A novel, unipotent progenitor population in the skin characterized by Atoh1 expression gives rise to Merkel cells both during development and adulthood.
Abstract
Resident progenitor cells in mammalian skin generate new cells as a part of tissue homeostasis. We sought to identify the progenitors of Merkel cells, a unique skin cell type that plays critical roles in mechanosensation. We found that some Atoh1-expressing cells in the hairy skin and whisker follicles are mitotically active at embryonic and postnatal ages. Genetic fate-mapping revealed that these Atoh1-expressing cells give rise solely to Merkel cells. Furthermore, selective ablation of Atoh1+ skin cells in adult mice led to a permanent reduction in Merkel cell numbers, demonstrating that other stem cell populations are incapable of producing Merkel cells. These data identify a novel, unipotent progenitor population in the skin that gives rise to Merkel cells both during development and adulthood.
Introduction
Mammalian skin is a dynamic organ that provides protection against a variety of environmental insults. Damage to the skin caused by these stressors must be repaired through constant skin cell replacement. Skin integrity is maintained by a heterogeneous population of resident progenitor cells capable of self-renewal and production of diverse cell types that make up hair follicles, glands, and interfollicular epidermis (Ghazizadeh and Taichman, 2001; Ito et al., 2005; Fuchs, 2007; Jaks et al., 2010; Solanas and Benitah, 2013).
In addition to its role as a barrier, skin also houses multiple somatosensory receptors, each tuned to detect different forms of mechanical stimuli. The Merkel cell–neurite complex is one such receptor located at the epidermal–dermal border of mammalian skin around whisker follicles, in hairy skin within specialized structures called touch domes and in glabrous (nonhairy) skin of the hands and feet (Halata et al., 2003). Embryologically, Merkel cells originate from epidermal progenitors and require expression of the basic helix-loop-helix transcription factor Atoh1 for their specification (Maricich et al., 2009; Morrison et al., 2009; Van Keymeulen et al., 2009). Atoh1 expression is maintained throughout development and in mature Merkel cells (Lumpkin et al., 2003).
Adult Merkel cells are postmitotic (Moll et al., 1995). However, quantitative, morphological, and fate-mapping studies suggest that Merkel cell numbers in adult hairy skin oscillate with the hair cycle, implying that Merkel cells turnover throughout an organism’s lifespan (Nafstad, 1987; Moll et al., 1996a; Nakafusa et al., 2006; Van Keymeulen et al., 2009). Mitotically active progenitors are the likely source of new Merkel cells, as a small percentage of Merkel cells are labeled several days after administration of nucleotide analogues (Mérot et al., 1987; Vaigot et al., 1987; Mérot and Saurat, 1988; Woo et al., 2010). Recent work in hairy skin has suggested that these progenitors are either multipotent stem cells located in the hair follicle bulge region or bipotent progenitors found among the touch dome keratinocytes (Van Keymeulen et al., 2009; Woo et al., 2010; Doucet et al., 2013). Accurate identification of Merkel cell progenitors is crucial because of the potential for these cells to act as the cellular origin of Merkel cell carcinoma (MCC), a rare but devastating disease that currently has no targeted therapies (Sidhu et al., 2005; Kuwamoto, 2011; Tilling and Moll, 2012).
Because Atoh1 expression is required by mitotic precursors of other Atoh1-lineal cell populations such as cerebellar granule cells, dorsal commissural interneurons, and secretory cells of the gut (Akazawa et al., 1995; Helms and Johnson, 1998; Yang et al., 2001), we hypothesized that the immediate Merkel cell progenitor would likewise express Atoh1. We used multiple techniques in different in vivo genetic mouse models to lineage trace and examine the proliferative capacity of Atoh1+ cells in hairy skin during embryogenesis and adulthood. We found that a subpopulation of Atoh1+ cells proliferates, contributes solely to the generation of Merkel cells, and cannot be replaced by other resident stem/progenitor cells in the skin. Our data identify a new progenitor population that is uniquely responsible for the generation and maintenance of Merkel cells.
Results
Adult Merkel cell precursors express Atoh1 and are unipotent
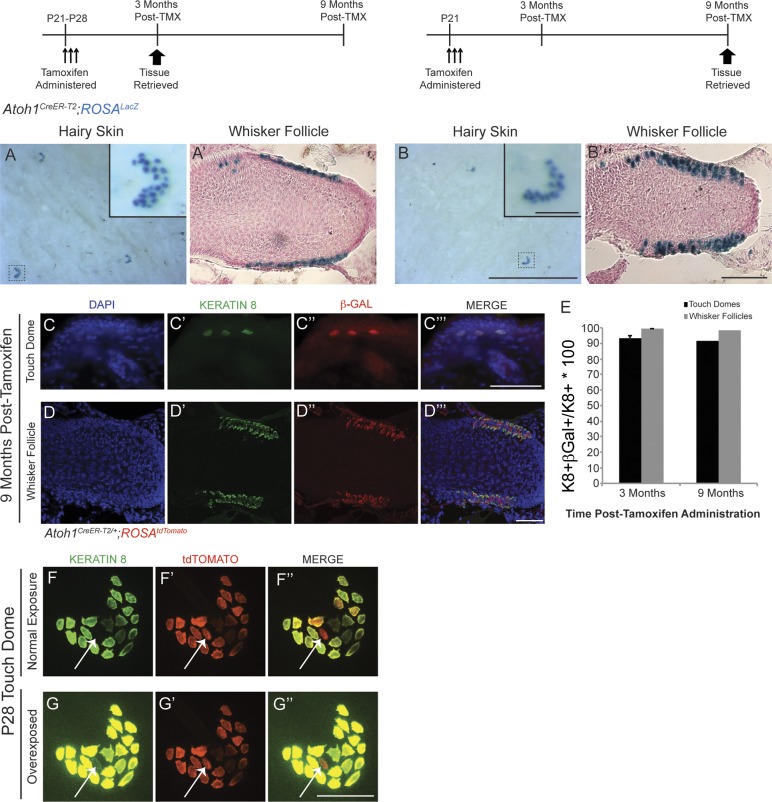
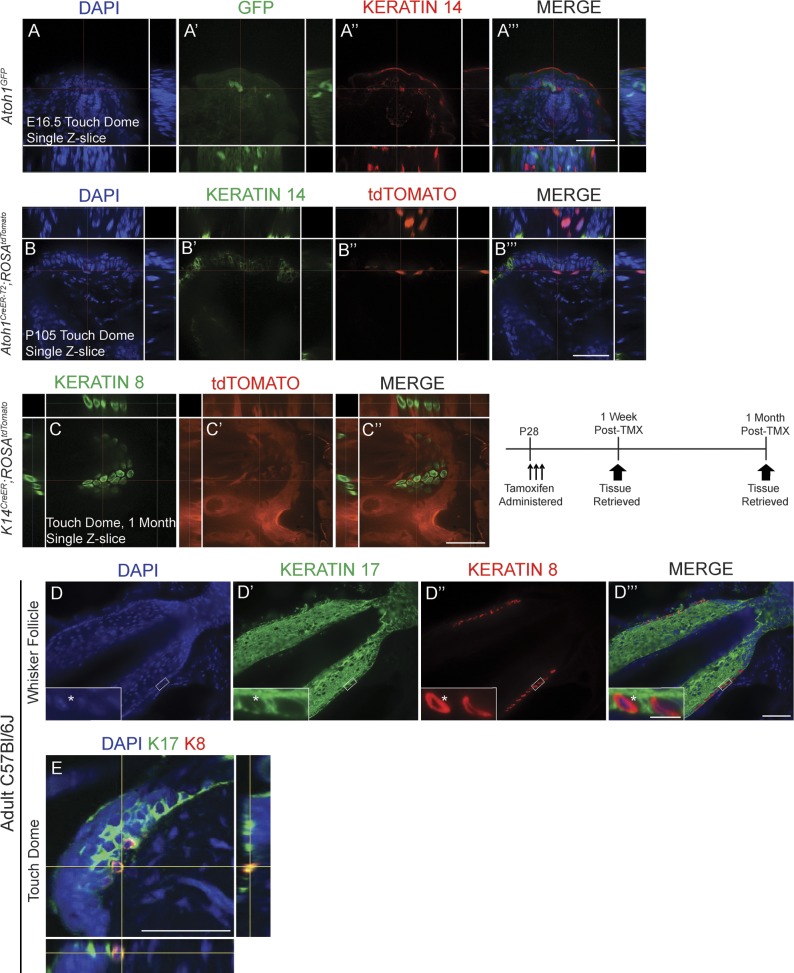
Several lines of evidence suggest that mature Merkel cells have a finite lifespan, implying that they are replaced by precursor cells located in the skin (Moll et al., 1996a; Nakafusa et al., 2006; Van Keymeulen et al., 2009; Doucet et al., 2013). To determine whether these precursors were Atoh1+, we lineage traced Atoh1+ cells in postnatal day 21–28 (P21–P28) Atoh1CreER-T2/+;ROSALacZ mice by administering high-dose tamoxifen (250 mg/kg) for a consecutive 3 d during the growth phase (anagen) of the first hair cycle. We found Xgal+ (5-bromo-4-chloro-indolyl-β-d-galactopyranoside) cells only in the expected locations for Merkel cells in the hairy skin and whisker pads 3 (n = 3) and 9 (n = 1) mo after tamoxifen administration (Fig. 1, A–B′), times after the completion of multiple hair cycles (Alonso and Fuchs, 2006). To confirm that these β-galactosidase (β-Gal)+ cells were Merkel cells, we coimmunostained for β-Gal and the Merkel cell marker Keratin 8 (K8; Fig. 1, C–D‴; Vielkind et al., 1995). 3 mo after tamoxifen administration, 93.5 ± 1.7% and 99.2 ± 0.4% of K8+ cells in hairy skin and whisker follicles coexpressed β-Gal, respectively; these percentages were 91.5% and 98.1% at 9 mo (≥200 hairy skin and ≥500 whisker follicle K8+ cells counted/mouse; Fig. 1 E). All β-Gal+ cells were also K8+, and nearly all K8+ cells (99.0 ± 0.4%, ≥150 K8+ cells/mouse, n = 3 mice) were also Keratin 20+ (K20; Fig. S1, A–A″″), in agreement with other studies (Eispert et al., 2009; Lesko et al., 2013). These data suggest that adult Merkel cells arise from Atoh1+ progenitors.
Figure 1.
Adult Merkel cell precursors express Atoh1 and are unipotent. In this and all figures, dosing and harvest paradigms are shown above the pertinent panels. (A–B′) Xgal staining of hairy skin (A and B) and whisker follicles (A′ and B′) shows the presence of labeled cells 3 (A and A′; n = 3 mice) and 9 (B and B′; n = 1 mouse) mo after tamoxifen. Insets in A and B are individual touch domes. (A′ and B′) Counterstain is Nuclear Fast red. (C–D‴) Touch domes (C–C‴) and whisker follicles (D–D‴) immunostained for K8 and β-Gal. (E) Percentages of K8+ cells that coexpress β-Gal at 3 (n = 3) and 9 (n = 1) mo after tamoxifen (TMX). Error bars show SEM. (F–G″) Hairy skin from a tamoxifen-treated P28 Atoh1CreER-T2/+;ROSAtdTomato mouse immunostained for K8 (n = 3 mice). tdTomato+ cell (arrows) that appears to be K8− at exposure times that identify other K8+ cells (F–F″) in fact expresses low levels of K8 (G–G″). Bars: (A and B, main images) 1 mm; (A and B, insets) 100 µm; (A′ and B′) 100 µm; (C–G″) 50 µm.
Previous studies concluded that K8+ cells are postmitotic (Vaigot et al., 1987; Mérot and Saurat, 1988; Moll et al., 1996b; Woo et al., 2010). Therefore, we were surprised that we never found β-Gal+/K8− cells in Atoh1CreER-T2/+;ROSALacZ mice. To determine whether this might be an issue with the β-Gal reporter, we examined K8 expression in the Atoh1 lineage by administering high-dose tamoxifen to P21 Atoh1CreER-T2/+;ROSAtdTomato mice and harvesting tissue 1 wk later. We found that all tdTomato+ cells were also K8+ but that 1.15 ± 0.5% of tdTomato+ cells expressed very low levels of K8 (>150 tdTomato+ hairy skin cells/mouse, n = 3 mice; Fig. 1, F–G″). This suggested that K8+ cells could proliferate (see next section).
Embryonic Merkel cell precursors express Atoh1 and are unipotent
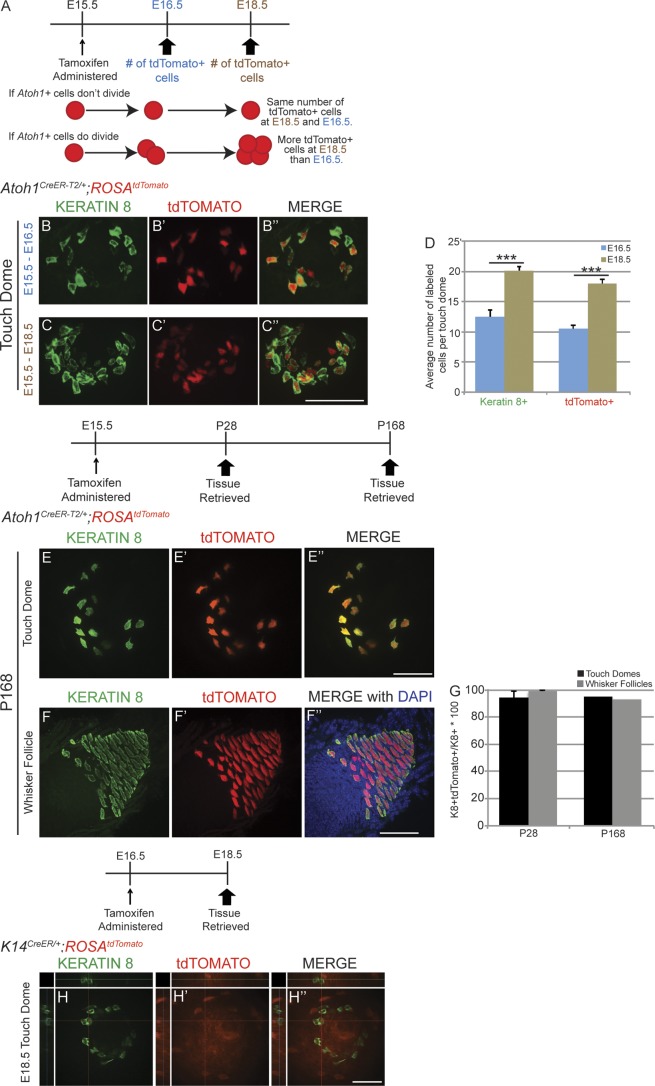
Atoh1+ cells are first observed in trunk skin and whisker follicles at embryonic day 14.5 (E14.5) and increase in number throughout late embryogenesis (Ben-Arie et al., 2000). We hypothesized that these early appearing Atoh1+ cells were progenitors responsible for Merkel cell generation. To test this possibility, we lineage traced Atoh1+ cells in Atoh1CreER-T2/+;ROSAtdTomato embryos. We limited recombination to the day of tamoxifen administration by administering a single low dose (10 mg/kg) to pregnant dams at E15.5 and then harvested tissue 1 (E16.5) or 3 (E18.5) d later. We found ∼71% more tdTomato+ cells/touch dome at E18.5 than at E16.5 (18.0 ± 1.2 vs. 10.5 ± 0.6; n = 20–30 touch domes/embryo from 3–6 embryos/age; P = 4 × 10−4, t test; Fig. 2, B–D), suggesting that Atoh1+ cells proliferated between these ages (Fig. 2 A). As expected, immunostaining for K8 demonstrated that the mean number of Merkel cells per touch dome also increased between E16.5 and E18.5 (13.8 ± 0.7 and 21.3 ± 0.8, respectively; P = 5.9 × 10−6, t test). The proportion of K8+ cells coexpressing K20 also increased between E16.5 and E18.5 (22.9 ± 0.7% and 46.8 ± 2.6%, respectively; n = 3 mice/age; P = 9.3 × 10−4, t test; Fig. S1, B–C‴). These data indicate that at least some Atoh1+ cells present at E15.5 are mitotically active and continue to divide after E16.5.
Figure 2.
Embryonic Merkel cell precursors express Atoh1, are unipotent, and give rise to the adult Atoh1+ population. (A) Experimental paradigm, outcomes, and interpretations. (B–C″) Whole-mount immunostaining for K8 (B and C), endogenous tdTomato signal (B′ and C′), and merged images (B″ and C″) in E16.5 (B–B″) and E18.5 (C–C″) Atoh1CreER-T2/+;ROSAtdTomato embryonic body skin. (D) K8+ and tdTomato+ cell numbers increase from E16.5 to E18.5 (n = 3–6 mice, t test). Error bars show SEM. (E–F″) Body skin (E–E″) and whisker follicle (F–F″) from a 24-wk-old (P168) Atoh1CreER-T2/+;ROSAtdTomato mouse that received high-dose tamoxifen at E15.5. (G) The vast majority of K8+ cells in touch domes and whisker follicles were tdTomato+ at P28 (n = 2) and P168 (n = 1) after tamoxifen administration at E15.5. Error bars show SEM. (H–H″) Single confocal z slice of touch dome whole-mount preparation from an E18.5 K14CreER;ROSAtdTomato mouse given tamoxifen at E16.5 and immunostained for K8 (H) shows that the two signals are not colocalized. ***, P < 0.001. Bars, 50 µm.
The Atoh1+ lineage separates from other skin lineages in late embryogenesis
Embryonic Atoh1+ cells are derived from the Keratin 14 (K14) lineage (Morrison et al., 2009; Van Keymeulen et al., 2009). Given our data suggesting that the Atoh1+ population expanded between E16.5 and E18.5, we wondered when Atoh1− skin precursor cells stopped producing Atoh1+ Merkel cell precursors. We administered high-dose tamoxifen to E15.5 Atoh1CreER-T2/+;ROSAtdTomato mice and harvested tissue at P28 (n = 2) and P168 (n = 1). If Atoh1− cells contributed to the Merkel cell lineage after E15.5, we expected to find a large proportion of K8+/tdTomato− cells. However, we found that the vast majority of K8+ cells were tdTomato+ at P28 and P168 (94.4 ± 0.04% and 95% K8+/tdTomato+ cells in touch domes; 98.9 ± 0.5% and 93.0% K8+/tdTomato+ cells in whisker follicles, respectively; >250 hairy skin and >500 whisker follicle K8+ cells counted/mouse; Fig. 2, E–G). Conversely, no K8+/tdTomato+ cells were found in E18.5 K14CreER/+;ROSAtdTomato embryos that received tamoxifen at E16.5 or E17.5 (>250 hairy skin and >500 whisker follicle K8+ cells counted/mouse, n = 2 mice/age; Fig. 2, H–H″). Tamoxifen administration at E14.5, when Atoh1+ cells first arise from the K14 lineage, did yield a subset of K8+/tdTomato+ cells at E18.5 (Fig. S2). These data suggest that the full complement of Atoh1+ Merkel cell progenitors are created in a 2–3-d period beginning with the appearance of the first Atoh1+ cells in the skin at E14.5.
A subset of Atoh1+ cells in hairy skin express mitotic markers
To confirm that a population of Atoh1+ cells was mitotically active, we examined several mitotic markers in Atoh1CreER-T2/+;ROSAtdTomato and Atoh1GFP mice (Lumpkin et al., 2003). We verified that the Atoh1GFP and Atoh1CreER-T2/+;ROSAtdTomato alleles labeled the same cells by generating Atoh1CreER-T2/+;Atoh1GFP/+;ROSAtdTomato/+ mice, administering high-dose tamoxifen by oral gavage at P21, and analyzing skin at P28. We found that 100% of GFP+ cells were tdTomato+ and that 98.6 ± 0.87% of tdTomato+ cells were GFP+ (>150 hairy skin K8+ cells/mouse, n = 3 mice; Fig. S3). Thus, these alleles are effectively interchangeable.
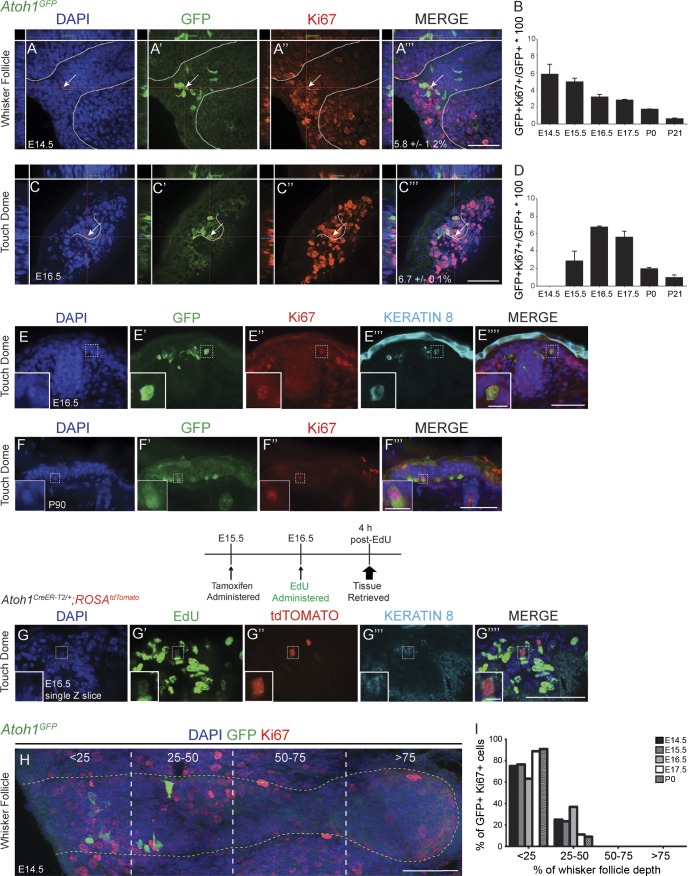
We immunostained hairy skin and whisker follicles from E14.5, E15.5, E16.5, E17.5, P0, and P21 Atoh1GFP mice for Ki67+, a marker of dividing cells (Fig. 3, A–D; Scholzen and Gerdes, 2000). GFP+/Ki67+ cells were present at all ages. The percentage of GFP+ cells that were also Ki67+ peaked at E14.5 in whisker follicles and at E16.5 in hairy skin and then decreased as the animals aged, reaching ∼1% at P21 (>500 cells/region/mouse, n = 2 mice/age; Fig. 3, B and D). GFP+/Ki67+ cells also expressed low levels of K8 (Fig. 3, E–E″″), consistent with our finding in Atoh1CreER-T2/+;ROSAtdTomato mice that all tdTomato+ cells were also K8+. A subset of GFP+ cells also expressed the M-phase marker phosphohistone H3 (PH3), suggesting that they were actively dividing (Fig. S4, A–A″″). To confirm this finding, we administered tamoxifen to Atoh1CreER-T2/+;ROSAtdTomato embryos at E15.5 (250 mg/kg) to label all Atoh1 lineage cells with tdTomato, then administered the nucleoside analogue 5-ethynyl-2’-deoxyuridine (EdU; 50 mg/kg) at E16.5 and harvested skin 4 h later to identify actively dividing cells. We found that 0.9 ± 0.1% of tdTomato+/K8+ cells in the whisker follicles and 1.1 ± 1.1% in the hairy skin incorporated EdU (>200 tdTomato+ cells/region/mouse, ∼1,500 tdTomato+ cells total; n = 2 mice; Fig. 3, G–G‴). These data indicate that embryonic Atoh1+ cells are mitotically active.
Figure 3.
A subset of Atoh1+ cells in hairy skin express cell proliferation markers. (A–C″) Confocal images of an E14.5 whisker follicle (A–A‴) and E16.5 touch dome (C–C‴) from Atoh1GFP mice immunostained for GFP (A′ and C′) and Ki67 (A″ and C″), counterstained with DAPI (A and C). Whisker (A–A‴) and guard hair (C–C‴) follicles are outlined with dashed lines. Crosshairs are over double-labeled cells, which are also indicated by arrows. Percentages ± SEM of GFP+/Ki67+ cells are shown in A‴ and C‴. (B and D) The percentages of GFP+/Ki67+ cells within the GFP+ population changed from E14.5 to P21. Error bars show SEM. (n = 2 mice/age.) (E–E″″) E16.5 Atoh1GFP touch dome immunostained for GFP, Ki67, and K8. Insets show a GFP+/Ki67+/K8+ cell. (F–F‴) P90 Atoh1GFP touch dome immunostained for GFP (F′) and Ki67 (F″). Insets show a GFP+/Ki67+ cell. (G–G″″) Single confocal z slice of touch dome from E16.5 Atoh1CreER-T2/+;ROSAtdTomato mouse given tamoxifen at E15.5, EdU at 16.5, and tissue retrieved 4 h after EdU administration. EdU (G′), tdTomato (G″), K8 (G‴), and merge (G″″) are shown. Insets show a K8+/tdTomato+/EdU+ cell. (H) Cross section of E14.5 Atoh1GFP whisker follicle illustrating how follicles were divided into quadrants. The dotted yellow line outlines a single whisker follicle. (I) GFP+/Ki67+ cells are clustered at the top of whisker follicles (n = 2 mice/age). Bars: (all main images) 50 µm; (E–F‴, insets) 10 µm; (G–G″″, insets) 5 µm.
We next examined 3 (n = 4)- and 6 (n = 2)-mo-old adult Atoh1GFP mice to determine whether GFP+/Ki67+ cells were present and whether the numbers of these cells changed during the natural hair cycle. Three of these mice were in the growth phase of the hair cycle (anagen), and three were in the resting phase (telogen). GFP+ cells in the body skin (250–500/mouse, ∼1,700 GFP+ cells total) and whisker follicles (1,500–5,000/mouse, ∼11,000 GFP+ cells total) were analyzed. We found one GFP+/Ki67+ cell in the body skin of a 3-mo-old mouse whose skin was in telogen (Fig. 3, F–F‴) and one GFP+/Ki67+ cell in the whisker follicle of a 6-mo-old mouse (Fig. S4, B–B‴). No K8+/EdU+ cells were found in the whisker follicles or back skin of P19–P24 mice (n = 3; >400 K8+ cells/mouse/region) after administration of EdU (50 mg/kg) and tissue harvest 4 h later. Collectively with our fate mapping data suggesting that a subpopulation of Atoh1+ cells continues to proliferate throughout the lifetime of the mouse, the low numbers of GFP+/Ki67+ cells and absence of K8+/EdU+ cells suggest that this proliferation occurs very slowly.
To determine where proliferative Atoh1+ cells were located, we divided whisker follicles into four equal segments and counted the number of GFP+/Ki67+ cells in each quadrant (Fig. 3 H). The vast majority of GFP+/Ki67+ cells were found in the most superficial 25% of the whisker follicle and never in the bottom 50% (Fig. 3 I). Similarly, GFP+/Ki67+ cells in guard hair follicles of the body skin were found mostly in the infundibulum (Fig. 3, C′ and C″). These data suggest that proliferative Atoh1+ cells are located in specific hair follicle regions.
Multiple Merkel cell progenitors are present in each touch dome and whisker follicle
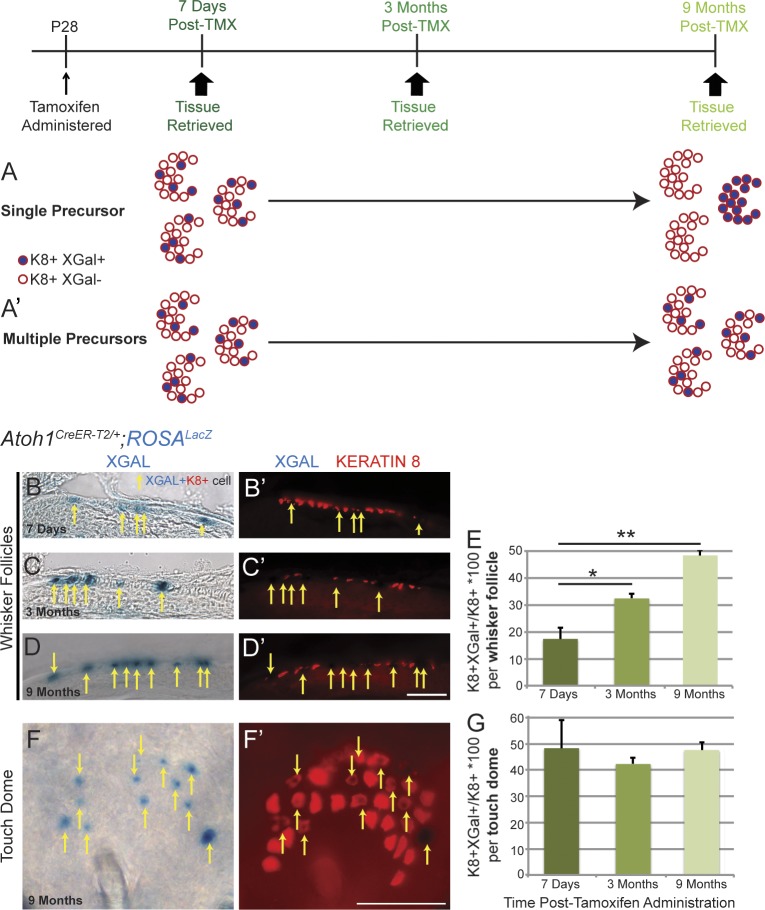
We wondered whether each Merkel cell niche (touch dome or whisker follicle) had a single designated progenitor or multiple progenitors. To investigate this, we randomly recombined only a fraction of the Atoh1+ population by administering a single very low dose of tamoxifen (0.1 mg/mouse, the lowest tested dose to activate recombination) to P28 Atoh1CreER-T2/+;ROSALacZ mice. We then harvested tissue 7 d, 3 mo, or 9 mo later and counted the number of K8+ cells colabeled with Xgal. We reasoned that if only one progenitor was present per niche (Fig. 4 A), over time, all cells in an individual touch dome or whisker follicle would be K8+/Xgal+ if recombination occurred in the progenitor or K8+/Xgal− if recombination did not occur. Conversely, if multiple progenitors were present in each niche there would continue to be a heterogeneous population of K8+/Xgal+ and K8+/Xgal− cells (Fig. 4 A′). We found that K8+/Xgal+ cells were randomly distributed in whisker follicles and touch domes at all time points (Fig. 4, B–D′, F, and F′). The percentage of K8+/Xgal+ cells in whisker follicles increased over time (17.4 ± 5.0% at 7 d, 32.4 ± 2.1% at 3 mo, and 48.3 ± 2.6% at 9 mo; >500 K8+ cells/mouse from n = 2–3 mice/time point; F(2,5) = 23.4, P < 0.01, one-way analysis of variance [ANOVA]) but remained constant in touch domes (48.3 ± 13.3% at 7 d, 42.2 ± 2.8% at 3 mo, and 47.6 ± 4.3% at 9 mo; >250 K8+ cells/mouse from n = 2–3 mice/time point; F(2,5) = 0.21, P = 0.82, one-way ANOVA; Fig. 4, E and G). These results suggest that multiple progenitors reside in each Merkel cell niche but that homeostasis might be achieved differently in different body regions.
Figure 4.
Multiple Merkel cell progenitors exist in adult touch domes and whisker follicles. (A and A′) Experimental paradigm, potential outcomes, and interpretations. (B–F′) Whisker follicles (B–D′) and body skin (F and F′) from Atoh1CreER-T2/+;ROSALacZ mice stained with Xgal and immunostained for K8 at 7 d (B and B′), 3 mo (C and C′), and 9 mo (D–F′) after tamoxifen (TMX) administration. Arrows show double-labeled cells. (E) The percentage of K8+/Xgal+ cells increased over time in the whiskers but not in the touch domes (n = 2–3 mice/time point, one-way ANOVA). Error bars show SEM. *, P < 0.05; **, P < 0.01. Bars, 100 µm.
Atoh1+ cells do not express K14, but a subset of Atoh1+ cells does express K17
Though embryonically derived from the K14 lineage, mature Merkel cells do not express K14 (Moll et al., 1993). To determine whether Atoh1+ Merkel cell progenitors retained K14 expression, we performed K14 immunostaining in hairy skin and whisker follicles from E16.5 Atoh1GFP and tamoxifen-treated adult Atoh1CreER-T2/+;ROSAtdTomato mice. All Atoh1+ cells were K14 negative (>250 GFP+ or tdTomato+ hairy skin and whisker follicle cells/mouse, n = 2 mice/genotype; Fig. 5, A–B‴). We postulated that K14 could be expressed in the Atoh1 lineage, but at low protein levels undetectable by immunostaining. To examine this possibility, we conditionally fate mapped the K14 lineage in adulthood by administering high-dose tamoxifen for a consecutive 3 d to P28 K14CreER/+;ROSAtdTomato mice and then harvesting tissue 1 and 4 wk later. Immunostaining for K8 revealed that all K8+ cells were tdTomato negative (>250 hairy skin and >500 whisker follicle K8+ cells counted/mouse, n = 2 mice/age; Fig. 5, C–C″). Collectively with our data from embryonic K14CreER/+;ROSAtdTomato mice (Fig. 2, H–H″), these data indicate that Atoh1+ cells do not express K14 at embryonic or postnatal ages.
Figure 5.
The Merkel cell lineage is K14−, but a subset of cells is K17+. (A–A‴) Single confocal z-slice image of a touch dome immunostained for GFP and K14 in the body skin of an E16.5 Atoh1GFP mouse. (B–B‴) All tdTomato+ cells in adult Atoh1CreER-T2/+;ROSAtdTomato mouse touch domes are K14−. (C–C″) Single confocal z-slice image of whole-mount touch dome preparation from a P60 K14CreER;ROSAtdTomato mouse given tamoxifen (TMX) at P28 and immunostained for K8 (C). There is no signal colocalization. (D) Whisker follicles from an adult C57BL/6J mouse immunostained for K17 (D′) and K8 (D″). Insets show one K8+K17+ cell (as indicated by asterisks) and one K8+K17− cell. (E) Single confocal z-slice image of touch dome from back skin of an adult C57BL/6J mouse immunostained for K8 and K17 showing signal colocalization. Bars: (main images) 50 µm; (D–D‴, insets) 10 µm.
A recent study suggested that bipotential K17+ progenitor cells located in touch domes give rise to touch dome keratinocytes and Merkel cells (Doucet et al., 2013). To determine whether K17 was expressed by cells already committed to the Merkel cell lineage, we coimmunostained adult C57BL/6J mouse hairy skin for K8 and K17. We found that 28.4 ± 6.0% and 9.2 ± 2.6% of K8+ cells in touch domes and whisker follicles, respectively (>250 hairy skin and >500 whisker follicle K8+ cells counted/mouse, n = 2–3 mice), were also K17+ (Fig. 5, D–E). Because all K8+ cells are also Atoh1+ (Fig. 1, F–G″), this demonstrates that some Atoh1+ cells coexpress K17.
Atoh1+ progenitors are the only source of Merkel cells in adult mice
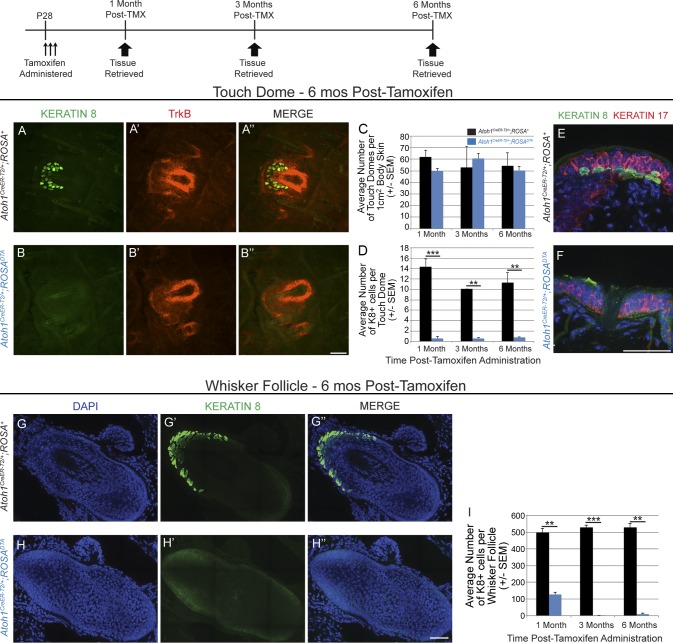
To determine whether other skin progenitor populations could produce Merkel cells in the absence of Atoh1+ progenitors, we genetically ablated Atoh1+ cells by administering high-dose tamoxifen for a consecutive 3 d to P28 Atoh1CreER-T2/+;ROSADTA mice and Atoh1CreER-T2/+;ROSA+ littermate controls. We used only Atoh1CreER-T2/+;ROSA+ mice as controls because they have slightly fewer K8+ cells per touch dome than their Atoh1+/+ siblings (18.1 ± 1.0 and 25.3 ± 1.8, respectively; >600 K8+ cells/mouse; n = 5 mice/genotype; P = 0.007, t test). Back and belly skin (1 cm2) and whisker pads were harvested 1, 3, and 6 mo after tamoxifen administration (n = 2 mice/genotype/time point). Touch domes in the hairy skin were identified by immunostaining for TrkB, which we serendipitously found to be a reliable marker of touch dome keratinocytes (Fig. 6, A–B″). Skin from Atoh1CreER-T2/+;ROSADTA mice and Atoh1CreER-T2/+;ROSA+ littermate controls had similar densities of touch domes (56.5 ± 2.8 vs. 53.7 ± 3.4 touch domes per 1 cm2 of hairy skin, respectively; F(2,6)=0.55, P = 0.6, two-way ANOVA; Fig. 6 C). However, 64%, 65%, and 60% of touch domes in Atoh1CreER-T2/+;ROSADTA mice 1, 3, and 6 mo after tamoxifen administration had no Merkel cells; this was verified on tissue sections double labeled with K8 and K17 (Fig. 6, E and F). Mean numbers of K8+ cells per touch dome were significantly decreased (F(1,6) = 183.7, P < 0.0001, two-way ANOVA) 1 (0.6 ± 0.4 vs. 14.4 ± 1.5 K8+ cells/touch dome, respectively, P = 6.0 × 10−4, Tukey’s post-hoc multiple comparisons test), 3 (0.6 ± 0.2 vs.10.1 ± 0.01, P = 4.5 × 10−3), and 6 (0.8 ± 0.1 vs. 11.3 ± 2.0, P = 2.6 × 10−3) mo after tamoxifen administration in Atoh1CreER-T2/+;ROSADTA mice compared with Atoh1CreER-T2/+;ROSA+ littermate controls (Fig. 6 D). The same was true in whisker follicles, in which Atoh1CreER-T2/+;ROSADTA mice had fewer K8+ cells/follicle than Atoh1CreER-T2/+;ROSA+ littermate controls (≥6 follicles/mouse; F(2,6) = 15.45, P = 4.0 × 10−3, two-way ANOVA) 1 (127.4 ± 14.6 vs. 499 ± 24.3; P = 5.8 × 10−3, Tukey’s post-hoc multiple comparisons test), 3 (1.6 ± 1.0 vs. 530 ± 12.9; P = 6.0 × 10−4), and 6 (8.37 ± 7.6 vs. 529.8 ± 22.2; P = 2.0 × 10−3) mo later (Fig. 6, G–I). Thus, deletion of Atoh1+ cells led to a persistent deficit in Merkel cells numbers, suggesting that the loss of Atoh1+ progenitors precludes the production of new Merkel cells in touch domes and whisker follicles of Atoh1CreER-T2/+;ROSADTA mice.
Figure 6.
Merkel cell generation in adult mice requires Atoh1+ progenitors. (A–B″) Whole-mount skin preparations immunostained for K8 (A and B) and TrkB (A′ and B′) show presence of touch domes 6 mo after tamoxifen (TMX) treatment in Atoh1CreER-T2/+;ROSA+ and Atoh1CreER-T2/+;ROSADTA mice. (C) Mean numbers of touch domes in Atoh1CreER-T2/+;ROSA+ and Atoh1CreER-T2;ROSADTA mice per 1 cm2 of body skin (n = 2 mice/genotype/time point, two-way ANOVA). (D) Mean numbers of K8+ cells per touch dome were markedly decreased in Atoh1CreER-T2/+;ROSADTA mice relative to Atoh1CreER-T2/+;ROSA+ littermate controls at all time points (n = 2 mice/genotype/time point, two-way ANOVA). (E and F) Cryosectioned hairy skin immunostained for K8 and K17 reveals touch domes that lack Merkel cells in Atoh1CreER-T2/+;ROSADTA mice. (G–H‴) Stitched images of cryosectioned whisker follicles immunostained for K8. (I) Mean numbers of K8+ cells per whisker follicle were decreased in Atoh1CreER-T2/+;ROSADTA mice relative to Atoh1CreER-T2/+;ROSA+ littermate controls at all time points (n = 2 mice/genotype/time point, two-way ANOVA). **, P < 0.01; ***, P < 0.001. Error bars show SEM. Bars, 50 µm.
Discussion
We have identified a novel, Atoh1+ progenitor population located in the infundibulum of guard hairs and whisker follicles that arises at embryonic ages, is maintained through adulthood, and produces only Merkel cells. The percentage of Atoh1+ cells that express mitotic markers is highest during embryonic development when Merkel cells are first produced and then steadily decreases with age, falling to scarcely detectable levels in adulthood. The low percentages of Atoh1+/Ki67+ cells in touch domes (0.06%) and whisker follicles (0.009%) and absence of K8+/EdU+ cells in adult mice are most likely secondary to a slow rate of precursor division, shortened cell cycle/S-phase duration, and/or a shortened period of Ki67 expression after reentry from G0 (Gerdes et al., 1984). Atoh1+ progenitors must continue to produce new Merkel cells in the whisker follicles of adult animals given our fate-mapping data in Atoh1CreER-T2/+;ROSALacZ mice given very low-dose tamoxifen (Fig. 4 E). Data from these experiments favor the interpretation that Merkel cell precursor division rates decrease as mice age, because the number of labeled cells doubled (17–34%) between 7 d and 3 mo after tamoxifen administration but then increased only ∼40% from 3 to 9 mo after tamoxifen (34–48%). These data suggest that mature Merkel cells in adult animals might have much longer lifespans than previously thought and that they are only rarely replaced. Our findings are consistent with previous studies that used morphology, marker expression, and incorporation of labeled nucleotide analogues to identify rare dividing Merkel cells in developing and adult animals (Mérot et al., 1987; Vaigot et al., 1987; Mérot and Saurat, 1988; Woo et al., 2010). One limitation of our study is that the use of the Atoh1GFP and Atoh1CreER-T2 alleles prevented us from separating progenitors and mature Merkel cells because both populations express Atoh1.
Previous work suggested that Merkel cell number is highest during anagen and lower during other stages of the hair cycle (Moll et al., 1996a; Nakafusa et al., 2006). We were unable to establish a connection between hair cycle stage and Atoh1+ cell proliferation, primarily because of the low number of GFP+/Ki67+ cells (one) that we found in adult hairy skin. It is notable, however, that this lone cell was found in telogen stage skin. This finding, coupled with our data from adolescent mice showing that ∼1% of GFP+ cells are Ki67+ at P21 during the first telogen (Fig. 3 D), suggests that Merkel cell precursors, though rarely mitotically active, can divide during the resting stage of the hair cycle.
Our data demonstrate that unipotent Atoh1+ Merkel cell progenitors are the only source of adult Merkel cells, because no new Merkel cells are formed after ablation of Atoh1+ cells in Atoh1CreER-T2/+;ROSADTA mice. This finding contrasts with a study that touch dome keratinocytes and Merkel cells share a common K17+ progenitor (Doucet et al., 2013). We found that ∼28% of K8+ touch dome cells coexpress K17, which coincides well with that study’s finding that ∼11% of K8+ Merkel cells were YFP+ just 24 h after tamoxifen administration to K17CreER-T2;ROSAYFP mice. Because we show that all K8+ cells are Atoh1+, and that the Atoh1+ lineage gives rise only to Merkel cells, the most parsimonious explanation is that touch domes contain two separate populations of K17+ precursors: one is Atoh1+/K8+/K17+ and gives rise only to Merkel cells, whereas the other is Atoh1−/K8−/K17+ and gives rise only to keratinocytes. Interestingly, we found that K8+/K17+ cells are not limited to touch domes, as ∼9% of K8+ cells in whisker follicles are also K17+. Therefore, we hypothesize that either all or a subset of the Atoh1+/K8+/K17+ cells are the Merkel cell progenitors. Further studies are needed to determine whether K17 is expressed only by Merkel cell progenitors or also by mature Merkel cells.
In contrast, we were unable to find evidence supporting the assertion that adult Merkel cell precursors express K14 (Van Keymeulen et al., 2009), as we never observed GFP+/K14+ cells in Atoh1GFP mice, K14+/tdTomato+ cells in Atoh1CreER-T2/+;ROSAtdTomato mice, or K8+/tdTomato+ cells in K14CreER/+;ROSAtdTomato mice at adult ages. As predicted, we did find K8+/tdTomato+ cells in K14CreER;ROSAtdTomato mice treated with tamoxifen at E14.5 when Atoh1+ cells first arise from the K14 lineage (Vielkind et al., 1995; Morrison et al., 2009), suggesting that our findings were not secondary to a technical issue of some sort. Notably, a microarray experiment conducted on early postnatal skin also failed to detect K14 expression in purified Merkel cells (Haeberle et al., 2004). One difference between our study and the previous study is that our analyses were restricted to hairy body skin and whiskers, whereas Van Keymuelen et al. (2009) analyzed the glabrous skin of the feet. So, it is possible that Atoh1+ precursors in different body regions express different markers. Regardless, if other Atoh1− precursor or stem cell populations (such as the K15+ bulge stem cell population also proposed by Van Keymuelen et al., 2009) were responsible for maintaining the adult Merkel cell population in whisker follicles and body skin, we would expect to find production of new Merkel cells after tamoxifen administration to Atoh1CreER-T2/+;ROSADTA mice. The absence of this compensation even with survival times of 6 mo after tamoxifen suggests that other skin precursor and stem cells lineages are either incapable of generating Merkel cells or can do so only under special conditions. Many skin stem cells have restricted lineages in adult animals under normal conditions but can give rise to additional lineages after wounding (Ghazizadeh and Taichman, 2001; Ito et al., 2005; Levy et al., 2005; Jaks et al., 2010). Whether skin stem cells can produce Merkel cells or their precursors after wounding is unknown, as is the ability of Merkel cell progenitors to give rise to other skin lineages; we are currently testing both of these possibilities.
Two lines of evidence illuminate potential differences in precursor allocation and/or Atoh1 expression levels in precursors and mature Merkel cells in touch domes versus whisker follicles. Very low-dose tamoxifen administration to adult Atoh1CreER-T2/+;ROSALacZ mice led to increasing percentages of K8+/Xgal+ cells in the whiskers at 1 wk (17%), 3 mo (34%), and 9 mo (48%) survival times (Fig. 4 E). Conversely, more K8+ cells were present in the whiskers of Atoh1CreER-T2/+;ROSADTA mice 28 d (127/follicle) than 3 mo (1.6/follicle) after treatment (Fig. 6 I). These data suggest that recombination occurred preferentially in Atoh1+ Merkel cell progenitors and that this population expanded in the Atoh1CreER-T2/+;ROSALacZ mice and was deleted in the Atoh1CreER-T2/+;ROSADTA mice. In contrast, ∼50% of touch dome cells were K8+/Xgal+ 1 wk, 3 mo, and 9 mo after treatment in Atoh1CreER-T2/+;ROSALacZ mice (Fig. 4 G), and very few K8+ cells remained in the touch domes of Atoh1CreER-T2/+;ROSADTA mice 1 mo after treatment (Fig. 6 D). This suggests that progenitors and mature Merkel cells in the touch domes underwent similar levels of recombination in both cases. One explanation for these results is that Atoh1+ Merkel cell progenitors in the whiskers express higher levels of Atoh1 than mature Merkel cells, making them more likely to undergo recombination at limiting doses of tamoxifen, whereas progenitors and mature Merkel cells in the touch domes express similar levels of Atoh1. Another possibility is that the percentage of Atoh1+ progenitor cells is higher in touch domes than in whisker follicles. Interestingly, the percentage of K8+/K17+ cells in touch domes (28%) is higher than that found in whiskers (9%), which would support our hypothesis that the Atoh1+/K8+/K17+ population is the progenitor population. A third explanation is that designated progenitors are present in the whisker follicles but that any Atoh1+ cell in the touch dome is capable of division. This explanation seems unlikely given that the number of YFP+ Merkel cells decreases over time after tamoxifen administration to K18CreER-T2;ROSAYFP mice, suggesting that K18+ cells (i.e., mature Merkel cells), all of which also express Atoh1, are incapable of division (Van Keymeulen et al., 2009). Ultimately, isolation of the precursor and mature Merkel cell populations followed by marker and gene expression analysis will allow us to distinguish between these possibilities.
The epidermal placodes that give rise to whisker follicles in the snout and tylotrich (guard hair) follicles in the hairy skin develop at E12.5 and E14.5, respectively, whereas Atoh1 expression is first seen in these regions at E14.5 (Vielkind et al., 1995; Ben-Arie et al., 2000; Morrison et al., 2009; Richardson et al., 2009). Our present results demonstrate that these early born Atoh1+ cells are mitotically active and that they give rise only to Merkel cells, suggesting that they form a self-renewing population of progenitors that is maintained through adulthood. The first appearance of these cells at E14.5–E15.5 in hairy skin makes the Merkel cell lineage one of the first committed lineages within the hair follicle, with specification taking place at the same time or before that of multipotent stem cells that inhabit the bulge region (Vidal et al., 2005; Nowak et al., 2008). Merkel cell progenitor commitment also occurs several days before specification of other unipotent progenitors such as those of the sebaceous gland lineage (Horsley et al., 2006). Thus, from the earliest times, the Atoh1+ lineage is a separate skin lineage.
Our study does not identify the factors that control the initial specification of Atoh1+ cells from the K14 lineage. Recent work suggests that the transcription factor Sox2 is a direct, positive regulator of Atoh1, whereas the Polycomb repressive complex negatively regulates Merkel cell specification through repression of Sox2 (Bardot et al., 2013; Lesko et al., 2013). Deletion of Sox2 in the developing skin reduces the number of Merkel cells in embryonic mice but does not preclude their production nor their expression of multiple canonical markers (Bardot et al., 2013; Lesko et al., 2013). Sox2 and Atoh1 are expressed concomitantly in the epidermis starting at E15.5; however, the genetic cascades and signaling molecules necessary for the initiation of Atoh1 and Sox2 expression are unknown. Future experiments are required to determine which Atoh1− cells in the developing skin ultimately give rise to the Merkel cell lineage.
Our findings have potential implications for understanding the genesis of MCC, a rare and devastating skin cancer for which there are no truly effective treatments aside from surgical excision. Although the cell type of origin of MCC tumors is unknown (Tilling and Moll, 2012), evidence that MCC arises from Merkel cells or their precursors comes from expression of Hath1, the human Atoh1 homologue, in MCC tumor lines and primary tumor cells, along with other Merkel cell markers such as K20, chromogranin A, synaptophysin, and neuron-specific enolase (Leonard et al., 2002; Heiskala et al., 2010). Assuming MCC arises from the Merkel cell lineage, it is plausible that Atoh1+ Merkel cell precursors might be the cell type of origin given their unipotency and mitotic activity. Further experiments are necessary to test this hypothesis.
Materials and methods
Mice
Atoh1GFP (JAX 013593; The Jackson Laboratory; Lumpkin et al., 2003), ROSALacZ (JAX 003474; Soriano, 1999), ROSAtdTomato (JAX 007914; Madisen et al., 2010), ROSADTA (JAX 009669; Voehringer et al., 2008), K14CreER (JAX 005107; Vasioukhin et al., 1999), and Atoh1CreER-T2 (open reading frame of the Atoh1 locus is replaced with CreER-T2; Fujiyama et al., 2009) mice were maintained in accordance with International Animal Care and Use Committee guidelines at the Case Western Reserve University and the Children’s Hospital of Pittsburgh of the University of Pittsburgh Medical Center. For embryonic ages, the plug date was designated as E0.5.
Tamoxifen and EdU administration
Tamoxifen (Sigma-Aldrich) was dissolved in a 9:1 corn oil/ethanol solution at a 1% or 5% concentration. Mice were briefly anesthetized with isoflurane, and tamoxifen was administered by oral gavage. For lineage tracing, tamoxifen was administered as a single dose of 0.1 mg (5 mg/kg; low-dose adult), 0.4 mg (∼10 mg/kg; low-dose embryonic), or 250 mg/kg (high dose) on either a consecutive 1 or 3 d as indicated in the text. EdU (Invitrogen) was dissolved in sterile PBS at a 10-mM concentration and administered as a single 50-mg/kg dose by intraperitoneal injection to adult mice or pregnant females.
Tissue processing
Mice were euthanized by cervical dislocation, and skin was dissected into cold PBS. Embryos were dissected from pregnant dams and decapitated before tissue dissection. Skin processed for immunohistochemistry was fixed in 4% PFA for 30 min (adult tissue or dissected embryonic skin) or overnight (whole embryos), washed in PBS, and cryopreserved in 30% sucrose/PBS. Skin for Xgal staining was fixed in cold 4% PFA for 15 min, washed in cold PBS, and stained in Xgal overnight at 37°C. Embryonic skin and whisker follicles were dissected before incubation in Xgal. Tissue was washed and postfixed for 2 h in 4% PFA before imaging. Tissue was also prepared for cryosectioning and counterstained with Nuclear Fast red (Sigma-Aldrich). Hair cycle stage was determined on hematoxylin and eosin–stained sectioned skin by analyzing hair follicle dimensions, depth into the subcutaneous tissue, and the shape and size of the dermal papilla and hair bulb as previously described (Müller-Röver et al., 2001).
Histology
Tissue was embedded in optimum cutting temperature (O.C.T.; Thermo Fisher Scientific) and serially sectioned on a cryostat (1950M; Leica) at 25 µm. Slides were vacuum dried, rehydrated in PBS, and blocked with 5% normal donkey serum or 3% nonfat dry milk in 0.3% PBS-T (PBS with Triton X-100). EdU was detected with an imaging kit (Click-iT EdU; Invitrogen). Slides were incubated overnight in blocking solution containing dilutions of the following primary antibodies: chicken anti-GFP (1:1,000; GFP-1010; Aves Labs), goat anti-TrkB (1:200; AF1494; R&D Systems), mouse anti–cytokeratin 20 (1:100; 182200; Life Technologies), rabbit anti-Ki67 (1:500; RM-9106-S1; Thermo Fisher Scientific), rat anti-K8 (1:20; TROMA-1; Developmental Studies Hybridoma Bank), chicken anti–β-Gal (1:1,000; BGL-1010; Aves Labs), rabbit anti-K14 (1:1,000; PRB-155P; Covance), rabbit anti–cytokeratin 17 (1:1,000; ab53707; Abcam), and rabbit anti-phospho–histone H3 (1:500; 06–570; EMD Millipore). Antigen retrieval was performed before Ki67 immunostaining by either heat-induced epitope retrieval with citrate buffer or proteolytic-induced epitope retrieval with trypsin. For heat-induced epitope retrieval, rehydrated tissue sections were incubated in sub-boiling 10 mM citrate buffer solution for 7 min followed by 10 min at room temperature. For proteolytic-induced epitope retrieval, rehydrated tissue sections were incubated at 37°C for 10 min in prewarmed 0.0125% trypsin in PBS. After primary antibody incubation, sections were washed and incubated for 30 min at room temperature in blocking solution containing the appropriate secondary antibodies obtained from Jackson ImmunoResearch Laboratories, Inc. (1:500): Alexa Fluor 488–conjugated donkey anti–rabbit (711-545-152), Alexa Fluor 488–conjugated donkey anti–rat (712-545-150), Alexa Fluor 488–conjugated donkey anti–chicken (703-545-155), Cy3-conjugated donkey anti–chicken (703-095-155), Cy3-conjugated donkey anti–goat (705-165-003), Cy3-conjugated donkey anti–mouse (715-165-150), Cy3-conjugated donkey anti–rabbit (711-165-152), Cy3-conjugated donkey anti–rat (712-165-150), Alexa Fluor 647–conjugated donkey anti–rabbit (711-605-152), and Alexa Fluor 647–conjugated donkey anti–rat (712-605-150). Sections were stained with the nuclear probe DAPI (1:1,000; Thermo Fisher Scientific) to visualize nuclei and mounted in ProLong gold (Invitrogen). Whole-mount immunostaining was performed by modifying previously published protocols (Li et al., 2011) on pelts of hairy skin. Fixed skin was dissected into small pieces, and the underlying adipose tissue was removed and washed for 5–8 h in 0.3% PBS-T. Tissue was incubated with primary antibodies for 4 d, washed for 5–8 h in 0.3% PBS-T, and then incubated with secondary antibodies for 2 d, all at room temperature. Antibodies were diluted in 20% dimethyl sulfoxide/5% normal donkey serum/0.3% PBS-T. Confocal images for Atoh1CreER-T2/+;ROSALacZ adult fate-mapping experiments were acquired with a laser-scanning confocal microscope (LSM 510 META; Carl Zeiss) using a 40× C-Apochromat, NA 1.2, water immersion objective. Images presented here are maximum intensity projections of a z series consisting of 1-µm optical slices collected every 0.5 µm (optimal interval setting determined by LSM 510 software, AIM 4.2). All other confocal images were acquired with an inverted microscope (Axio Observer; Carl Zeiss) on a spinning-disc confocal (UltraVIEW VoX; PerkinElmer) with a C-Apochromat 40×, 1.1 NA water immersion objective, a camera (C9100-13; Hamamatsu Photonics), and Volocity software (PerkinElmer). Images presented here are maximum intensity projections of a z series or single z slices (as noted in the figures) consisting of 1-µm optical slices collected every 0.35 µm. Images in Fig. 6 (G–H‴) are stitched from z-stacked 40× images. Multiple 3D images were acquired for the desired region of interest with 10% overlap between adjacent images. Images were compiled and stitched using Volocity software with the standard brightness correction. Nonconfocal images were acquired with a fluorescent scope (DM5500 B; Leica) using an HCX Plan Apochromat 40×, 1.25 NA and an HC Plan Apochromat 10×, 0.4 NA objective, camera (DFC420; Leica), and Leica Acquisition Software v4.2. Images were cropped, and brightness and contrast were enhanced for publication quality with Photoshop and/or Illustrator (Adobe).
Cell counts
Cell counts of Atoh1GFP mice (n = 2/age) were performed on a microscope (DM5000 B) with a dual red/green fluorescent filter using an HCX Plan Apochromat 40×, 0.75 NA objective at room temperature. All GFP+ cells from one whisker pad were counted per animal, amounting to >500, >1,700, >6,000, >1,000, and >1,500 GFP+ cells for each mouse at E14.5, E15.5, E16.5, E17.5, P0, and P21, respectively. GFP+ cells from hairy skin were counted from a total of 15 slides/mouse from three different body levels, amounting to >40, >75, >2,500, >3,500, >3,000, and >250 GFP+ cells for each mouse at E14.5, E15.5, E16.5, 17.5, P0, and P21, respectively. Greater than 1,500 and >250 GFP+ cells were counted in the whisker follicles and hairy skin, respectively, for each Atoh1GFP mouse at 3 and 6 mo of age. Together, we analyzed >95,000 GFP+ cells in these experiments. Cell counts from all other mice were performed at room temperature on a fluorescent microscope (DM5500 B) using an HCX Plan Apochromat 40×, 1.25 NA objective. In our adult Atoh1CreER-T2/+;ROSALacZ fate-mapping experiment, >200 and >500 K8+ cells in the body skin and whisker follicles were counted per mouse at 3 (n = 3) and 9 mo (n = 1) after tamoxifen administration. In our P28 Atoh1CreER-T2;ROSAtdTomato analysis, >200 tdTomato+ cells in body skin were counted per mouse (n = 3 mice). 20 touch domes from 3–6 mice/time point were counted in embryonic Atoh1CreER-T2/+;ROSAtdTomato mice, amounting to >2,500 and >2,900 tdTomato+ cells at E16.5 and E18.5, respectively. In our embryonic tamoxifen administration and adult survival experiment with Atoh1CreER-T2/+;ROSAtdTomato mice, >250 and >500 tdTomato+ cells were counted in the body skin and whisker follicles, respectively, per mouse at P28 (n = 2) and P168 (n = 1). In our embryonic EdU incorporation experiment, >200 tdTomato+ cells/mouse were counted each in the body skin and whisker follicles (n = 2 mice), amounting to ∼1,500 tdTomato+ cells. In our adult EdU incorporation experiment, >400 K8+ cells/mouse were counted in each the body skin and whisker follicles (n = 3 mice), amounting to ∼16,500 K8+ cells. 20 touch domes from two mice per age were counted in embryonic K14CreER;ROSAtdTomato mice, amounting to >1,000 K8+ cells. In our low-dose experiment with Atoh1CreER-T2/+;ROSALacZ mice, >250 and >500 K8+ cells were counted per mouse, amounting to >2,000 and >550 K8+ cells in the body skin and >16,000 and >7,000 K8+ cells in the whisker follicles at 1 wk/3 mo (n = 3 mice/time point) and 9 mo (n = 2 mice) survival times, respectively. In our analysis of K14 expression in Atoh1CreER-T2;ROSAtdTomato mice, >250 tdTomato+ cells were counted per mouse (n = 2 mice). In our K14CreER;ROSAtdTomato fate-mapping experiment, we counted >250 and >500 K8+ cells in body skin and whisker follicles, respectively, at 1 wk and 1 mo after tamoxifen administration (n = 2 mice). In our analysis of K17 expression, we counted >250 and >500 K8+ cells in body skin and whisker follicles of adult C57BL/6J mice (n = 2–3). In our genetic ablation experiment with Atoh1CreER-T2/+;ROSADTA mice, ∼50 touch domes from 1 cm2 of hairy skin (half from the back and belly) and >6 full, reconstructed whisker follicles were quantified from each mouse (n = 2 mice/age/genotype), amounting to >4,000 and >33,000 K8+ cells analyzed in body skin and whisker follicles, respectively. We quantified >150 K8+ cells/mouse in our analysis of K20 expression (n = 3 mice/age) and in our verification of the Atoh1GFP and Atoh1CreER-T2;ROSAtdTomato alleles (n = 3 mice). Throughout all of these experiments, we counted >52,000 cells in the body skin and >168,000 cells in the whisker follicles, culminating in our analysis of >221,000 total K8+ or Atoh1-lineal cells. Cell counts were compared using independent sample two-tailed t tests (Excel; Microsoft) or one- or two-way ANOVA with post-hoc Tukey’s multiple comparisons test (Prism 6; GraphPad Software).
Online supplemental material
Fig. S1 shows colocalization of K8 and K20 in embryonic and postnatal touch domes. Fig. S2 demonstrates labeling of E18.5 K8+ cells by K14CreER;ROSAtdTomato when tamoxifen is administered at E14.5. Fig. S3 verifies interchangeability of the Atoh1GFP and Atoh1CreER-T2/+;ROSAtdTomato alleles in labeling the same population of cells in the skin. Fig. S4 provides further evidence for proliferative Atoh1+ cells with embryonic PH3 and adult Ki67 labeling in the whisker follicles. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201407101/DC1.
Supplementary Material
Acknowledgments
We thank members of the Maricich laboratory and Drs. Heather Broihier, Evan Deneris, Radhika Atit, and Sharyl Fyffe-Maricich for their thoughtful discussions and input. We thank Dr. Stefanie Altieri for help with statistics. Confocal imaging was performed at the Case Western Reserve University Neurosciences Imaging Center and at the Children’s Hospital of Pittsburgh with the generous assistance of Dr. Tim Sanders.
This work was supported by National Institutes of Health grant T32 GM008056 (M.C. Wright), National Institutes of Health grant AR059114 (S.M. Maricich), and the Richard King Mellon Foundation Institute for Pediatric Research (S.M. Maricich).
The authors declare no competing financial interests.
Footnotes
Abbreviations used in this paper:
- ANOVA
- analysis of variance
- β-Gal
- β-galactosidase
- EdU
- 5-ethynyl-2’-deoxyuridine
- MCC
- Merkel cell carcinoma
References
- Akazawa C., Ishibashi M., Shimizu C., Nakanishi S., and Kageyama R.. 1995. A mammalian helix-loop-helix factor structurally related to the product of Drosophila proneural gene atonal is a positive transcriptional regulator expressed in the developing nervous system. J. Biol. Chem. 270:8730–8738 10.1074/jbc.270.15.8730 [DOI] [PubMed] [Google Scholar]
- Alonso L., and Fuchs E.. 2006. The hair cycle. J. Cell Sci. 119:391–393 10.1242/jcs02793 [DOI] [PubMed] [Google Scholar]
- Bardot E.S., Valdes V.J., Zhang J., Perdigoto C.N., Nicolis S., Hearn S.A., Silva J.M., and Ezhkova E.. 2013. Polycomb subunits Ezh1 and Ezh2 regulate the Merkel cell differentiation program in skin stem cells. EMBO J. 32:1990–2000 10.1038/emboj.2013.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Arie N., Hassan B.A., Bermingham N.A., Malicki D.M., Armstrong D., Matzuk M., Bellen H.J., and Zoghbi H.Y.. 2000. Functional conservation of atonal and Math1 in the CNS and PNS. Development. 127:1039–1048. [DOI] [PubMed] [Google Scholar]
- Doucet Y.S., Woo S.-H., Ruiz M.E., and Owens D.M.. 2013. The touch dome defines an epidermal niche specialized for mechanosensory signaling. Cell Reports. 3:1759–1765 10.1016/j.celrep.2013.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eispert A.C., Fuchs F., Brandner J.M., Houdek P., Wladykowski E., and Moll I.. 2009. Evidence for distinct populations of human Merkel cells. Histochem. Cell Biol. 132:83–93 10.1007/s00418-009-0578-0 [DOI] [PubMed] [Google Scholar]
- Fuchs E.2007. Scratching the surface of skin development. Nature. 445:834–842 10.1038/nature05659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama T., Yamada M., Terao M., Terashima T., Hioki H., Inoue Y.U., Inoue T., Masuyama N., Obata K., Yanagawa Y., et al. . 2009. Inhibitory and excitatory subtypes of cochlear nucleus neurons are defined by distinct bHLH transcription factors, Ptf1a and Atoh1. Development. 136:2049–2058 10.1242/dev.033480 [DOI] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H.H., Schwab U., and Stein H.. 1984. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 133:1710–1715. [PubMed] [Google Scholar]
- Ghazizadeh S., and Taichman L.B.. 2001. Multiple classes of stem cells in cutaneous epithelium: a lineage analysis of adult mouse skin. EMBO J. 20:1215–1222 10.1093/emboj/20.6.1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeberle H., Fujiwara M., Chuang J., Medina M.M., Panditrao M.V., Bechstedt S., Howard J., and Lumpkin E.A.. 2004. Molecular profiling reveals synaptic release machinery in Merkel cells. Proc. Natl. Acad. Sci. USA. 101:14503–14508 10.1073/pnas.0406308101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halata Z., Grim M., and Bauman K.I.. 2003. Friedrich Sigmund Merkel and his “Merkel cell”, morphology, development, and physiology: review and new results. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 271A:225–239 10.1002/ar.a.10029 [DOI] [PubMed] [Google Scholar]
- Heiskala K., Arola J., Heiskala M., and Andersson L.C.. 2010. Expression of Reg IV and Hath1 in neuroendocrine neoplasms. Histol. Histopathol. 25:63–72. [DOI] [PubMed] [Google Scholar]
- Helms A.W., and Johnson J.E.. 1998. Progenitors of dorsal commissural interneurons are defined by MATH1 expression. Development. 125:919–928. [DOI] [PubMed] [Google Scholar]
- Horsley V., O’Carroll D., Tooze R., Ohinata Y., Saitou M., Obukhanych T., Nussenzweig M., Tarakhovsky A., and Fuchs E.. 2006. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 126:597–609 10.1016/j.cell.2006.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Liu Y., Yang Z., Nguyen J., Liang F., Morris R.J., and Cotsarelis G.. 2005. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 11:1351–1354 10.1038/nm1328 [DOI] [PubMed] [Google Scholar]
- Jaks V., Kasper M., and Toftgård R.. 2010. The hair follicle-a stem cell zoo. Exp. Cell Res. 316:1422–1428 10.1016/j.yexcr.2010.03.014 [DOI] [PubMed] [Google Scholar]
- Kuwamoto S.2011. Recent advances in the biology of Merkel cell carcinoma. Hum. Pathol. 42:1063–1077 10.1016/j.humpath.2011.01.020 [DOI] [PubMed] [Google Scholar]
- Leonard J.H., Cook A.L., Van Gele M., Boyle G.M., Inglis K.J., Speleman F., and Sturm R.A.. 2002. Proneural and proneuroendocrine transcription factor expression in cutaneous mechanoreceptor (Merkel) cells and Merkel cell carcinoma. Int. J. Cancer. 101:103–110 10.1002/ijc.10554 [DOI] [PubMed] [Google Scholar]
- Lesko M.H., Driskell R.R., Kretzschmar K., Goldie S.J., and Watt F.M.. 2013. Sox2 modulates the function of two distinct cell lineages in mouse skin. Dev. Biol. 382:15–26 10.1016/j.ydbio.2013.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy V., Lindon C., Harfe B.D., and Morgan B.A.. 2005. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev. Cell. 9:855–861 10.1016/j.devcel.2005.11.003 [DOI] [PubMed] [Google Scholar]
- Li L., Rutlin M., Abraira V.E., Cassidy C., Kus L., Gong S., Jankowski M.P., Luo W., Heintz N., Koerber H.R., et al. . 2011. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 147:1615–1627 10.1016/j.cell.2011.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin E.A., Collisson T., Parab P., Omer-Abdalla A., Haeberle H., Chen P., Doetzlhofer A., White P., Groves A., Segil N., and Johnson J.E.. 2003. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr. Patterns. 3:389–395 10.1016/S1567-133X(03)00089-9 [DOI] [PubMed] [Google Scholar]
- Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R., et al. . 2010. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13:133–140 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricich S.M., Wellnitz S.A., Nelson A.M., Lesniak D.R., Gerling G.J., Lumpkin E.A., and Zoghbi H.Y.. 2009. Merkel cells are essential for light-touch responses. Science. 324:1580–1582 10.1126/science.1172890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mérot Y., and Saurat J.H.. 1988. Proliferation of Merkel cells in the skin. Acta Derm. Venereol. 68:366–367. [PubMed] [Google Scholar]
- Mérot Y., Carraux P., and Saurat J.H.. 1987. Merkel cell mitoses in vibrissae: an ultrastructural study. J. Anat. 153:241–244. [PMC free article] [PubMed] [Google Scholar]
- Moll I., Troyanovsky S.M., and Moll R.. 1993. Special program of differentiation expressed in keratinocytes of human haarscheiben: an analysis of individual cytokeratin polypeptides. J. Invest. Dermatol. 100:69–76 10.1111/1523-1747.ep12354535 [DOI] [PubMed] [Google Scholar]
- Moll I., Kuhn C., and Moll R.. 1995. Cytokeratin 20 is a general marker of cutaneous Merkel cells while certain neuronal proteins are absent. J. Invest. Dermatol. 104:910–915 10.1111/1523-1747.ep12606183 [DOI] [PubMed] [Google Scholar]
- Moll I., Paus R., and Moll R.. 1996a. Merkel cells in mouse skin: intermediate filament pattern, localization, and hair cycle-dependent density. J. Invest. Dermatol. 106:281–286 10.1111/1523-1747.ep12340714 [DOI] [PubMed] [Google Scholar]
- Moll I., Zieger W., and Schmelz M.. 1996b. Proliferative Merkel cells were not detected in human skin. Arch. Dermatol. Res. 288:184–187 10.1007/BF02505222 [DOI] [PubMed] [Google Scholar]
- Morrison K.M., Miesegaes G.R., Lumpkin E.A., and Maricich S.M.. 2009. Mammalian Merkel cells are descended from the epidermal lineage. Dev. Biol. 336:76–83 10.1016/j.ydbio.2009.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Röver S., Handjiski B., van der Veen C., Eichmüller S., Foitzik K., McKay I.A., Stenn K.S., and Paus R.. 2001. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol. 117:3–15 10.1046/j.0022-202x.2001.01377.x [DOI] [PubMed] [Google Scholar]
- Nafstad P.H.1987. Evidence of turnover of mammalian Merkel cells. J. Anat. 151:57–64. [PMC free article] [PubMed] [Google Scholar]
- Nakafusa J., Narisawa Y., Shinogi T., Taira K., Tanaka T., Inoue T., and Misago N.. 2006. Changes in the number of Merkel cells with the hair cycle in hair discs on rat back skin. Br. J. Dermatol. 155:883–889 10.1111/j.1365-2133.2006.07441.x [DOI] [PubMed] [Google Scholar]
- Nowak J.A., Polak L., Pasolli H.A., and Fuchs E.. 2008. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 3:33–43 10.1016/j.stem.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson G.D., Bazzi H., Fantauzzo K.A., Waters J.M., Crawford H., Hynd P., Christiano A.M., and Jahoda C.A.B.. 2009. KGF and EGF signalling block hair follicle induction and promote interfollicular epidermal fate in developing mouse skin. Development. 136:2153–2164 10.1242/dev.031427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen T., and Gerdes J.. 2000. The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 182:311–322 [DOI] [PubMed] [Google Scholar]
- Sidhu G.S., Chandra P., and Cassai N.D.. 2005. Merkel cells, normal and neoplastic: an update. Ultrastruct. Pathol. 29:287–294 10.1080/01913120590951284 [DOI] [PubMed] [Google Scholar]
- Solanas G., and Benitah S.A.. 2013. Regenerating the skin: a task for the heterogeneous stem cell pool and surrounding niche. Nat. Rev. Mol. Cell Biol. 14:737–748 10.1038/nrm3675 [DOI] [PubMed] [Google Scholar]
- Soriano P.1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21:70–71 10.1038/5007 [DOI] [PubMed] [Google Scholar]
- Tilling T., and Moll I.. 2012. Which are the cells of origin in merkel cell carcinoma? J. Skin Cancer. 2012:1–6 10.1155/2012/680410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaigot P., Pisani A., Darmon Y.M., and Ortonne J.P.. 1987. The majority of epidermal Merkel cells are non-proliferative: a quantitative immunofluorescence analysis. Acta Derm. Venereol. 67:517–520. [PubMed] [Google Scholar]
- Van Keymeulen A., Mascre G., Youseff K.K., Harel I., Michaux C., De Geest N., Szpalski C., Achouri Y., Bloch W., Hassan B.A., and Blanpain C.. 2009. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J. Cell Biol. 187:91–100 10.1083/jcb.200907080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V., Degenstein L., Wise B., and Fuchs E.. 1999. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc. Natl. Acad. Sci. USA. 96:8551–8556 10.1073/pnas.96.15.8551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal V.P.I., Chaboissier M.-C., Lützkendorf S., Cotsarelis G., Mill P., Hui C.-C., Ortonne N., Ortonne J.-P., and Schedl A.. 2005. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr. Biol. 15:1340–1351 10.1016/j.cub.2005.06.064 [DOI] [PubMed] [Google Scholar]
- Vielkind U., Sebzda M.K., Gibson I.R., and Hardy M.H.. 1995. Dynamics of Merkel cell patterns in developing hair follicles in the dorsal skin of mice, demonstrated by a monoclonal antibody to mouse keratin 8. Acta Anat. (Basel). 152:93–109 10.1159/000147688 [DOI] [PubMed] [Google Scholar]
- Voehringer D., Liang H.-E., and Locksley R.M.. 2008. Homeostasis and effector function of lymphopenia-induced “memory-like” T cells in constitutively T cell-depleted mice. J. Immunol. 180:4742–4753 10.4049/jimmunol.180.7.4742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S.-H., Stumpfova M., Jensen U.B., Lumpkin E.A., and Owens D.M.. 2010. Identification of epidermal progenitors for the Merkel cell lineage. Development. 137:3965–3971 10.1242/dev.055970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Bermingham N.A., Finegold M.J., and Zoghbi H.Y.. 2001. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 294:2155–2158 10.1126/science.1065718 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.