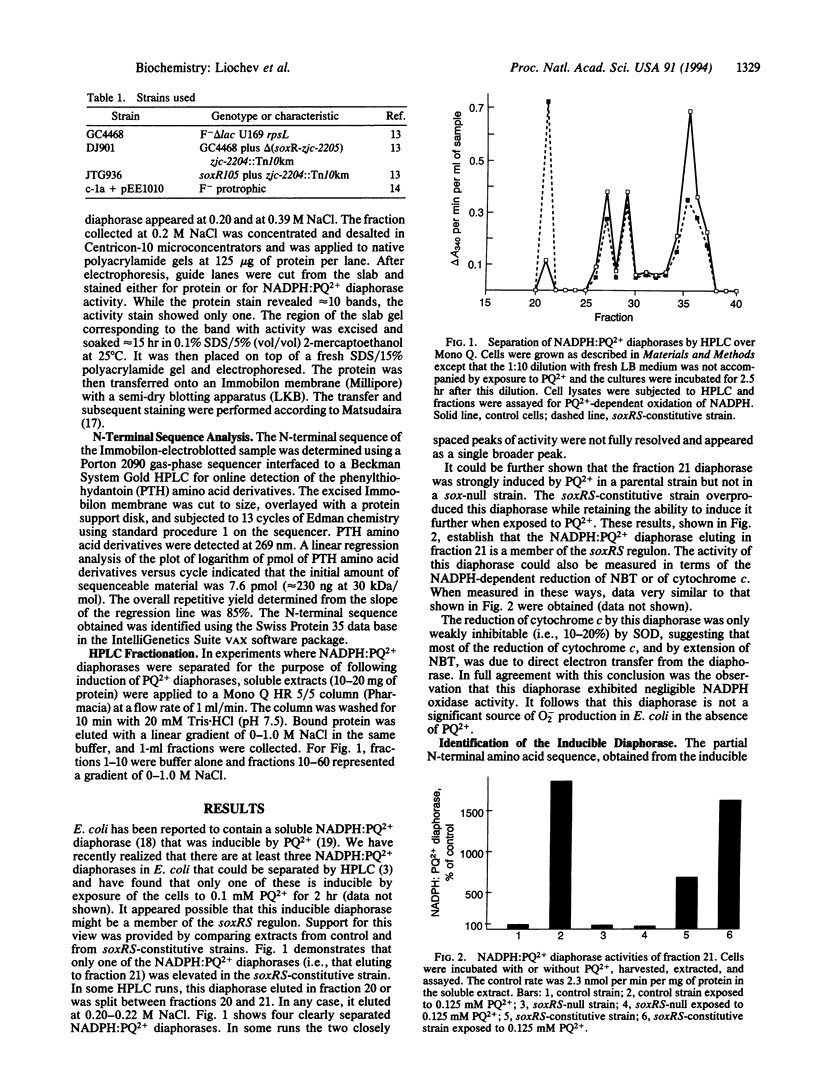
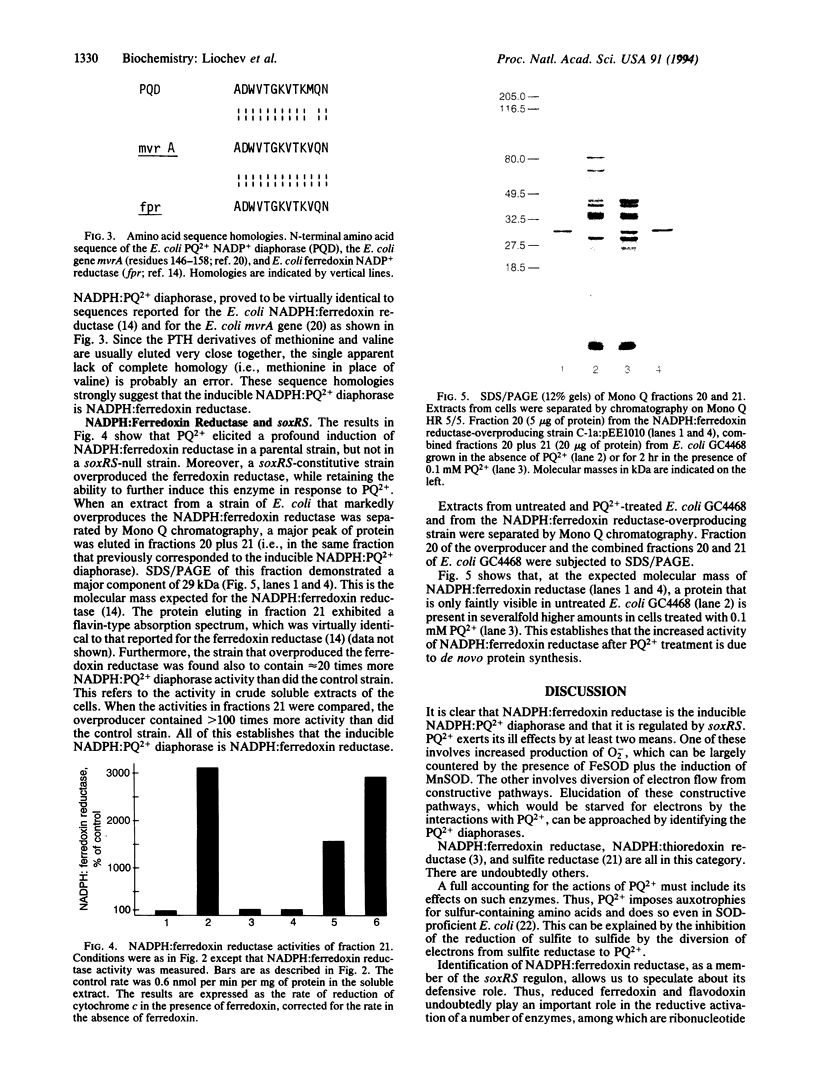
Soluble extracts of Escherichia coli contain four NADPH:paraquat diaphorases that were separable by anion-exchange HPLC over Mono Q. One of these was induced when the cells were exposed to paraquat. This was the case in a soxRS-competent strain but not in a soxRS-null strain, while a soxRS-constitutive strain overexpressed this diaphorase without the stimulus of exposure to paraquat. This NADPH:paraquat diaphorase could use cytochrome c or nitroblue tetrazolium as an electron acceptor, whereas O2 was a relatively poor acceptor. This diaphorase was identified as the NADPH:ferredoxin reductase. A role for reduced ferredoxin and flavodoxin in the adaptive soxRS response to oxidative stress and in the regulation of the redox status of soxR is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianchi V., Reichard P., Eliasson R., Pontis E., Krook M., Jörnvall H., Haggård-Ljungquist E. Escherichia coli ferredoxin NADP+ reductase: activation of E. coli anaerobic ribonucleotide reduction, cloning of the gene (fpr), and overexpression of the protein. J Bacteriol. 1993 Mar;175(6):1590–1595. doi: 10.1128/jb.175.6.1590-1595.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschkowski H. P., Neuer G., Ludwig-Festl M., Knappe J. Routes of flavodoxin and ferredoxin reduction in Escherichia coli. CoA-acylating pyruvate: flavodoxin and NADPH: flavodoxin oxidoreductases participating in the activation of pyruvate formate-lyase. Eur J Biochem. 1982 Apr;123(3):563–569. [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Amábile-Cuevas C. F. Redox redux: the control of oxidative stress responses. Cell. 1991 Nov 29;67(5):837–839. doi: 10.1016/0092-8674(91)90355-3. [DOI] [PubMed] [Google Scholar]
- Fujii K., Huennekens F. M. Activation of methionine synthetase by a reduced triphosphopyridine nucleotide-dependent flavoprotein system. J Biol Chem. 1974 Nov 10;249(21):6745–6753. [PubMed] [Google Scholar]
- Gardner P. R., Fridovich I. NADPH inhibits transcription of the Escherichia coli manganese superoxide dismutase gene (sodA) in vitro. J Biol Chem. 1993 Jun 15;268(17):12958–12963. [PubMed] [Google Scholar]
- Gardner P. R., Fridovich I. Superoxide sensitivity of the Escherichia coli 6-phosphogluconate dehydratase. J Biol Chem. 1991 Jan 25;266(3):1478–1483. [PubMed] [Google Scholar]
- Gardner P. R., Fridovich I. Superoxide sensitivity of the Escherichia coli aconitase. J Biol Chem. 1991 Oct 15;266(29):19328–19333. [PubMed] [Google Scholar]
- Greenberg J. T., Demple B. A global response induced in Escherichia coli by redox-cycling agents overlaps with that induced by peroxide stress. J Bacteriol. 1989 Jul;171(7):3933–3939. doi: 10.1128/jb.171.7.3933-3939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. T., Monach P., Chou J. H., Josephy P. D., Demple B. Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6181–6185. doi: 10.1073/pnas.87.16.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Paraquat and Escherichia coli. Mechanism of production of extracellular superoxide radical. J Biol Chem. 1979 Nov 10;254(21):10846–10852. [PubMed] [Google Scholar]
- Imlay J. A., Fridovich I. Suppression of oxidative envelope damage by pseudoreversion of a superoxide dismutase-deficient mutant of Escherichia coli. J Bacteriol. 1992 Feb;174(3):953–961. doi: 10.1128/jb.174.3.953-961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao S. M., Hassan H. M. Biochemical characterization of a paraquat-tolerant mutant of Escherichia coli. J Biol Chem. 1985 Sep 5;260(19):10478–10481. [PubMed] [Google Scholar]
- Kerscher L., Oesterhelt D. Purification and properties of two 2-oxoacid:ferredoxin oxidoreductases from Halobacterium halobium. Eur J Biochem. 1981 Jun 1;116(3):587–594. doi: 10.1111/j.1432-1033.1981.tb05376.x. [DOI] [PubMed] [Google Scholar]
- Kerscher L., Oesterhelt D. The catalytic mechanism of 2-oxoacid:ferredoxin oxidoreductases from Halobacterium halobium. One-electron transfer at two distinct steps of the catalytic cycle. Eur J Biochem. 1981 Jun 1;116(3):595–600. doi: 10.1111/j.1432-1033.1981.tb05377.x. [DOI] [PubMed] [Google Scholar]
- Knappe J., Neugebauer F. A., Blaschkowski H. P., Gänzler M. Post-translational activation introduces a free radical into pyruvate formate-lyase. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1332–1335. doi: 10.1073/pnas.81.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. F., Mashino T., Fridovich I. alpha, beta-Dihydroxyisovalerate dehydratase. A superoxide-sensitive enzyme. J Biol Chem. 1987 Apr 5;262(10):4724–4727. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liochev S. I., Fridovich I. Effects of overproduction of superoxide dismutases in Escherichia coli on inhibition of growth and on induction of glucose-6-phosphate dehydrogenase by paraquat. Arch Biochem Biophys. 1992 Apr;294(1):138–143. doi: 10.1016/0003-9861(92)90147-o. [DOI] [PubMed] [Google Scholar]
- Liochev S. I., Fridovich I. Fumarase C, the stable fumarase of Escherichia coli, is controlled by the soxRS regulon. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5892–5896. doi: 10.1073/pnas.89.13.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Limited N-terminal sequence analysis. Methods Enzymol. 1990;182:602–613. doi: 10.1016/0076-6879(90)82047-6. [DOI] [PubMed] [Google Scholar]
- Mito S., Zhang Q. M., Yonei S. Isolation and characterization of Escherichia coli strains containing new gene fusions (soi::lacZ) inducible by superoxide radicals. J Bacteriol. 1993 May;175(9):2645–2651. doi: 10.1128/jb.175.9.2645-2651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimyo M. Isolation and characterization of methyl viologen-sensitive mutants of Escherichia coli K-12. J Bacteriol. 1988 May;170(5):2136–2142. doi: 10.1128/jb.170.5.2136-2142.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulliez E., Fontecave M., Gaillard J., Reichard P. An iron-sulfur center and a free radical in the active anaerobic ribonucleotide reductase of Escherichia coli. J Biol Chem. 1993 Feb 5;268(4):2296–2299. [PubMed] [Google Scholar]
- Natvig D. O., Imlay K., Touati D., Hallewell R. A. Human copper-zinc superoxide dismutase complements superoxide dismutase-deficient Escherichia coli mutants. J Biol Chem. 1987 Oct 25;262(30):14697–14701. [PubMed] [Google Scholar]
- Nunoshiba T., Hidalgo E., Amábile Cuevas C. F., Demple B. Two-stage control of an oxidative stress regulon: the Escherichia coli SoxR protein triggers redox-inducible expression of the soxS regulatory gene. J Bacteriol. 1992 Oct;174(19):6054–6060. doi: 10.1128/jb.174.19.6054-6060.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalle C. T., Fridovich I. Anaerobic biosynthesis of the manganese-containing superoxide dismutase in Escherichia coli. Effects of diazenedicarboxylic acid bis(N,N'-dimethylamide) (diamide). J Biol Chem. 1990 Dec 15;265(35):21966–21970. [PubMed] [Google Scholar]
- Privalle C. T., Kong S. E., Fridovich I. Induction of manganese-containing superoxide dismutase in anaerobic Escherichia coli by diamide and 1,10-phenanthroline: sites of transcriptional regulation. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2310–2314. doi: 10.1073/pnas.90.6.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavone J. R., Hassan H. M. The role of redox in the regulation of manganese-containing superoxide dismutase biosynthesis in Escherichia coli. J Biol Chem. 1988 Mar 25;263(9):4269–4273. [PubMed] [Google Scholar]
- Siegel L. M., Davis P. S., Kamin H. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. 3. The Escherichia coli hemoflavoprotein: catalytic parameters and the sequence of electron flow. J Biol Chem. 1974 Mar 10;249(5):1572–1586. [PubMed] [Google Scholar]
- Tsaneva I. R., Weiss B. soxR, a locus governing a superoxide response regulon in Escherichia coli K-12. J Bacteriol. 1990 Aug;172(8):4197–4205. doi: 10.1128/jb.172.8.4197-4205.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkup L. K., Kogoma T. Escherichia coli proteins inducible by oxidative stress mediated by the superoxide radical. J Bacteriol. 1989 Mar;171(3):1476–1484. doi: 10.1128/jb.171.3.1476-1484.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Weiss B. Two-stage induction of the soxRS (superoxide response) regulon of Escherichia coli. J Bacteriol. 1992 Jun;174(12):3915–3920. doi: 10.1128/jb.174.12.3915-3920.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]