Abstract
Introduction
Altered local mechanical loading may disrupt normal cartilage homeostasis and play a role in the progression of osteoarthritis. Currently, there is limited data quantifying local cartilage strains in response to dynamic activity in normal or injured knees.
Purpose
The purpose of this study was to directly measure local tibiofemoral cartilage strains in response to a dynamic hopping activity in normal healthy knees. We hypothesize that local regions of cartilage will exhibit significant compressive strains in response to hopping, while overall compartmental averages may not.
Study Design
Controlled laboratory study.
Methods
Both knees of eight healthy subjects were MR imaged before and immediately after a dynamic hopping activity. Images were segmented and then used to create 3D surface models of bone and cartilage. These pre- and post-activity models were then registered using an iterative closest point technique to enable site-specific measurements of cartilage strain (defined as the normalized change in cartilage thickness before and after activity) on the femur and tibia.
Results
Significant strains were observed in both the medial and lateral tibial cartilage, with each compartment averaging a decrease of 5%. However, these strains varied with location within each compartment, reaching a maximum compressive strain of 8% on the medial plateau and 7% on the lateral plateau. No significant averaged compartmental strains were observed in the medial or lateral femoral cartilage. However, local regions of the medial and lateral femoral cartilage experienced significant compressive strains, reaching maximums of 6% and 3% respectively.
Conclusion
Local regions of both the femur and tibia experienced significant cartilage strains as a result of dynamic activity. An understanding of changes in cartilage strain distributions may help to elucidate the biomechanical factors contributing to cartilage degeneration after joint injury.
Key Terms: knee, cartilage, osteoarthritis, strain, tibiofemoral, hopping, jumping, magnetic resonance imaging, stress test, biomechanics
Introduction
Normal physiologic loading of chondrocytes is important for the development and maintenance of healthy cartilage2. However, altered mechanical stresses and strains, which may occur after ligament or meniscus injury 4, 44, may disrupt normal cartilage homeostasis and play a role in the progression of osteoarthritis (OA)1, 14. Therefore, a number of studies have sought to understand how cartilage is loaded during activities of daily living. For example, some studies have used skin markers and in-ground force plates to estimate loading distributions between the medial and lateral compartments of the tibiofemoral joint7, 36. Other studies have used biplanar radiographic images to measure joint kinematics and changes in joint space during activities such as walking and running18, 40. To provide additional information regarding cartilage function, some recent studies have incorporated measurements of cartilage geometry with biplanar radiographic imaging to approximate acute cartilage strains during quasi-static loading and gait5, 6, 25, 44. However, there is limited data directly measuring cartilage strains in response to dynamic activities such as running and jumping.
During activities of daily living, the knee joint experiences loads of several times body weight23, 29, 42. As the cartilage deforms under these loads, water is extruded28. After the load is removed, the low-permeability matrix of cartilage results in a time dependent recovery of deformation as fluid returns to the tissue. This sustained deformation can be measured using magnetic resonance (MR) imaging immediately before and after loading11, 13. Previous studies have used this principle to measure changes in knee cartilage volume as a result of different dynamic activities, including running8, 20, 21, 30, drop landings30, and knee bends12. While these studies contribute important information to the literature, compartmental volumetric changes may not be sensitive to variations in cartilage strain at different locations within the joint. Currently, there are limited site-specific measurements of cartilage strains in response to dynamic activities of daily living. Since mechanical loading can influence chondrocyte metabolism 16, 22, 32, 37, local measurements of cartilage strain are important to understanding normal cartilage physiology and may provide critical insights into the mechanisms leading to cartilage degeneration. Furthermore, such data may also provide baseline data for future studies evaluating cartilage loading in populations at high risk for the development of OA. Therefore, the objective of this study was to directly measure site-specific cartilage strains in vivo as a result of dynamic activity in normal knees. We hypothesized that local regions of cartilage will exhibit significant compressive strains in response to hopping, while compartmental averages of strain may not.
Methods
Institutional Review Board (IRB) approval was obtained before initiation of this study. Eight male subjects (mean age: 26.3 years, age range: 24–30 years) with no history of injury or surgery to either knee participated in this study. The mean body mass index (BMI) of these subjects was 22.8 kg/m2 (range 21.1 to 25.1 kg/m2). Both knees of each subject were studied, for a total of 16 knees.
All subjects participated in this study on the morning of their testing day to reduce the potential for diurnal differences in cartilage thickness11, 48. Subjects were asked to not perform any strenuous lower body activity the night prior to or the morning of their participation. Subjects first lay supine on a stretcher immediately outside of the MR suite for 45 minutes to minimize cartilage deformation prior to scanning6, 33, 39. Subjects were then moved to a wheelchair without any weightbearing and transported to the MR suite where they underwent pre-activity imaging. Imaging was performed using a 3T scanner (Trio Tim, Siemens) with an eight-channel knee coil and the patient in a supine position with the knee relaxed. Sagittal plane images (field of view 16×16 cm, 512×512 pixels) of 1 mm thickness were generated using a double-echo steady state sequence (DESS, flip angle: 25°, TR: 17 ms, TE: 6 ms)11, 48. Total scan time was approximately 9 minutes for each knee.
Subjects were transported via wheelchair to the hall adjacent to the MR suite and performed 60 single-legged hops of 0.6m on the tested leg. For each subject, the order of testing was alternated between left and right knees. During this hopping activity, the contralateral knee was kept in a flexed position off the ground. After completing the hopping activity, subjects were seated in a wheelchair and immediately transported into the MR suite to undergo post-activity MR imaging. The time from completion of the activity to the initiation of the post-activity MRI averaged 3.5 minutes (range: 3–4 minutes). After post-activity imaging was completed, testing was repeated on the contralateral knee.
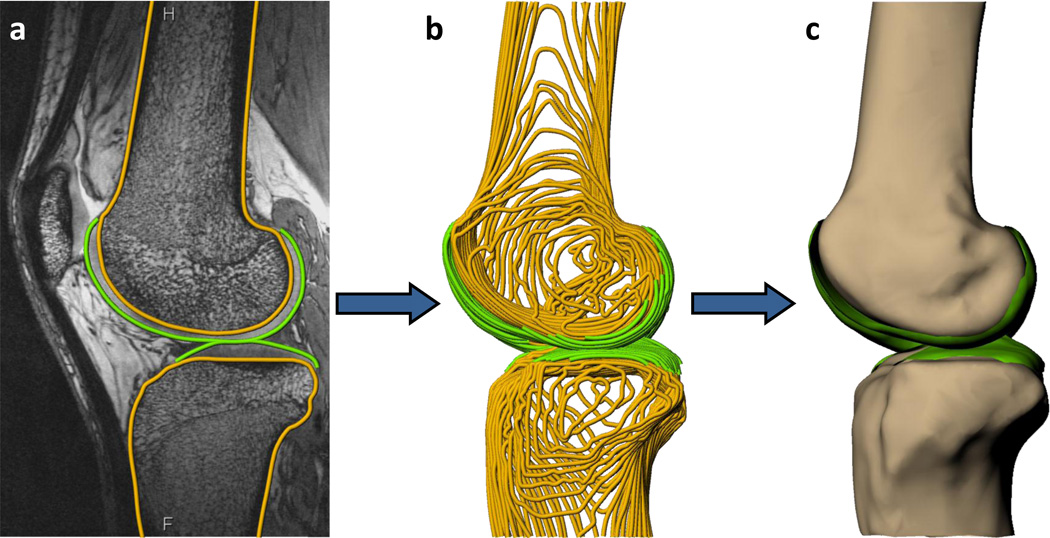
The sagittal MR images were imported into solid modeling software (Rhinoceros, Robert McNeel and Associates, Seattle, Washington) and the outer bony cortex and cartilage surfaces of the femur and tibia were manually traced using non-uniform rational B-spline (NURBS) curves on each MR image by a single investigator (Figure 1a). These curves (Figure 1b) were then used to create a 3D surface mesh model of both bone and cartilage (Geomagic Studio, Morrisville, NC) (Figure 1c). This methodology has been previously validated for measuring cartilage thickness44. Furthermore, a recent study indicated that the coefficient of repeatability for measuring cartilage thickness using this methodology is 0.03mm, which corresponds to a difference in cartilage thickness of 1% 11.
Figure 1.
a) The surfaces of the tibia, femur, and articular cartilage were segmented for each sagittal 3T MR slice, b) stacked to form a wireframe model, and c) converted to 3D surface mesh models.
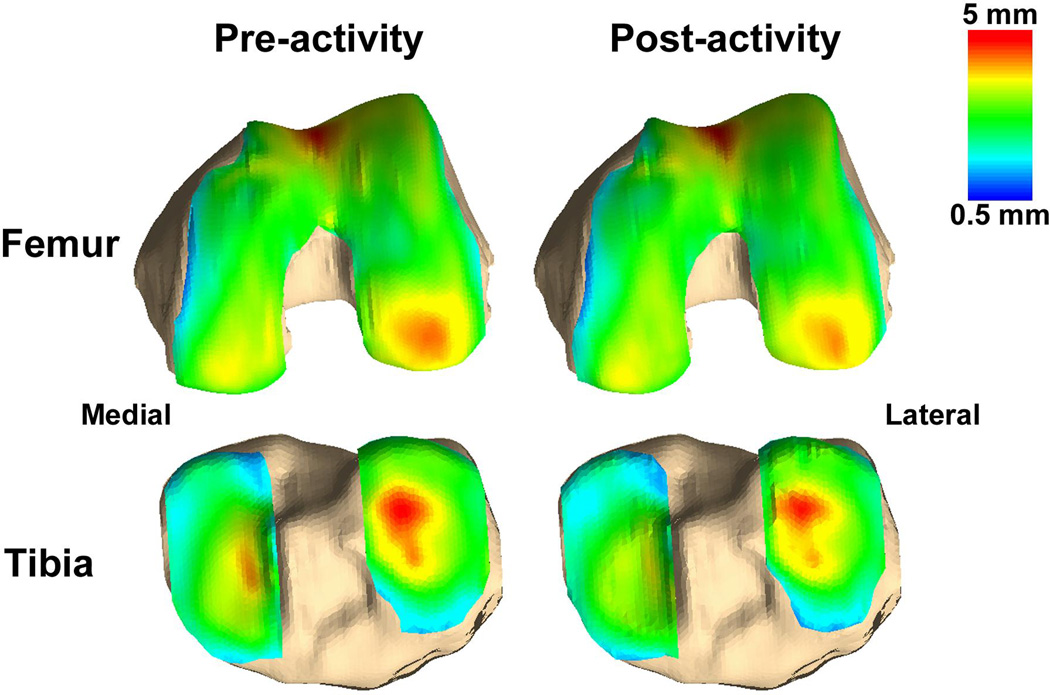
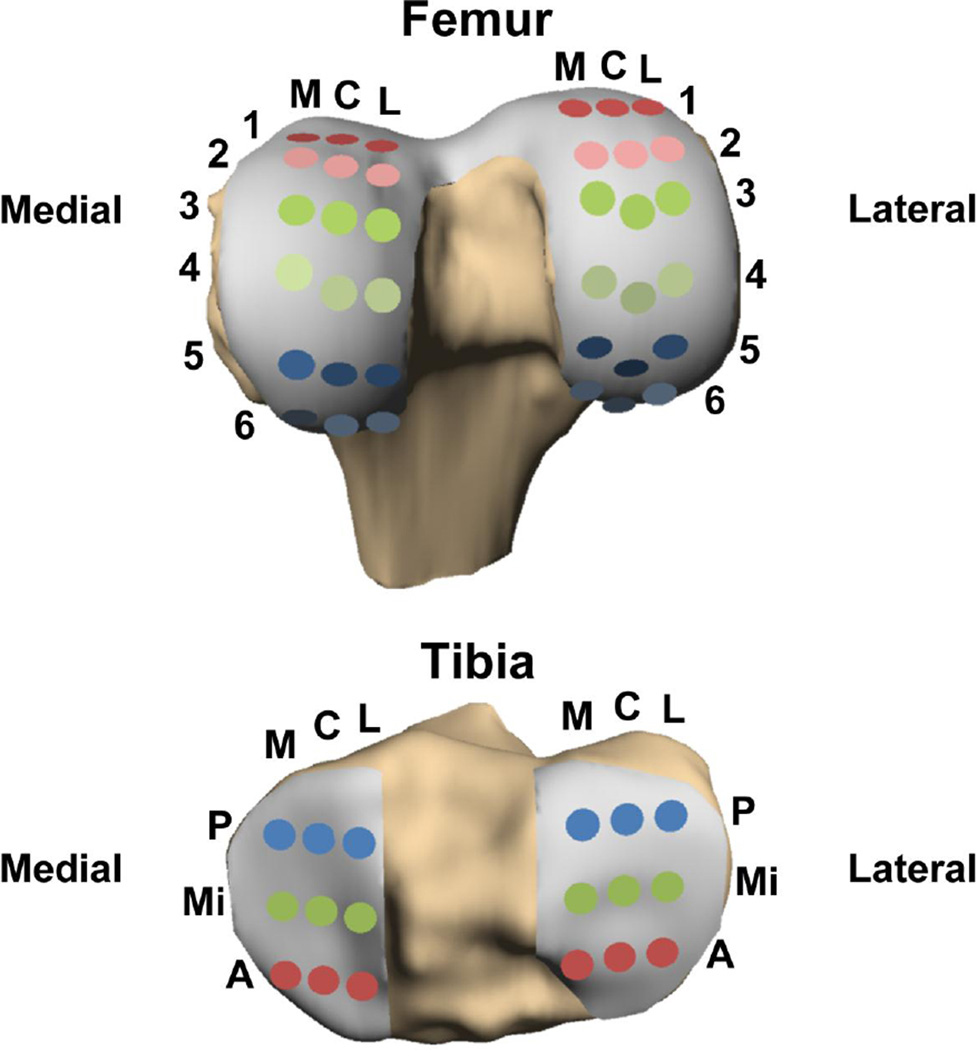
Cartilage thickness was calculated as the distance between each point on the cartilage surface and the nearest point on the bony surface. These calculations were used to generate cartilage thickness maps for each knee (Figure 2). The femoral and tibial bony surfaces of the pre-activity and post-activity models were aligned using an iterative closest point technique11, 33, 48. This registration process enabled the site-specific measurement of cartilage strain using the pre-activity and post-activity models. Next, a grid system was created to sample strain at points spanning the femoral and tibial cartilage surfaces (Figure 3)11, 33, 48. Nine evenly spaced grid points were created on each medial and lateral tibial plateau and 18 points were created on each medial and lateral femoral condyle. Strain was defined as the normalized change in thickness before and after activity and was calculated as the average across all of the points on model of the cartilage surface within a 2.5 mm radius of the respective grid point11, 48. Overall compartmental strains were calculated as the average of the sampled points in a given tibial or femoral compartment.
Figure 2.
Representative cartilage thickness maps of pre-activity and post-activity femoral and tibial models. Thickness is represented in color, with thicker cartilage in red, and thinner cartilage in blue.
Figure 3.
Femoral and tibial strain grids: 18 points on each femoral condyle and 9 points on each tibial plateau. L=lateral, C=center, M=medial, A=anterior, Mi=middle, P=posterior.
Statistical analysis was performed using Statistica (StatSoft, Tulsa, OK). Single sample t-tests were used to determine whether averaged compartmental and local strains were different from zero. Differences were considered statistically significant where p < 0.05.
Results
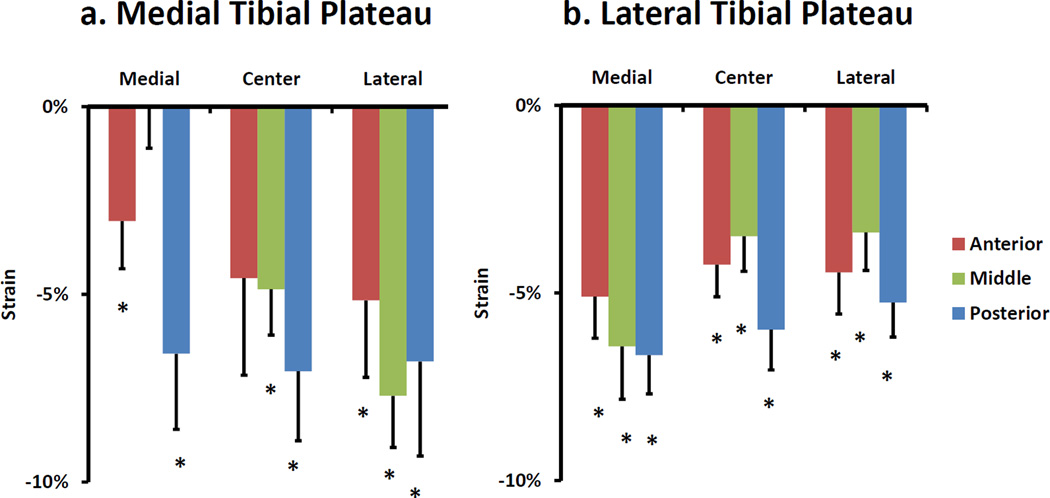
Statistically significant compressive strains were exhibited in both the medial and lateral tibial plateaus as a result of single-legged hopping. In the medial compartment, there was an overall compressive strain of 5 ± 1% (mean ± standard error of the mean) (p < 0.001). However, some regions exhibited significant local strains, while others did not (Figure 4a). For example, local compressive strain reached a maximum of 8 ± 1% (p < 0.001) in the lateral portion of the medial compartment. In the lateral compartment, an overall compressive strain of 5 ± 1% (p < 0.001) was exhibited. However, as was observed in the medial compartment, some regions exhibited significant strains while others did not. For example, local compressive strain reached a maximum of 7 ± 1% (p < 0.001) in the medial portion of the lateral compartment of the tibia (Figure 4b).
Figure 4.
Local strain results for a) medial and b) lateral tibial plateaus. Please see Figure 3 for point location legend. Error bars represent standard error of the mean. * p<0.05 different from 0.
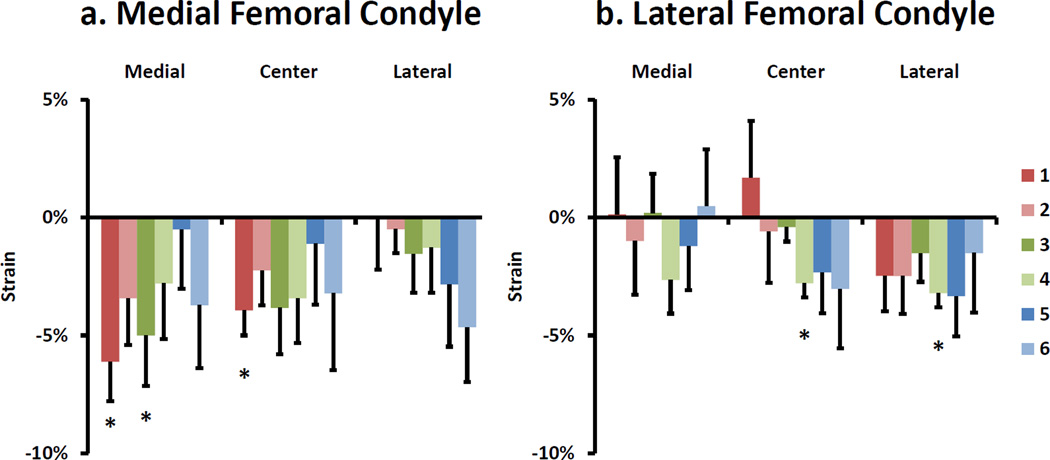
Overall, statistically significant averaged compartmental strains were not observed in either the medial or lateral femoral condyles as a result of single-legged hopping. In the medial compartment, an average compressive strain of 2 ± 1% (p = 0.11) was measured. However, local compressive strain reached a maximum of 6 ± 2% (p < 0.01) (Figure 5a) on the most anterior and medial portion of the medial femoral condyle. In the lateral compartment, an average compressive strain of 1 ± 1% (p = 0.29) was measured. However, there were significant compressive strains of 3 ± 1% (p < 0.001) (Figure 5b) in two local regions of the lateral femoral condyle.
Figure 5.
Local strain results for a) medial and b) lateral femoral condyles. Please see Figure 3 for point location legend. Error bars represent standard error of the mean. * p<0.05 different from 0.
Discussion
Mechanical loading plays an important role in normal cartilage homeostasis. Disruptions to the normal cartilage stress and strain distributions, which can occur after ligament or meniscus injury3, 4, 44, can alter chondrocyte metabolism 16, 22, 32, 37, and potentially predispose the knee to degenerative changes1, 15. Thus, baseline data characterizing the local mechanical environment of cartilage in response to in vivo loading conditions may provide valuable insights into the mechanisms contributing to the development and progression of OA. The present study measured variations in cartilage strains with location in the joint in response to a dynamic hopping activity. Specifically, we found significant averaged strains in both tibial compartments. However, some local regions of the tibia experienced significant strains, while other regions did not. Additionally, while no average compartmental changes were observed on either femoral condyle, some local regions of femoral cartilage experienced significant strains. These results suggest that site-specific measurements of strain may provide important information regarding the local mechanical environment of cartilage that volumetric measurements of cartilage deformation may not be able to detect.
In comparison, our results regarding the average compartmental strains are consistent with previous studies measuring volumetric changes in cartilage in response to activity. For example, a previous study by Eckstein et al12 measured compartmental volumetric changes as a result of a high impact single-legged jump landing activity. They found significant changes in the tibial cartilage volume in both compartments, with greater volume change in the lateral tibial plateau. They found no significant volumetric changes in the femoral cartilage in either compartment, which was similar to our finding of no significant averaged compartmental strains in the present study. Another study by Niehoff et al30 investigating the effects of a high-impact double-legged jump landing also found significant average compartmental changes in cartilage thickness in both the medial (−2.2%) and lateral (−1.8%) tibial compartments. Again, no changes in the overall compartmental thickness were observed in either femoral condyle, which was also consistent with the averaged compartmental results of the present study. In contrast, the present study also observed local regions where significant cartilage strains were experienced on both the femur and the tibia. Together, these findings suggest that the site-specific measurements described in the present study may be more sensitive to variations in cartilage strain at different locations within the joint than volumetric deformations.
Our finding that there were fewer areas of significant local strains and lower compartmental strains exhibited in the femoral cartilage compared to the tibial cartilage may be explained by differences in loading and mechanical properties within the joint. For example, this may be a result of the larger area of femoral cartilage through which load is transmitted during dynamic flexion-extension movements25, 47. In contrast, smaller areas of cartilage may be more consistently loaded throughout knee motion in the tibial cartilage. These differences in strain between the femur and tibia may also be due to the heterogeneity of mechanical properties of articular cartilage in the knee. Treppo et al43 reported that femoral cartilage had a significantly greater equilibrium modulus and dynamic stiffness (at 0.1Hz) than tibial cartilage. As a result, the femoral cartilage may exhibit less strain than tibial cartilage when experiencing dynamic loads through the knee joint.
Interestingly, there were higher local compressive strains near the tibial spine compared to the peripheral regions in both tibial compartments. This pattern was most apparent in the middle region of both compartments. We believe that this may be due, in part, to the presence of the meniscus, which distributes load in the peripheral region of the tibial plateau19, 47. Thus, regions of cartilage covered by the meniscus may experience less strain than regions where cartilage to cartilage contact occurs.
The single-legged hopping activity is one of many activities with which this method could be used to assess cartilage strains. For the purposes of this study, single-legged hops were chosen for multiple reasons. Single-legged hops are believed to place higher demands on the knee than walking or jogging and may represent dynamic movements such as jumping and cutting during athletic activities35. In addition, single-legged hops are used as a clinical tool to assess knee injury rehabilitation and as a return to sport evaluation31, 35. Consideration was also given to the time sensitive nature in which the post-activity MR scan needed to be performed and the ability to ensure the safety of the subjects. This required that the activity could be performed in close proximity to the MR suite and in a controlled environment. We concluded that a hopping activity fulfilled these requirements. However, future studies might investigate the local strain patterns as a result of other dynamic activities, such as walking or running.
The method used in this study requires two measurements of cartilage: before the activity is performed and immediately after the activity is completed. Consequently, the measured deformation and calculated strain is the cumulative result of the entire activity. Therefore, though these are direct measurements of cartilage deformation, they may differ from the instantaneous cartilage strain induced throughout dynamic activity5, 6, 25. Additionally, due to time required for subject transport, positioning and initiation of the imaging scan, some cartilage deformation may be recovered and our measurements may underestimate the true strains induced by the activity. However, we were able to begin imaging all knees within four minutes after the hopping activity was completed, which is consistent with prior studies30. Furthermore, this amount of time appears to be relatively small compared to the time scale required for cartilage to completely recover from activity13, 45. For example, a previous study indicated that 90 minutes was required for full volumetric recovery of the patellar cartilage after performing 100 deep knee bends13. Future studies might also use this methodology to evaluate the recovery of cartilage strain at various time points after exercise.
The current study investigated cartilage strains in young healthy male subjects only during a hopping activity. However, the strain patterns seen in normal healthy knees may be different between males and females due to anatomical differences between sexes, such as Q angle17. Future studies might evaluate differences in strain patterns between males and females. Additionally, future studies might use marker-based motion capture techniques38, 41 to investigate the influence of different motion patterns on cartilage deformation.
The ability to directly measure site-specific cartilage strains also has applications in the study of abnormal cartilage loading following ligament or meniscus injury. For example, this protocol could be used as a “stress test” to evaluate changes in the mechanical response of cartilage in these patient populations. Understanding how joint injury alters the mechanical environment of cartilage is important because these changes could directly affect chondrocyte metabolism16, 22, 32, 37 and disrupt normal cartilage homeostasis1, 14. Thus, this methodology could be used to investigate cartilage strains in patients with ligament or meniscus injuries to provide insight into the mechanisms contributing to the high prevalence and early onset of post-traumatic OA in these patient populations 26, 27, 34, 46. Future studies may also seek to combine this method with other MR imaging modalities, such as T1ρ or T2 mapping24, which may allow for the measurement of zone-specific changes in cartilage strain due to variations in cartilage composition, structure, and mechanical properties with depth9, 10, 49. The fusion of these modalities may also allow for the correlation of mechanical deformation and changes in water content as a result of dynamic activity39. Additionally, such data may also help to explain the interplay between altered strains and biochemical changes in cartilage and their role in the progression of OA24.
In conclusion, our study demonstrated that local regions of tibiofemoral cartilage experienced significant compressive strains in response to a dynamic hopping activity. These local tissue strains varied from the compartmental averages, suggesting that site-specific measurements of cartilage strain may provide additional information regarding changes in the local mechanical environment that volumetric measures may not be sensitive enough to detect. In the future, this methodology and data could be used to evaluate the effects of soft tissue injuries (such as ligament or meniscus injuries) on cartilage strain distributions in response to dynamic activities of daily living. An understanding of these cartilage strain distributions may help to elucidate the biomechanical factors contributing to cartilage degeneration.
Clinical relevance.
Site-specific measurements of in vivo cartilage strains are important because altered loading is believed to be a factor contributing to the development and progression of osteoarthritis. Specifically, this methodology and data could be used to evaluate the effects of soft tissue injuries (such as ligament or meniscus tears) on cartilage strains in response to dynamic activities of daily living.
Acknowledgments
This work was supported by grants from the NIH (AR063325 and AR065527).
References
- 1.Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 2.Beaupre GS, Stevens SS, Carter DR. Mechanobiology in the development, maintenance, and degeneration of articular cartilage. J Rehabil Res Dev. 2000;37(2):145–151. [PubMed] [Google Scholar]
- 3.Bedi A, Chen T, Santner TJ, El-Amin S, Kelly NH, Warren RF, Maher SA. Changes in dynamic medial tibiofemoral contact mechanics and kinematics after injury of the anterior cruciate ligament: a cadaveric model. Proc Inst Mech Eng H. 2013;227(9):1027–1037. doi: 10.1177/0954411913490387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedi A, Kelly N, Baad M, Fox AJ, Ma Y, Warren RF, Maher SA. Dynamic contact mechanics of radial tears of the lateral meniscus: implications for treatment. Arthroscopy. 2012;28(3):372–381. doi: 10.1016/j.arthro.2011.08.287. [DOI] [PubMed] [Google Scholar]
- 5.Bingham JT, Papannagari R, Van de Velde SK, Gross C, Gill TJ, Felson DT, Rubash HE, Li G. In vivo cartilage contact deformation in the healthy human tibiofemoral joint. Rheumatology (Oxford) 2008;47(11):1622–1627. doi: 10.1093/rheumatology/ken345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bischof JE, Spritzer CE, Caputo AM, Easley ME, DeOrio JK, Nunley JA, 2nd, DeFrate LE. In vivo cartilage contact strains in patients with lateral ankle instability. J Biomech. 2010;43(13):2561–2566. doi: 10.1016/j.jbiomech.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blazek K, Favre J, Asay J, Erhart-Hledik J, Andriacchi T. Age and obesity alter the relationship between femoral articular cartilage thickness and ambulatory loads in individuals without osteoarthritis. J Orthop Res. 2014;32(3):394–402. doi: 10.1002/jor.22530. [DOI] [PubMed] [Google Scholar]
- 8.Boocock M, McNair P, Cicuttini F, Stuart A, Sinclair T. The short-term effects of running on the deformation of knee articular cartilage and its relationship to biomechanical loads at the knee. Osteoarthritis Cartilage. 2009;17(7):883–890. doi: 10.1016/j.joca.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Butz KD, Chan DD, Nauman EA, Neu CP. Stress distributions and material properties determined in articular cartilage from MRI-based finite strains. J Biomech. 2011;44(15):2667–2672. doi: 10.1016/j.jbiomech.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Chan DD, Neu CP. Probing articular cartilage damage and disease by quantitative magnetic resonance imaging. J R Soc Interface. 2013;10(78):20120608. doi: 10.1098/rsif.2012.0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman JL, Widmyer MR, Leddy HA, Utturkar GM, Spritzer CE, Moorman CT, 3rd, Guilak F, Defrate LE. Diurnal variations in articular cartilage thickness and strain in the human knee. J Biomech. 2013;46(3):541–547. doi: 10.1016/j.jbiomech.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckstein F, Lemberger B, Gratzke C, Hudelmaier M, Glaser C, Englmeier KH, Reiser M. In vivo cartilage deformation after different types of activity and its dependence on physical training status. Ann Rheum Dis. 2005;64(2):291–295. doi: 10.1136/ard.2004.022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckstein F, Tieschky M, Faber S, Englmeier KH, Reiser M. Functional analysis of articular cartilage deformation, recovery, and fluid flow following dynamic exercise in vivo. Anat Embryol (Berl) 1999;200(4):419–424. doi: 10.1007/s004290050291. [DOI] [PubMed] [Google Scholar]
- 14.Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev. 2005;33(4):195–200. doi: 10.1097/00003677-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol. 2011;25(6):815–823. doi: 10.1016/j.berh.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guilak F, Meyer BC, Ratcliffe A, Mow VC. The effects of matrix compression on proteoglycan metabolism in articular cartilage explants. Osteoarthritis Cartilage. 1994;2(2):91–101. doi: 10.1016/s1063-4584(05)80059-7. [DOI] [PubMed] [Google Scholar]
- 17.Horton MG, Hall TL. Quadriceps femoris muscle angle: normal values and relationships with gender and selected skeletal measures. Phys Ther. 1989;69(11):897–901. doi: 10.1093/ptj/69.11.897. [DOI] [PubMed] [Google Scholar]
- 18.Hosseini A, Van de Velde SK, Kozanek M, Gill TJ, Grodzinsky AJ, Rubash HE, Li G. In-vivo time-dependent articular cartilage contact behavior of the tibiofemoral joint. Osteoarthritis Cartilage. 2010;18(7):909–916. doi: 10.1016/j.joca.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kazemi M, Li LP, Savard P, Buschmann MD. Creep behavior of the intact and meniscectomy knee joints. J Mech Behav Biomed Mater. 2011;4(7):1351–1358. doi: 10.1016/j.jmbbm.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Kersting UG, Stubendorff JJ, Schmidt MC, Bruggemann GP. Changes in knee cartilage volume and serum COMP concentration after running exercise. Osteoarthritis Cartilage. 2005;13(10):925–934. doi: 10.1016/j.joca.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Kessler MA, Glaser C, Tittel S, Reiser M, Imhoff AB. Volume changes in the menisci and articular cartilage of runners: an in vivo investigation based on 3-D magnetic resonance imaging. Am J Sports Med. 2006;34(5):832–836. doi: 10.1177/0363546505282622. [DOI] [PubMed] [Google Scholar]
- 22.Kim YJ, Sah RL, Grodzinsky AJ, Plaas AH, Sandy JD. Mechanical regulation of cartilage biosynthetic behavior: physical stimuli. Arch Biochem Biophys. 1994;311(1):1–12. doi: 10.1006/abbi.1994.1201. [DOI] [PubMed] [Google Scholar]
- 23.Kutzner I, Heinlein B, Graichen F, Bender A, Rohlmann A, Halder A, Beier A, Bergmann G. Loading of the knee joint during activities of daily living measured in vivo in five subjects. J Biomech. 2010;43(11):2164–2173. doi: 10.1016/j.jbiomech.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, Ries MD, Horvai A, Link TM, Majumdar S. Quantitative MRI using T1rho and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging. 2011;29(3):324–334. doi: 10.1016/j.mri.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu F, Kozanek M, Hosseini A, Van de Velde SK, Gill TJ, Rubash HE, Li G. In vivo tibiofemoral cartilage deformation during the stance phase of gait. J Biomech. 2010;43(4):658–665. doi: 10.1016/j.jbiomech.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 27.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 28.Mow VC, Holmes MH, Lai WM. Fluid transport and mechanical properties of articular cartilage: a review. J Biomech. 1984;17(5):377–394. doi: 10.1016/0021-9290(84)90031-9. [DOI] [PubMed] [Google Scholar]
- 29.Mundermann A, Dyrby CO, D'Lima DD, Colwell CW, Jr, Andriacchi TP. In vivo knee loading characteristics during activities of daily living as measured by an instrumented total knee replacement. J Orthop Res. 2008;26(9):1167–1172. doi: 10.1002/jor.20655. [DOI] [PubMed] [Google Scholar]
- 30.Niehoff A, Muller M, Bruggemann L, Savage T, Zaucke F, Eckstein F, Muller-Lung U, Bruggemann GP. Deformational behaviour of knee cartilage and changes in serum cartilage oligomeric matrix protein (COMP) after running and drop landing. Osteoarthritis Cartilage. 2011;19(8):1003–1010. doi: 10.1016/j.joca.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Noyes FR, Barber SD, Mangine RE. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am J Sports Med. 1991;19(5):513–518. doi: 10.1177/036354659101900518. [DOI] [PubMed] [Google Scholar]
- 32.O'Conor CJ, Leddy HA, Benefield HC, Liedtke WB, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci U S A. 2014;111(4):1316–1321. doi: 10.1073/pnas.1319569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okafor EC, Utturkar GM, Widmyer MR, Abebe ES, Collins AT, Taylor DC, Spritzer CE, Moorman CT, 3rd, Garrett WE, DeFrate LE. The effects of femoral graft placement on cartilage thickness after anterior cruciate ligament reconstruction. J Biomech. 2014;47(1):96–101. doi: 10.1016/j.jbiomech.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3(4):261–267. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 35.Rudolph KS, Axe MJ, Snyder-Mackler L. Dynamic stability after ACL injury: who can hop? Knee Surg Sports Traumatol Arthrosc. 2000;8(5):262–269. doi: 10.1007/s001670000130. [DOI] [PubMed] [Google Scholar]
- 36.Russell EM, Miller RH, Umberger BR, Hamill J. Lateral wedges alter mediolateral load distributions at the knee joint in obese individuals. J Orthop Res. 2013;31(5):665–671. doi: 10.1002/jor.22248. [DOI] [PubMed] [Google Scholar]
- 37.Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7(5):619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 38.Scanlan SF, Chaudhari AM, Dyrby CO, Andriacchi TP. Differences in tibial rotation during walking in ACL reconstructed and healthy contralateral knees. J Biomech. 2010;43(9):1817–1822. doi: 10.1016/j.jbiomech.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subburaj K, Kumar D, Souza RB, Alizai H, Li X, Link TM, Majumdar S. The acute effect of running on knee articular cartilage and meniscus magnetic resonance relaxation times in young healthy adults. Am J Sports Med. 2012;40(9):2134–2141. doi: 10.1177/0363546512449816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tashman S, Anderst W, Kolowich P, Havstad S, Arnoczky S. Kinematics of the ACL-deficient canine knee during gait: serial changes over two years. J Orthop Res. 2004;22(5):931–941. doi: 10.1016/j.orthres.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Taylor KA, Cutcliffe HC, Queen RM, Utturkar GM, Spritzer CE, Garrett WE, DeFrate LE. In vivo measurement of ACL length and relative strain during walking. J Biomech. 2013;46(3):478–483. doi: 10.1016/j.jbiomech.2012.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor KA, Terry ME, Utturkar GM, Spritzer CE, Queen RM, Irribarra LA, Garrett WE, DeFrate LE. Measurement of in vivo anterior cruciate ligament strain during dynamic jump landing. J Biomech. 2011;44(3):365–371. doi: 10.1016/j.jbiomech.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Treppo S, Koepp H, Quan EC, Cole AA, Kuettner KE, Grodzinsky AJ. Comparison of biomechanical and biochemical properties of cartilage from human knee and ankle pairs. J Orthop Res. 2000;18(5):739–748. doi: 10.1002/jor.1100180510. [DOI] [PubMed] [Google Scholar]
- 44.Van de Velde SK, Bingham JT, Hosseini A, Kozanek M, DeFrate LE, Gill TJ, Li G. Increased tibiofemoral cartilage contact deformation in patients with anterior cruciate ligament deficiency. Arthritis Rheum. 2009;60(12):3693–3702. doi: 10.1002/art.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Ginckel A, Verdonk P, Victor J, Witvrouw E. Cartilage status in relation to return to sports after anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(3):550–559. doi: 10.1177/0363546512473568. [DOI] [PubMed] [Google Scholar]
- 46.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63(3):269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker PS, Erkman MJ. The role of the menisci in force transmission across the knee. Clin Orthop Relat Res. 1975;109:184–192. doi: 10.1097/00003086-197506000-00027. [DOI] [PubMed] [Google Scholar]
- 48.Widmyer MR, Utturkar GM, Leddy HA, Coleman JL, Spritzer CE, Moorman CT, 3rd, DeFrate LE, Guilak F. High body mass index is associated with increased diurnal strains in the articular cartilage of the knee. Arthritis Rheum. 2013;65(10):2615–2622. doi: 10.1002/art.38062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong BL, Sah RL. Mechanical asymmetry during articulation of tibial and femoral cartilages: local and overall compressive and shear deformation and properties. J Biomech. 2010;43(9):1689–1695. doi: 10.1016/j.jbiomech.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]