Abstract
Objectives
Numerous studies suggest important roles of the chemokine, fractalkine (CX3CL1) in acute/chronic pancreatitis, however the possible mechanisms of the effects are unclear. Pancreatic stellate cells (PSCs) can play important roles in pancreatitis, secreting inflammatory cytokines/chemokines, as well as proliferation. Therefore, we investigated CX3CL1 receptor (CX3CR1) occurrence in normal pancreas and pancreatitis (acute/chronic) tissues, and the effects of CX3CL1 on activated-PSCs.
Methods
CX3CR1 expression/localization in normal pancreas and pancreatitis (acute/chronic) tissues were evaluated with immunohistochemical analysis. CX3CR1 expression and effects of CX3CL1 on activated-PSCs were examined with realtime-PCR, BrdU assays and Western Blotting.
Results
In normal pancreas, acinar cells expressed CX3CR1 within granule-like-formations in the cytoplasm, whereas in acute/chronic pancreatitis, acinar, ductal and activated-PSCs expressed CX3CR1 on cell membranes. With activation of normal PSCs, CX3CR1 is increased. CX3CL1 activated multiple signaling cascades in PSCs. CX3CL1, did not induce inflammatory-genes expression in activated-PSCs, but induced proliferation.
Conclusions
CX3CR1s are expressed in normal pancreas. Expression is increased in acute/chronic pancreatitis and the CX3CR1s are activated. CX3CL1 induces proliferation of activated-PSCs without increasing release of inflammatory-mediators. These results suggest that CX3CR1 activation of PSCs could be important in their effects in pancreatitis, especially to PSCs proliferation in pancreatitis where CX3CL1 levels are elevated.
Keywords: fractalkine, fractalkine receptor, chemokine, pancreatic stellate cells, pancreatitis, signal transduction pathways
Introduction
Chemokines play an essential role in normal physiology by recruiting inflammatory cells in damaged tissues and maintaining homeostasis in various organs via chemokine receptors1,2. Chemokines also play a critical role in the pathogenesis of many inflammatory diseases 3–8. Fractalkine (CX3CL1), one of the most important chemokines, was discovered as a different subclass of CX3C from the known chemokines in late 1990s and designated as CX3CL1 9. CX3CL1 plays a key role in the pathogenesis of many inflammatory diseases via its receptor, CX3CR110 (including hepatitis 11,12, atherosclerosis 13,14, neuropathic pain 15, and rheumatoid arthritis 16).
In the case of pancreatitis, various chemokines have been reported to play critical roles in the progression of pancreatitis 17–26. Among these various chemokines, CX3CL1 may be especially important in pancreatitis, because serum levels of CX3CL1 are increased in rats with severe acute pancreatitis 27 and in patients with chronic pancreatitis 28. Alcohol consumption in patients with chronic pancreatitis increases the serum level of CX3CL1 28 and ethanol addition to PSCs synergistically stimulates the release of CX3CL1 to the cell-culture supernatant29. Furthermore, CX3CL1 expression in human chronic pancreatitis correlates with the clinical pain score, which led to the suggestion that the increased serum level of CX3CL1 might play a pathogenic role in chronic pancreatitis30,31. However, the expression of CX3CL1 receptor, CX3CR1, has not been fully examined in both acute and chronic pancreatitis and it is unclear the exact mechanisms by which CX3CL1 might effect pancreatic function or cause pancreatic diseases.
Recent studies have established the important roles played by various resident cells of the pancreas (particularly acinar cells and PSCs) in various aspects of pancreatitis (acute and chronic) and pancreatic cancer, especially in inflammatory leukocyte attraction via secretion of chemokines and cytokines and expression of adhesion molecules 32–42. PSCs, not only can secrete various inflammatory cytokines and chemokines 29,43,44, which can participate in the inflammatory processes, they can also proliferate and secrete collagen, thereby playing an essential role in tissue regeneration and fibrosis45,46. Whereas a number of stimulants can affect PSCs, including activation of some G-protein coupled receptors (PGE2, angiotensin, CCK-A, CCK-B) 47–52, it is unknown if the fractalkine receptor (CX3CR1) occurs on PSCs either in normal pancreas or whether CX3CR1 on PSCs are activated in acute or chronic pancreatitis, or if this does occur, whether the CX3CR1 agonists affect the PSCs signaling cascade, growth or ability to release inflammatory mediators. To address these questions, in the present study we have examined the occurrence of the CX3CR1 in PSCs in acute and chronic pancreatitis models, as well as the ability of the CX3CR1 agonist, CX3CL1, to alter function of PSCs and effect PSCs behavior.
Materials and Methods
Materials
Forskolin and lipopolysaccharide (LPS) were obtained from Sigma–Aldrich (St Louis, MO, USA). Recombinant rat CX3CL1, MCP-1 and PDGF-BB were obtained from R&D Systems (Minneapolis, Minn). Various antibodies (rabbit anti-rat phosphorylated extracellular signal-regulated kinase (ERK), rabbit anti-rat phosphorylated JNK, rabbit anti-rat phosphorylated p38, rabbit anti-rat phosphorylated Akt, rabbit anti-rat phosphorylated CREB, rabbit anti-rat β-tubulin, anti-rabbit IgG HRP conjugated and anti-mouse IgG HRP conjugated) were from Cell Signaling Technology (Beverly, MA, USA). Rabbit anti-CX3CR1, mouse anti-glial fibrillary acidic protein (GFAP) and mouse anti-alpha smooth muscle actin (α-SMA) antibodies were from Abcam PLC (Cambridge, UK). Anti-rabbit IgG Alexa 488 conjugated antibody, anti-mouse IgG Alexa 555 conjugated antibody and Hoechst 33342 were from Invitrogen (Carlsbad, CA, USA).
Animals
For acute pancreatitis studies, 15-week-old adult male Wistar rats and, for chronic pancreatitis studies, Wistar/Bonn Kobori (KOB) rats (KBT Oriental, Saga, Japan) were used. All animal procedures were performed in accordance with the guidelines of the Committee on Animal Care of Kyushu University. Acute pancreatitis was induced in male Wistar rats by administrating a single intraperitoneal injection of 4.0 g/kg body weight L-arginine monohydrochloride in 0.9% sodium chloride (pH 7.0) as described previously 53. Rats were fed ad libitum after the treatment and killed 72 h after the injection of arginine. To study results in chronic pancreatitis, a Male Wistar/Bonn Kobori (KOB) rats were used because they develop chronic pancreatitis by the age of 3 months and diabetes mellitus occurs at 9 months 54. KOB rats have been widely accepted as a rodent model of chronic pancreatitis 55.
Isolation of quiescent PSCs and cell culture
PSCs were isolated from the pancreas of normal male Wistar rats (weighing 180–200 g) by a density-gradient centrifugation method as previously described 56. Briefly, purity of PSCs was more than 90% as evaluated by the typical star-like configuration and by detecting vitamin A autofluorescence. Cells were maintained in complete DMEM/F-12: this is a mixture of DMEM (Dulbecco’s modified Eagle medium) and Ham’s F-12 nutrient mixture (from Wako Pure Chemicals, Osaka, Japan) supplemented with 10% fetal bovine serum, 50 units/mL of penicillin, and 50 mg/mL of streptomycin (from Invitrogen, Carlsbad, CA, USA). All experiments using PSCs were performed with cells between passages 1 and 5. Unless otherwise specified, PSCs were incubated in serum-free medium for 24 h before the addition of experimental reagents.
Immunofluorescent staining
Immunofluorescent staining was performed as previously described 56. Briefly, double immunofluorescent staining for CX3CR1 and GFAP (quiescent PSCs) or α-SMA (staining activated PSCs) was performed as previously described 29. Tissue sections were deparaffinized and rehydrated in PBS. Following antigen retrieval, the slides were blocked with 2 % BSA and incubated with rabbit anti-CX3CR1 antibody (at 1:100 dilution) and mouse anti-α-SMA antibody (at 1:400 dilution) overnight at 4 °C. After washing, the slides were incubated for 1 h with Alexa 488-labeled anti-rabbit IgG antibody (at 1:1000 dilution) and Alexa 555-labeled anti-mouse IgG antibody (at 1:1000 dilution). After washing, the slides were analyzed for fluorescence using a fluorescent microscopy. Nuclear counterstaining was performed using Hoechst 33342. For in vitro staining, PSCs were incubated without serum for 24 hours at 37°C and fixed in 4% paraformaldehyde. After blocking with 1% normal bovine serum albumin, cells were incubated with rabbit anti-rat CX3CR1 antibody (at 1:100 dilution) and mouse anti-α-SMA antibody (at 1:400 dilution) overnight at 4°C. After washing, cells were incubated with anti-rabbit Alexa488-conjugated IgG and Alexa 555-labeled anti-mouse IgG antibody for 1 h, washed again with PBS and then samples were analyzed for fluorescence under a confocal laser scanning microscope (Nikon A1/C1, Tokyo, Japan). For a negative control, the primary antibody was replaced with 2% BSA or polyclonal rabbit IgG (Abcam). The degree of cellular localization of CX3CR1 was calculated using ImageJ (NIH).
Expressional changes of CX3CR1 and cytokines/chemokines mRNAs in pancreatic tissues and PSCs: real-time reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from the pancreatic tail and from PSCs using an RNeasy Mini Kit (Qiagen, Valencia, CA) as previously described 29,57. Briefly, for RT-PCR, 100 ng of total RNA was reverse transcribed into first-strand complementary DNA (cDNA) using a PrimeScript RT Reagent Kit (Takara Bio, Inc, Otsu, Shiga, Japan) according to the manufacturer’s instructions. RT-PCR was performed using a LightCycler Real-Time PCR system (Roche, Switzerland) according to the manufacturer’s instructions. The reaction mixture (20 µL) contained SYBR Premix Ex Taq II (TLi RNAseH Plus; Takara Bio, Inc, Otsu, Shiga, Japan), 4 mM MgCl2, 0.5 mM of the upstream and downstream PCR primers (Table 1) and 2 µL of first-strand cDNA template. To control for variations in the reactions, all PCR data were normalized against GAPDH expression.
Table 1.
Sequences of primers used in this study
| Gene | strand | Sequence (5’ to 3’) |
|---|---|---|
| rat CX3CR1 | sense | CTCCACAACGCCATGTGC |
| anti-sense | CCGGTTGTTCATGGAGTTGG | |
| rat IL-6 | sense | CCACCAGGAACGAAAGTCAA |
| anti-sense | CAGTCCCAAGAAGGCAACTG | |
| rat IL-1β | sense | GCACAGTTCCCCAACTGGTA |
| anti-sense | CCGACCATTGCTGTTTCCTA | |
| rat CINC-1 | sense | CCACACTCAAGAATGGTCGCG |
| anti-sense | AGACGCCATCGGTGCAATC | |
| rat TNF-α | sense | CTGGTGGTACCAGCAGATGG |
| anti-sense | GGAGGCTGACTTTCTCCTGG | |
| rat GAPDH | sense | GCTCTCTGCTCCTCCCTGTT |
| anti-sense | CACACCGACCTTCACCATCT |
Western Blotting
Western blot analysis was performed as previously described 56. Briefly, cells were lysed in RIPA buffer (Nacalai Tesque, Kyoto, Japan) and cellular proteins (approximately 50 µg) were fractionated by electrophoresis on a 10% sodium dodecyl sulfate polyacrylamide gel (Bio-Rad, Hercules, Calif). The proteins were then transferred onto a nitrocellulose membrane (Bio-Rad, Hercules, Calif), and the membrane was incubated for 2 to 6 h with primary antibodies (at 1:1000 to 1:4000 dilutions). After incubating with HRP-conjugated anti-rabbit or anti-mouse IgG antibody (at 1:10000 dilution), the proteins were visualized by using an ECL kit from Perkin Elmer (Waltham, MA, USA) and ImageQuant™ LAS 4000 mini (GE Healthcare Japan Corporation, Tokyo, Japan). Levels of phosphorylated ERK, JNK, p38, Akt, CREB and β-tubulin were determined by General-Purpose Analysis Software Multi Gauge (Fujifilm, Tokyo, Japan).
Quantification of soluble MCP-1: MCP-1 ELISA
After 24 h of incubation, the levels of MCP-1 in the culture supernatants were measured by ELISA (Rat MCP-1 ELISA from Thermo Scientific, Rockford, IL, USA) performed as described previously 29.
Cell proliferation assay (5-Bromo-2-deoxyuridine ELISA): BrdU incorporation assay
DNA synthesis was measured by the incorporation of 5-bromo-2-deoxyuridine (BrdU) using a BrdU cell proliferation assay kit (Calbiochem; Darmstadt, Germany) performed as described previously 56. Briefly, BrdU was added to wells of the plate during the final 2 h of culture and was incorporated into the DNA of any dividing cells. Cells were fixed and permeabilized, and anti-BrdU monoclonal antibody was pipetted into the wells and allowed to incubate for 1 h while it binds to any incorporated BrdU. Unbound antibody was washed away, and HRP-conjugated goat anti-mouse was added. The HRP catalyzes the conversion of the chromogenic substrate from a colorless solution into a solution with an intensity that is proportional to the amount of incorporated BrdU in the cells. The colored reaction product was quantified by using a spectrophotometer.
Cell viability assay: MTS assay
Cell viability was assessed by the MTS assay (CellTiter 96® Aqueous One Solution Cell Proliferation Assay, Madison, WI, USA). After treatment with CX3CL1 for 24 h, MTS solution was added to the cells and the incubation continued at 37 °C for 1 h. After the incubation period, cell viability was quantified by the differences in absorbance at wavelengths of 570 and 690 nm.
Statistical analysis
Results are expressed as the means ± SEM of 3–4 separate cell preparations per experimental protocol. The two-tailed unpaired Student’s t-test was used for the statistical analyses. P values of <0.05 were considered statistically significant.
Results
Comparison of expression of the CX3CR1 in pancreas of rats with acute pancreatitis and normal controls (Fig. 1)
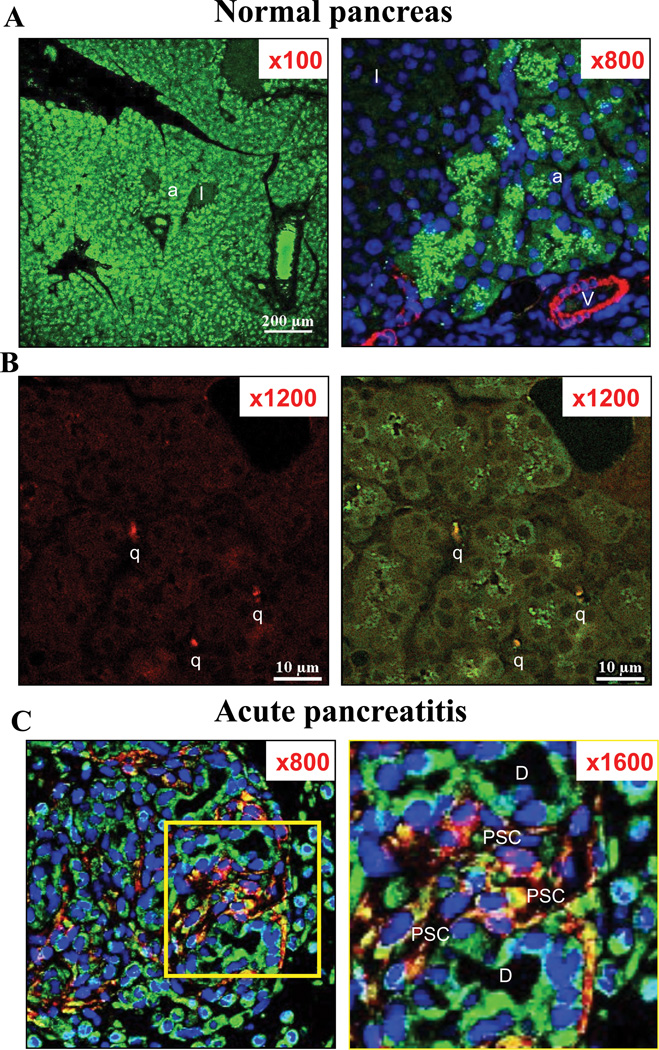
Figure 1. Differences of intracellular fractalkine receptor (CX3CR1) distribution in normal pancreas (Panel A, B) and in L-arginine induced acute pancreatitis (Panel C).
Expression of CX3CR1 (in green); glial fibrillary acidic protein (GFAP; in red) [quiescent pancreatic stellate cells (PSCs)], and alpha-smooth muscle actin (α-SMA; in red) [activated PSCs] in the pancreas of 15-week-old Wistar rats and the pancreas from L-arginine induced acute pancreatitis are examined by immunofluorescence staining. In the top two panels (A: in normal pancreas), figures are x100 and x800 each. α-SMA (in red) and CX3CR1 expression (in green) are shown. This figure demonstrates that CX3CR1 is expressed diffusely in acinar (a) and was also seen in intra-lobular duct cells but CX3CR1 is minimally expressed in the cytoplasm and the cell surface membrane of these cells in normal pancreas. Islets (I) and blood vessel cells (V) do not express CX3CR1. Blood vessel cells (V) express α-SMA, but no activated pancreatic stellate cells are seen. In the middle panels (B: in normal pancreas), figures are x1200 (left and right), and show a magnification of an area containing quiescent PSCs (q). GFAP (in red) and CX3CR1 expression (in green) are shown. Co-localization of CX3CR1 and GFAP is shown in yellow [CX3CR1 positive quiescent PSCs] In the bottom panels (C: in acute pancreatitis tissues), figures are x800 (left) and on the right x1600 (right). The area seen is shown a magnification of an area containing an increased numbers of activated PSCs (PSC) with acute pancreatitis. In the square on the left is shown at 1600× magnification on the right (Fig. 1C). Intracellular localization of CX3CR1differs from normal in that it is expressed on the cell surface membrane of acinar, duct (D) and activated PSCs (PSC). Co-localization of CX3CR1 and α-SMA shows yellow staining[CX3CR1 positive activated PSCs]. These pictures are representative immunofluorescent confocal microscopy images of four experiments.
Initially, we performed immunofluorescent staining to evaluate the expression of the CX3CR1 in normal pancreas from male Wistar rats. High expression of the CX3CR1 was observed in pancreatic acinar cells and intra-lobular duct cells (Fig. 1A). On high magnification (Fig. 1A, right panel, ×800), CX3CR1 was expressed in the cytoplasm of these cells, but not on the cell surface membrane, presenting with diffusely scattered intracellular granule-like formations. GFAP positive PSCs (quiescent PSCs: q) (Fig. 1B, right) showed CX3CR1, however the precise localization of the receptor was difficult to see because of the cell size. No expression of CX3CR1 was seen in pancreatic islets (I) or in α-SMA positive vessel cells (V). In the normal pancreatic specimen, no α-SMA positive PSCs (activated myofibroblast-like cells) were seen. In contrast, an increasing numbers of activated PSCs (PSC) were observed in pancreas from L-arginine induced acute pancreatitis (Fig. 1C). Furthermore, the cellular localization of CX3CR1 was altered and distributed on the cell surface membrane in activated PSCs, acinar cells and duct cells (D) (Fig. 1C, right). We found none of the normal cells expressed CX3CR1 on their cell surface. However, we also found in acute pancreatitis (Fig. 1C) that 94 % of the cells (including activated PSCs, acinar, ductal and inflammatory cells) expressed CX3CR1 on cell surface (6% of the other cells did not express it on cell surface). No intracellular granule-like formations were seen in these cells.
Expression of CX3CR1 in chronic pancreatitis (Fig. 2)
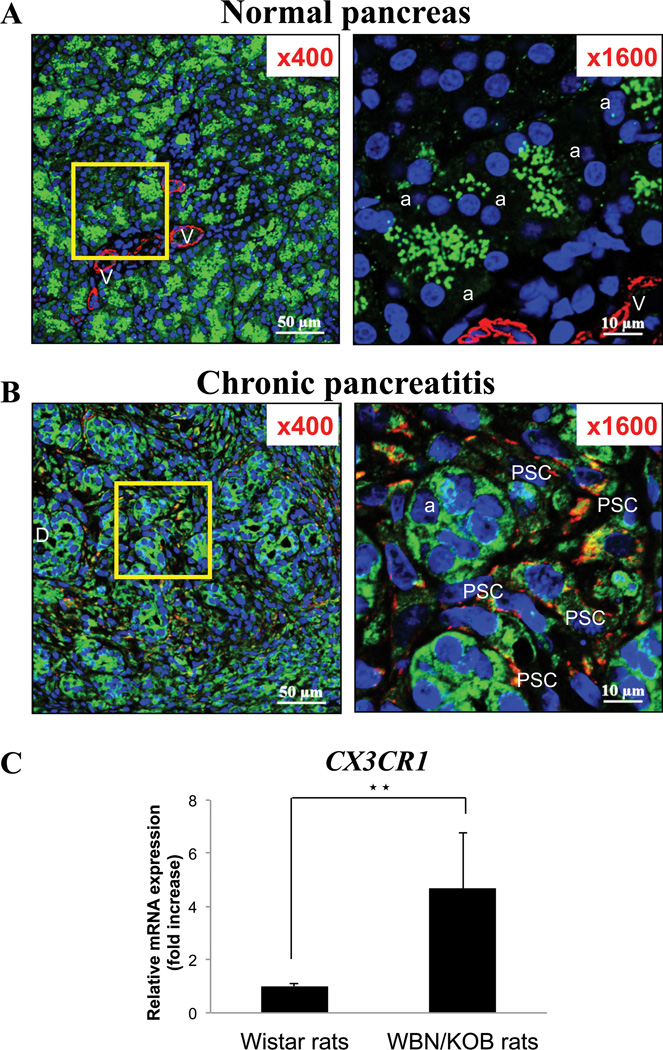
Figure 2. Increased and altered distribution of fractalkine receptor (CX3CR1) expression in chronic pancreatitis (Panel B) compared to normal pancreas (Panel A).
Expression of CX3CR1 and alpha-smooth muscle actin (α-SMA) [activated pancreatic stellate cells (PSCs)] in the pancreas of 15-week-old Wistar rats (normal) and Wistar/Bonn kobori rats (chronic pancreatitis) are examined by immunofluorescence staining. In the top two panels (A: in normal pancreas), figures are x400 and x1600 each. α-SMA (in red) and CX3CR1 expression (in green) are shown. This figure demonstrates that CX3CR1 is expressed diffusely in acinar (a) and intra-lobular duct cells, but not express on the cell surface membrane of these cells in normal pancreas. Vascular cells (V) do not express CX3CR1 but express α-SMA and no activated PSCs are seen. The middle panels (B) show the staining of the pancreas from rats with chronic pancreatitis, magnification of which are x400 (left) and x1600 (right). The right panel is an increased magnification of the area in the square in the left panel and shows an increased numbers of activated PSCs (PSC). Intracellular localization of CX3CR1 is changed with chronic pancreatitis in that it is expressed on the cell surface membrane of acinar, duct (D) and activated PSCs in chronic pancreatitis. Co-localization of CX3CR1 and α-SMA shows yellow staining [CX3CR1 positive activated PSCs]. These pictures are representative immunofluorescent confocal microscopy images of four experiments. In the bottom panel (C), the quantitative PCR result of CX3CR1 expression in normal and chronic pancreatitis in the pancreas is shown. The result demonstrates that CX3CR1 expression is increased in chronic pancreatitis at the mRNA level (★★=P<0.01, comparing normal pancreas and chronic pancreatitis). These results were representative of four experiments.
We next investigated whether CX3CR1 expression was present or altered in the pancreas of rats with chronic pancreatitis (Fig. 2). To accomplish this, double staining for α-SMA (to localize activated PSCs) and the CX3CR1 was performed in pancreatic tissue sections from male Wistar/Bonn Kobori rats, which are a well-established model of chronic pancreatitis (Fig. 2B). In the pancreas from rats with chronic pancreatitis, a marked increase in CX3CR1 expression was seen, and throughout the pancreas was seen prominent fibrosis, and a marked increase in the number of α-SMA positive cells (activated PSCs), especially in the interstitial area (Fig. 2B, left). Double-positive staining cells (yellow for CX3CR1 and α-SMA) were observed supporting the conclusion that CX3CR1 was present on α-SMA positive cells (activated PSCs) (Fig. 2B, right). Acinar (a) and tubular complexes also expressed the CX3CR1 (Fig. 2B, right) on the cell surface membrane. Similar to acute pancreatitis (Fig. 1C), in Fig. 2B, we found 92 % of the cells have CX3CR1 on cell surface. These data demonstrated that by far the majority of activated PSCs and other cells in an acute (Fig. 1C) and chronic (Fig. 2B) inflammatory models of pancreatitis have CX3CR1 on their cell surface. To quantitate the mRNA expression of CX3CR1, we performed realtime PCR. The result showed a 5-fold increase in expression of CX3CR1 in pancreas from rats with chronic pancreatitis (Fig. 2C).
Expression of CX3CR1 in quiescent and activated PSCs in vitro (Fig. 3)
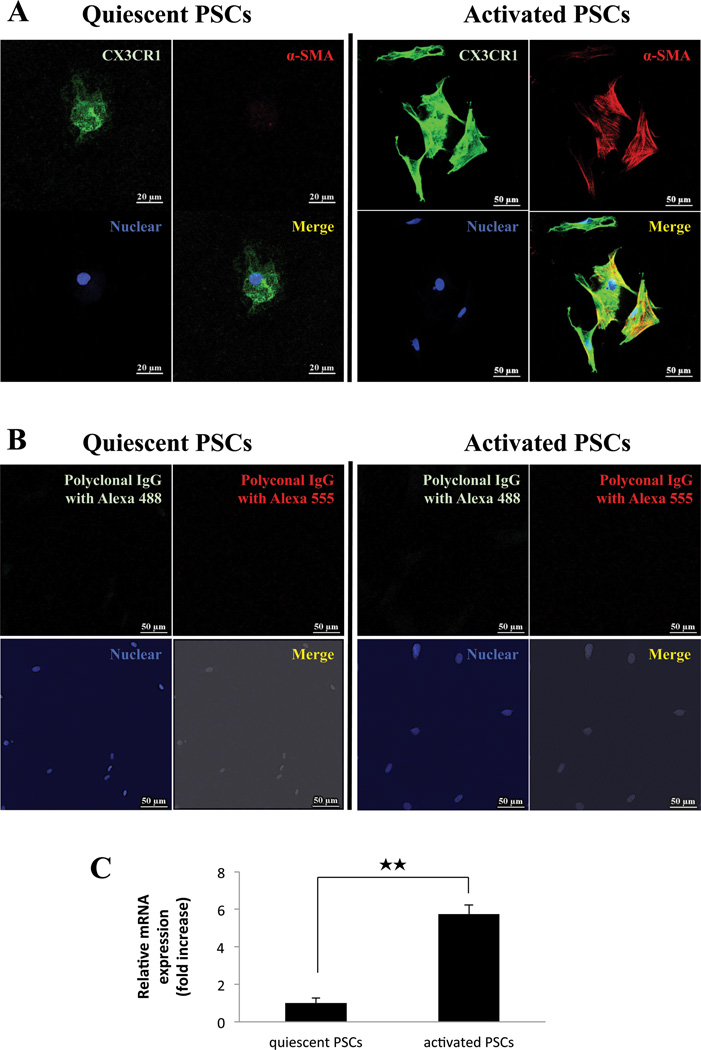
Figure 3. Both quiescent and activated normal pancreatic stellate cells (PSCs) express CX3CR1 in vitro and the expression increases with activation.
Panel A show the expression of CX3CR1 protein in quiescent (2 days after isolation; Magnification is x1000; left panels) and culture-activated PSCs (3rd passage. Magnification is x400; right panels). In Panel A, CX3CR1 is stained in green, α-SMA (activated PSCs) in red, nuclei in blue and co-localization of α-SMA and CX3CR1 in yellow. Panel B is the negative control for Panel A (incubated with Polyclonal IgG antibody and stained with Alexa 488green, IgG with Alexa 555 in red and nuclei in blue). Panel C shows the quantitation of CX3CR1 expression by RT-PCR in quiescent and activated normal PSCs. The result is from 4 experiments for passages 1 and 3. ★★=P<0.01, comparing passage 1 and 3. These results were representative of four experiments.
To further investigate the expression of CX3CR1 in normal pancreas and the result of its activation, we studied PSCs isolated from normal pancreas in both a quiescent phase (1st passage: 2 days after isolated) (Fig. 3A, top/left) and an activated phase (3rd passage) in vitro (Fig. 3A, top/right). Quiescent and activated PSCs expressed CX3CR1 (Fig. 3A). Activation of the PSCs during the 3rd passage was confirmed by demonstrating the presence of immunofluorescent staining for α-SMA (Fig. 3A, top/right). We also confirmed the specificity of CX3CR1 antibody using isotype anti-rat IgG as a negative control (Fig. 3A, bottom). To elucidate further the level of expression of CX3CR1 mRNA in quiescent and activated PSCs, whether PSCs expressed CX3CR1 mRNA and whether the amount varies with PSCs activation, we performed RT-PCR in cultured PSCs after the 1st passage (quiescent) and the 3rd passage (activated) (Fig. 3B). CX3CR1 was expressed in PSCs in both quiescent and activated phase (Fig. 3B). Quantitative realtime PCR revealed expression of CX3CR1 mRNA in activated PSCs from the 3rd passage (activated) compared to quiescent PSCs from the 1st passage (quiescent) increased almost 6-fold (Fig. 3B).
Effect of activation of the CX3CR1 by the specific agonist, CX3CL1, on various cell signaling cascades in PSCs
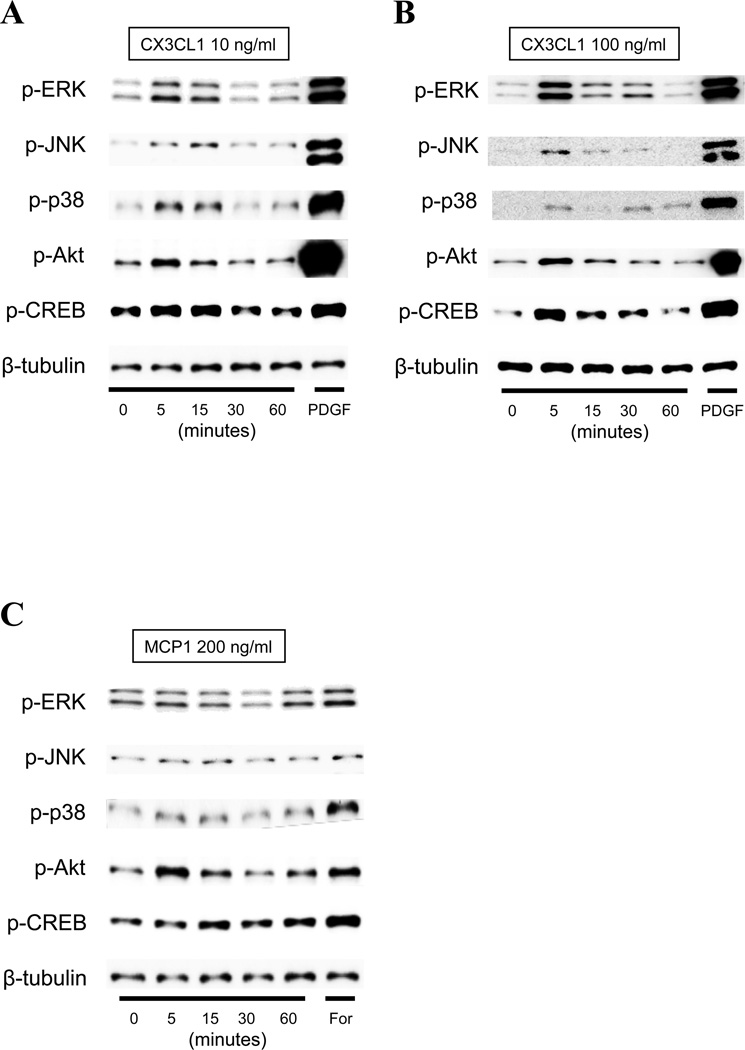
To evaluate which cell signaling cascades was activated by CX3CL1 in PSCs, we next employed Western blotting analysis after incubation of PSCs with CX3CL1 or with the chemokine, MCP-1 (Fig. 4). CX3CL1 rapidly stimulated (by 5 min) phosphorylation of MAPKs (ERK, JNK and p38), Akt and CREB with a maximal response between 5–15 min at 10 ng/ml (Fig. 4A). Furthermore, increased and prolonged (phosphorylated ERK and CREB seen at 30 min) activation was observed with increased concentration of CX3CL1 (100 ng/ml) (Fig. 4B). On the other hand, MCP-1 stimulated these cascades weakly (Fig. 4C). PDGF-BB (PDGF) and forskolin (For) were used as positive controls and each activated all of the cell signaling pathways.
Figure 4. Time course of the effect of fractalkine (CX3CL1: 10–100 ng/ml) on various cell signaling cascades (Panel A and B) and comparison with effect of MCP-1 (200 ng/ml) (Panel C).
Results for phospho-ERK, phospho-JNK, phospho-p38, phospho-Akt and phospho-CREB are determined using specific phospho-antibodies. The results are determined at the indicated times with CX3CL1 and MCP-1, and after a 5 min incubation with the positive controls, PDGF-BB (PDGF, top panel) or forskolin (For, bottom panel). This experiment is representative of 4 experiments.
Effect of activation of CX3CR1 by CX3CL1 on stimulation of the expression of various inflammatory cytokines/chemokines in PSCs
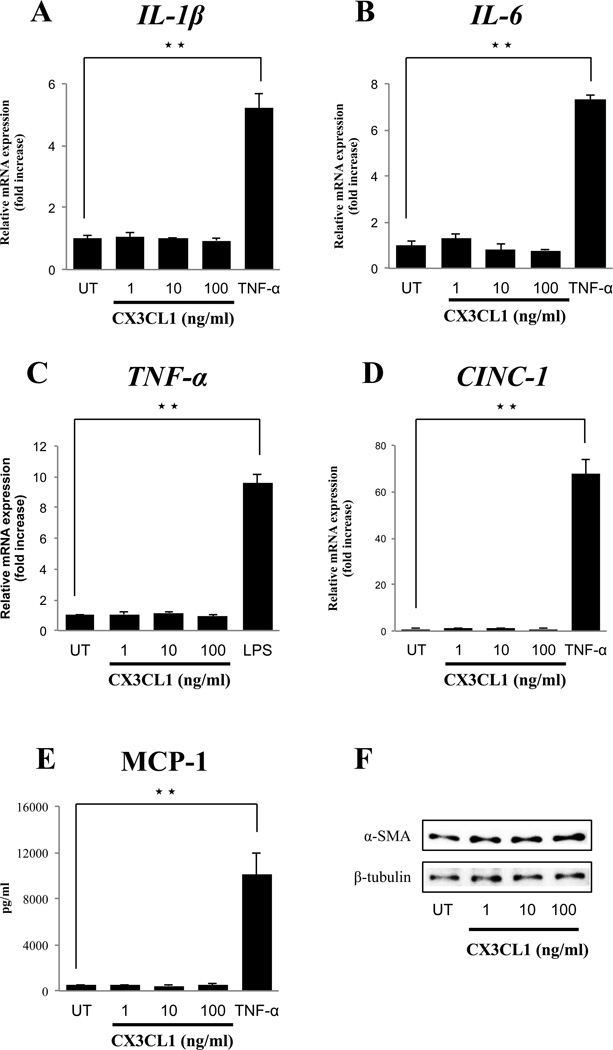
We next examined whether the CX3CR1 agonist, CX3CL1, stimulated inflammatory responses in PSCs using realtime PCR (Fig. 5A–E). CX3CL1 (1–100 ng/ml) did not alter the mRNA expression of the inflammatory cytokines (IL-1β, IL-6, TNF-α) or the chemokines (CINC-1) (Fig. 5A–D). CX3CL1 also did not stimulate MCP-1 release from PSCs (Fig. 5E). However, α-SMA expression, which is cell activation marker, was also not increased by CX3CL1 treatment (Fig. 5F).
Figure 5. Effect of the fractalkine receptor (CX3CR1) agonist, fractalkine (CX3CL1), on inflammatory mediators and cell activation of PSCs.
Panel A-D show the RT-PCR results from 4 experiments for activated PSCs (passages 3–5) treated with various concentrations of CX3CL1 or TNF-α (10 ng/ml) or LPS (1 µg/ml) or IL-1β (10 ng/ml) compared with untreated (UT) activated PSCs for 6 hours. Panel E shows the quantification of soluble MCP-1 release in PSCs treated similar to demonstrated in Panel A-D but for 24 hours. Panel F shows the Western Blotting results in activated PSCs from 4 experiments for passage 1 (2 days after isolation), comparing untreated (UT) with treatment with CX3CL1 (1–100 ng/ml) for 24 hours. ★★=P<0.01, comparing untreated and treated with CX3CL1 or LPS. These results were representative of four experiments.
Effects of CX3CL1 or MCP-1 on growth of PSCs
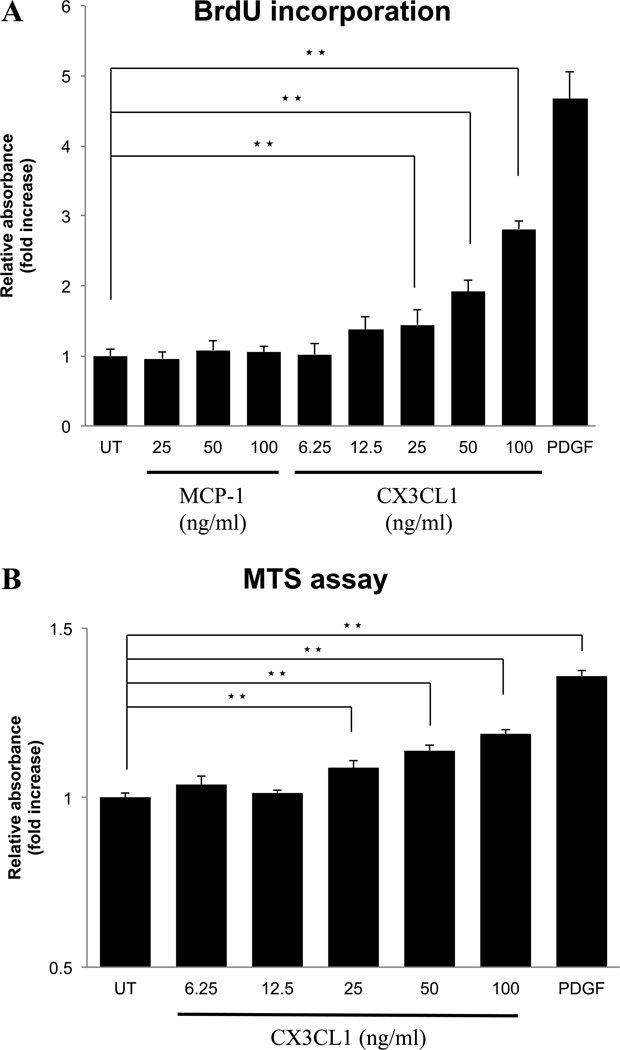
In vascular smooth muscle cells, activation of the CX3CR1 causes cell proliferation 58, in contrast, the chemokine, MCP-1, in some cells stimulates proliferation, however, it is reported to inhibit proliferation in rat PSCs 59. To investigate their effect on cell growth of normal PSCs, we compared the effects of different concentrations of both CX3CL1 and MCP-1 on proliferation in PSCs. CX3CL1 stimulated PSCs proliferation in a dose-dependent manner (Fig. 6A). However, MCP-1 had no effect on proliferation, either stimulatory or inhibitory (Fig. 6A). We additionally performed the MTS assay to evaluate cell viability (Fig. 6B). In this assay, CX3CL1 induced cell proliferation dose-dependently with results similar to that seen with the BrdU assay (Fig. 6A).
Figure 6. Effect of the fractalkine receptor (CX3CR1) agonist, fractalkine (CX3CL1), on cell proliferation of PSCs.
Activated PSCs (passages 3 – 5) (2000 cells/well) were pre-incubated for 24 hours in serum free medium and then incubated for 24 hours with the indicated concentrations of CX3CL1 (1–100 ng/ml), MCP-1 (25–100 ng/ml), PDGF-BB (25 ng/ml), or untreated (UT). Growth was determined using a BrdU incorporation assay (A) and the MTS cell viability assay (B) using a coloriometric microplate reader. This figure shows a concentration-dependent effect of CX3CL1 on cell proliferation after 24 hours. ★★=P<0.01, comparing untreated and treated with CX3CL1 or PDGF. These results were the means of four experiments.
Discussion
Chemokines play a key role in the pathogenesis of many inflammatory diseases 1–5,10 (including hepatitis 6,11,12, atherosclerosis 13,14, neuropathic pain 7,15, and rheumatoid arthritis 8,16). In the case of pancreatitis, various chemokines have been reported to play critical roles in the progression of pancreatitis 17–20,22–26,60. Specifically, MCP-1 is reported to play an essential role in both acute and chronic pancreatitis by recruiting a number of inflammatory cells, especially monocytes 17,21,61–63. Cytokine induced neutrophil chemoattractant-1 (CINC-1) is also reported to be important in acute pancreatitis, especially in pancreatitis-induced acute lung injury by recruiting excessive neutrophils 17,64–67. Among these various chemokines, CX3CL1, may be especially important in pancreatitis, because serum levels of CX3CL1 are increased in rats with severe acute pancreatitis 27 and in patients with chronic pancreatitis 28. Alcohol consumption in patients with chronic pancreatitis increases the serum level of CX3CL1 28 and ethanol addition to PSCs stimulates the release of CX3CL1 to the cell-culture supernatant29, which might contribute to increasing the local CX3CL1 concentration around pancreatitis tissues. CX3CL1 is a novel chemokine, whose release correlates with the alcohol or ethanol exposure both in vivo an in vitro28,29. CX3CL1 expression in human chronic pancreatitis correlates with the clinical pain score, which led to the suggestion that the increased serum level of CX3CL1 might play a pathogenic role in chronic pancreatitis 30,31. However, the expression of CX3CL1 receptor, CX3CR1, has not been fully examined in both acute and chronic pancreatitis and it is unclear the exact mechanisms by which CX3CL1 might effect pancreatic function or cause pancreatic diseases.
Recent studies have established the important roles played by various resident cells of the pancreas (particularly acinar cells and PSCs) in various aspects of pancreatitis (acute and chronic), especially in inflammatory leukocyte attraction via secretion of chemokines and cytokines and expression of adhesion molecules 32–40. PSCs, not only can secrete various inflammatory cytokines and chemokines 29,43,44, which can participate in the inflammatory processes, they can also proliferate and secrete collagen, thereby playing an essential role in tissue regeneration and fibrosis45,46. Whereas a number of stimulants can affect PSCs, including activation of some G-protein coupled receptors (PGE2, angiotensin, CCK-A, CCK-B) 47–52, it is unknown if the CX3CR1 occurs on PSCs either in normal pancreas or whether CX3CR1 on PSCs are activated in acute or chronic pancreatitis, or if this does occur, whether the CX3CR1 agonists affect the PSCs signaling cascade, growth or ability to release inflammatory mediators. To address these questions, in the present study we have examined the occurrence of the CX3CR1 in PSCs in acute and chronic pancreatitis models, as well as the ability of the CX3CR1 agonist, CX3CL1, to alter function of PSCs and effect PSCs behavior.
We first examined the expression of the CX3CR1 in normal pancreas, followed by its expression in the pancreas of various pancreatitis models (acute and chronic). Our results show that the CX3CR1 is present predominantly in acinar cells in normal pancreas. We did not find it present in endocrine cells in islets or vascular cells, which is consistent with results of a previous study30. We also found CX3CR1 expressed not on the cell membrane in the steady state, but present within scattered granule-like formations in the cytoplasm, which differs from previous reports with CX3CR1, which used human normal pancreatic specimens30. However, our result showing CX3CR1 only in the cytoplasmic location in the steady state, is not a novel finding, because similar distribution of other chemokine receptors (CXCR3, CXCR4) have been reported in the steady state 68,69. In acute and chronic pancreatitis, we found two important changes, in CX3CR1 distribution. We found in both acute and chronic pancreatitis CX3CR1 was redistributed to cell membranes of both acinar cells and duct cells. There have been a number of studies reporting phenotypic changes of the duct cells in inflammatory conditions70–74. The phenotype of the duct cells changed in pancreatitis tissue as to CX3CR1 occurrence. Furthermore, it was expressed in increased amount on the cell membrane of PSCs, which were also markedly increased in number. A number of our results, in both the acute and chronic pancreatitis models, are consistent with the conclusion that CX3CR1 is expressed in activated PSCs in these models and that the CX3CR1 is increased on the cell surface membrane, a finding which is not seen in normal pancreas. First, we observed an increased numbers of activated PSCs in L-arginine induced acute pancreatitis, which is consistent with findings from a previous report 75,76, which demonstrated increased α-SMA expression (a marker for activated PSCs) coincided with TGF-β1 activation in this model. Second, in the chronic pancreatitis model (WBN/KOB rat), we also demonstrated increased number of PSCs, which is consistent with findings in other studies77,78. Third, we found in the acute and chronic pancreatitis models a marked overexpression of CX3CR1 and that this overexpression generally co-localized with the location of the activated PSCs or with areas of marked increased in PSCs activity in the acute and chronic pancreatitis model with enhanced fibrosis. We do not know the exact functional significance of the CX3CR1 redistribution to the membrane in pancreatitis, however, it is reported with some chemokine receptors that they are prominently detected in an intracellular location in steady state conditions and that they underwent redistribution to the cell membrane after activation 79. This would suggest that the CX3CR1 is being activated on redistributing to the cell membrane during acute and chronic pancreatitis. Because of the known role of activated PSCs in both acute and chronic pancreatitis, our findings that they are increased in both pancreatitis models, and that CX3CR1 is also increased and co-expressed with the activated PSCs, suggests that CX3CL1 could be having an effect on the PSCs in both models of pancreatitis and play an important role in pathogenesis of the pancreatitis.
Our results show for the first time that in both quiescent and activated PSCs, CX3CR1 is expressed. This result is similar to findings reported with pancreatic acinar cell line, AR42J, in which CX3CR1 was also found to be expressed 27. In culture-activated PSCs, our results demonstrate with their activation after continued culture, there is a marked increase in the expression of CX3CR1. This finding differs from another study which reported decreased CX3CR1 expression with activation and increased expression with deactivation in microglial cells80. However, this result is similar to the finding with some G-protein coupled receptor (GPCR: AT1R 49, ETR 81, PAR2 82) on PSCs, which also demonstrated an increased expression when PSCs are activated. These results support the proposal that CX3CR1, like these other GPCRs, could be playing an important role in conditions where PSCs are activated such as acute and chronic pancreatitis, and may contribute to the various changes seen with these diseases 49,81,82.
We found that several cellular signaling cascades are activated by CX3CL1 in PSCs including ERK, JNK, p38, PI3K–Akt and CREB. These results are similar to the effect in vascular smooth muscle cells and renal mesangial cells where CX3CR1 activation stimulated growth through an ERK dependent mechanism, as well as in renal mesangial cells where it stimulated p38 MAPK activation which mediated cell growth 83,84. JNK MAPK activation by CX3CL1 was also reported in endothelial cells which induced cell migration 85. In contrast to our results, in neurons, CX3CR1 activation stimulated only ERK with no activation of either JNK or p38 86. Our finding that the PI3K–Akt pathway is activated by CX3CL1 is similar to the effect of activation of CX3CL1 in smooth muscle cells and ovarian cancer cells, where PI3K activation mediated CX3CL1’s anti-apoptotic and proliferative effects 83. Also similar to our findings, CREB activation by CX3CL1 has been reported in neurons which induces an anti-apoptotic effect 86.
With CX3CL1 treatment, we did not find an increased expression of inflammatory mRNA’s of cytokines (IL-1β, IL-6, TNF-α) or chemokines (MCP-1, CINC-1) in activated PSCs. CX3CL1 has been reported to induce release of the chemokine, MCP-1, in peripheral blood monocytes 87 and induced TNF-α production in AR42J cells (pancreatic acinar cell line) 27, which differs from our results showing no increased expressions of these inflammatory mediators in PSCs. PSCs specific function, such as α-SMA and type I collagen expression were not induced by CX3CL1 in PSCs, which is consistent with findings in a previous study with human PSCs, but differs from those with microglial cells where CX3CR1 activation stimulated reorganization of the actin skeleton88.
A number of studies demonstrate that CX3CL1 has cell proliferative and anti-apoptotic effects in various cells (microglial cells 89, neurons86, synovicites90, vascular smooth muscle cells83, mesangial cells84 and cancer cells91). Our results in PSCs are consistent with these findings in other cells, because we found the CX3CR1 agonist, CX3CL1 stimulated cell proliferation of PSCs. These results show that effect of activation in PSCs by CX3CR1 differ from that cause activation by the chemokine, MCP-1, with the former having a proliferative effect, but not the latter 59. However they are similar to the effects of activation of two other GPCRs on PSCs, the AT1 receptor and PAR2 receptor, which also result in PSCs cell proliferation 48,49,82,92. In contrast, activation of ET-1 receptors on PSCs does not have proliferative effects 81,93. The role of the proliferative effect on PSCs of CX3CL1 and the other GPCRs in acute or chronic pancreatitis at present is unknown. However, in our CX3CL1 results raise the possibility that the prolonged increases in serum levels of CX3CL1 reported in patients with acute and chronic pancreatitis 27,28, could lead to increased fibrosis due to enhanced PSCs growth.
Very recently, the role of CX3CL1/CX3CR1 axis in the maintenance of normal β cell function has been reported 94. Some studies report cellular cross-talk between PSCs and pancreatic β cells with activation of PSCs leading to apoptosis and a decrease in β cell function 95. In clinical CP, β cell function is disturbed, supporting the concept of Kikuta et al. 95 that CX3CL1 have a proliferative or anti-apoptotic effect in pancreatic β cells, and also proliferative effect on pancreatic stellate cells. If serum CX3CL1 concentration is elevated pathologically by environmental factors, such as alcohol consumption, increased number of activated PSCs by themselves affect the β cells survival resulting in loss of islet functions (β cell dysfunction in type 2 diabetes). Both CX3CL1 concentration and the number of PSCs might affect β cell survival.
In conclusion, we demonstrated the CX3CR1 expression in normal pancreas occurs primarily in pancreatic acinar cells within granule-like formations. However, in acute and chronic pancreatitis there is marked increase in CX3CR1 primarily in pancreatic acinar cells, ductal cells and activated PSCs, as well as redistribution to the cell membrane, capable with its activation. Furthermore, with activation of normal PSCs, there is marked increase in CX3CR1 level. However, in activated PSCs, CX3CL1, a CX3CR1 agonist, did not stimulate release of inflammatory cytokines or chemokines, however, it did stimulate PSCs proliferation. CX3CR1 on PSCs might play a role in tissue remodeling with proliferation, but prolonged increased CX3CL1 levels, occurs with increased ethanol intake, can possibly induce the increased number of activated PSCs resulting in tissue fibrosis and β cells dysfunction. However, at present, it remains unclear whether these changes in PSCs contribute to the development of pancreatitis seen in patients with continued elevation of serum CX3CL1 level, or whether they could lead to complications of the increased PSCs proliferation due to the prolonged increase in serum CX3CL1 levels reported in patients with pancreatitis 28.
Supplementary Material
Acknowledgment
We appreciate for the technical supports from the Research Support Center, Graduate School of Medical Sciences, Kyushu University.
Grant support: This work was supported by a grant from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (20590808, Ito T) and the Research Committee provided by the Ministry of Health, Labour, and Welfare Japan (50253448, Ito T). Also partially supported by intramural research funds of NIDDK, NIH.
Glossary
- PSCs
pancreatic stellate cells
- MCP-1
monocyte chemoattractant protein-1
- CINC-1
Cytokine induced neutrophil chemoattractant-1
- CX3CL1
fractalkine
- CX3CR1
fractalkine receptor
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
All of the authors declare no conflict of interest.
Competing interests None
Ethics approval These studies were approved by the Committee on Animal Care of Kyushu University, Fukuoka, Japan.
References
- 1.Baggiolini M, Dewald B, Moser B, editors. Human chemokines: An update. 1997. Annual Review of Immunology; No. 15. [DOI] [PubMed] [Google Scholar]
- 2.Rossi D, Zlotnik A, editors. The biology of chemokines and their receptors. 2000. Annual Review of Immunology; No. 18. [DOI] [PubMed] [Google Scholar]
- 3.Luster AD. Mechanisms of disease: Chemokines - Chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 4.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 5.Charo IF, Ransohoff RM. Mechanisms of disease: The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 6.Wasmuth HE, Tacke F, Trautwein C. Chemokines in liver inflammation and fibrosis. Semin Liver Dis. 2010;30:215–225. doi: 10.1055/s-0030-1255351. [DOI] [PubMed] [Google Scholar]
- 7.Watkins LR, Maier SF. Beyond neurons: Evidence that immune and glial cells contribute to pathological pain states. Physiol Rev. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- 8.Szekanecz Z, Kim J, Koch AE. Chemokines and chemokine receptors in rheumatoid arthritis. Semin Immunol. 2003;15:15–21. doi: 10.1016/s1044-5323(02)00124-0. [DOI] [PubMed] [Google Scholar]
- 9.Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 10.Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–642. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 11.Efsen E, Grappone C, DeFranco RMS, et al. Up-regulated expression of fractalkine and its receptor CX3CR1 during liver injury in humans. J Hepatol. 2002;37:39–47. doi: 10.1016/s0168-8278(02)00065-x. [DOI] [PubMed] [Google Scholar]
- 12.Isse K, Harada K, Zen Y, et al. Fractalkine and CX3CR1 are involved in the recruitment of intraepithelial lymphocytes of intrahepatic bile ducts. Hepatology. 2005;41:506–516. doi: 10.1002/hep.20582. [DOI] [PubMed] [Google Scholar]
- 13.Combadière C, Potteaux S, Gao J-, et al. Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation. 2003;107:1009–1016. doi: 10.1161/01.cir.0000057548.68243.42. [DOI] [PubMed] [Google Scholar]
- 14.Lesnik P, Haskell CA, Charo IF. Decreased atherosclerosis in CX3CR1‒/‒ mice reveals a role for fractalkine in atherogenesis. J Clin Invest. 2003;111:333–340. doi: 10.1172/JCI15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardona AE, Pioro EP, Sasse ME, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 16.Ruth JH, Volin MV, Haines GK, III, et al. Fractalkine, a novel chemokine in rheumatoid arthritis and in rat adjuvant-induced arthritis. Arthritis Rheum. 2001;44:1568–1581. doi: 10.1002/1529-0131(200107)44:7<1568::AID-ART280>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 17.Brady M, Bhatia M, Christmas S, et al. Expression of the chemokines MCP-1/JE and cytokine-induced neutrophil chemoattractant in early acute pancreatitis. Pancreas. 2002;25:260–269. doi: 10.1097/00006676-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Goecke H, Forssmann U, Uguccioni M, et al. Macrophages infiltrating the tissue in chronic pancreatitis express the chemokine receptor CCR5. Surgery. 2000;128:806–814. doi: 10.1067/msy.2000.108613. [DOI] [PubMed] [Google Scholar]
- 19.Grady T, Liang P, Ernst SA, et al. Chemokine gene expression in rat pancreatic acinar cells is an early event associated with acute pancreatitis. Gastroenterology. 1997;113:1966–1975. doi: 10.1016/s0016-5085(97)70017-9. [DOI] [PubMed] [Google Scholar]
- 20.Ito T. Can measurement of chemokines become useful biological and functional markers of early-stage chronic pancreatitis? J Gastroenterol. 2007;42:72–77. doi: 10.1007/s00535-006-1929-4. [DOI] [PubMed] [Google Scholar]
- 21.Zhao HF, Ito T, Gibo J, et al. Anti-monocyte chemoattractant protein 1 gene therapy attenuates experimental chronic pancreatitis induced by dibutyltin dichloride in rats. Gut. 2005;54:1759–1767. doi: 10.1136/gut.2004.049403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreno C, Nicaise C, Gustot T, et al. Chemokine receptor CCR5 deficiency exacerbates cerulein-induced acute pancreatitis in mice. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2006;291:G1089–G1099. doi: 10.1152/ajpgi.00571.2005. [DOI] [PubMed] [Google Scholar]
- 23.Rau B, Baumgart K, Krüger CM, et al. CC-chemokine activation in acute pancreatitis: Enhanced release of monocyte chemoattractant protein-1 in patients with local and systemic complications. Intensive Care Med. 2003;29:622–629. doi: 10.1007/s00134-003-1668-4. [DOI] [PubMed] [Google Scholar]
- 24.Saluja AK, Steer ML. Pathophysiology of pancreatitis. Role of cytokines and other mediators of inflammation. Digestion. 1999;60:27–33. doi: 10.1159/000051450. [DOI] [PubMed] [Google Scholar]
- 25.Saurer L, Reber P, Schaffner T, et al. Differential expression of chemokines in normal pancreas and in chronic pancreatitis. Gastroenterology. 2000;118:356–367. doi: 10.1016/s0016-5085(00)70218-6. [DOI] [PubMed] [Google Scholar]
- 26.Sun J, Bhatia M. Blockade of neurokinin-1 receptor attenuates CC and CXC chemokine production in experimental acute pancreatitis and associated lung injury. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2007;292:G143–G153. doi: 10.1152/ajpgi.00271.2006. [DOI] [PubMed] [Google Scholar]
- 27.Huang LY, Chen P, Xu LX, et al. Fractalkine as a marker for assessment of severe acute pancreatitis. Journal of Digestive Diseases. 2012;13:225–231. doi: 10.1111/j.1751-2980.2012.00580.x. [DOI] [PubMed] [Google Scholar]
- 28.Yasuda M, Ito T, Oono T, et al. Fractalkine and TGF-β1 levels reflect the severity of chronic pancreatitis in humans. World Journal of Gastroenterology. 2008;14:6488–6495. doi: 10.3748/wjg.14.6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uchida M, Ito T, Nakamura T, et al. ERK pathway and sheddases play an essential role in ethanol-induced CX3CL1 release in pancreatic stellate cells. Lab Invest. 2013;93:41–53. doi: 10.1038/labinvest.2012.156. [DOI] [PubMed] [Google Scholar]
- 30.Ceyhan GO, Deucker S, Demir IE, et al. Neural fractalkine expression is closely linked to pain and pancreatic neuritis in human chronic pancreatitis. Laboratory Investigatio. 2009;89:347–361. doi: 10.1038/labinvest.2008.170. [DOI] [PubMed] [Google Scholar]
- 31.D’Haese JG, Demir IE, Kehl T, et al. The impact of MFG-E8 in chronic pancreatitis: Potential for future immunotherapy? BMC Gastroenterology. 2013:13. doi: 10.1186/1471-230X-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorelick FS. Pancreas cell physiology and pancreatitis cell biology: Summary of a symposium held at the joint meeting of the EPC and the IAP, Heidelberg 2002. Pancreatology. 2003;3:207–208. doi: 10.1159/000070730. [DOI] [PubMed] [Google Scholar]
- 33.Hoque R, Sohail M, Malik A, et al. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology. 2011;141:358–369. doi: 10.1053/j.gastro.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoque R, Malik AF, Gorelick F, et al. Sterile inflammatory response in acute pancreatitis. Pancreas. 2012;41:353–357. doi: 10.1097/MPA.0b013e3182321500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977;373:97–117. doi: 10.1007/BF00432156. [DOI] [PubMed] [Google Scholar]
- 36.Vonlaufen A, Apte MV, Imhof BA, et al. The role of inflammatory and parenchymal cells in acute pancreatitis. J Pathol. 2007;213:239–248. doi: 10.1002/path.2231. [DOI] [PubMed] [Google Scholar]
- 37.Pandol SJ, Raraty M. Pathobiology of alcoholic pancreatitis. Pancreatology. 2007;7:105–114. doi: 10.1159/000104235. [DOI] [PubMed] [Google Scholar]
- 38.Erkan M, Adler G, Apte MV, et al. StellaTUM: Current consensus and discussion on pancreatic stellate cell research. Gut. 2012;61:172–178. doi: 10.1136/gutjnl-2011-301220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Omary MB, Lugea A, Lowe AW, et al. The pancreatic stellate cell: A star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50–59. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pandol S, Gukovskaya A, Edderkoui M, et al. Epidemiology, risk factors, and the promotion of pancreatic cancer: Role of the stellate cell. J Gastroenterol Hepatol. 2012;27:127–134. doi: 10.1111/j.1440-1746.2011.07013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masamune A, Shimosegawa T. Pancreatic stellate cells - Multi-functional cells in the pancreas. Pancreatology. 2013;13:102–105. doi: 10.1016/j.pan.2012.12.058. [DOI] [PubMed] [Google Scholar]
- 42.Erkan M. The role of pancreatic stellate cells in pancreatic cancer. Pancreatology. 2013;13:106–109. doi: 10.1016/j.pan.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Andoh A, Takaya H, Saotome T, et al. Cytokine regulation of chemokine (IL-8, MCP-1, and RANTES) gene expression in human pancreatic periacinar myofibroblasts. Gastroenterology. 2000;119:211–219. doi: 10.1053/gast.2000.8538. [DOI] [PubMed] [Google Scholar]
- 44.Masamune A, Kikuta K, Watanabe T, et al. Pancreatic stellate cells express Toll-like receptors. J Gastroenterol. 2008;43:352–362. doi: 10.1007/s00535-008-2162-0. [DOI] [PubMed] [Google Scholar]
- 45.Apte MV, Haber PS, Applegate TL, et al. Periacinar stellate shaped cells in rat pancreas: Identification, isolation, and culture. Gut. 1998;43:128–133. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bachem MG, Schneider E, Gross H, et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421–432–491–492. doi: 10.1016/s0016-5085(98)70209-4. [DOI] [PubMed] [Google Scholar]
- 47.Charo C, Holla V, Arumugam T, et al. Prostaglandin E2 Regulates Pancreatic Stellate Cell Activity Via the EP4 Receptor. Pancreas. 2012 doi: 10.1097/MPA.0b013e318264d0f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hama K, Ohnishi H, Yasuda H, et al. Angiotensin II stimulates DNA synthesis of rat pancreatic stellate cells by activating ERK through EGF receptor transactivation. Biochem Biophys Res Commun. 2004;315:905–911. doi: 10.1016/j.bbrc.2004.01.155. [DOI] [PubMed] [Google Scholar]
- 49.Reinehr R, Zoller S, Klonowski-Stumpe H, et al. Effects of angiotensin II on rat pancreatic stellate cells. Pancreas. 2004;28:129–137. doi: 10.1097/00006676-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Yamada T, Kuno A, Masuda K, et al. Candesartan, an angiotensin II receptor antagonist, suppresses pancreatic inflammation and fibrosis in rats. J Pharmacol Exp Ther. 2003;307:17–23. doi: 10.1124/jpet.103.053322. [DOI] [PubMed] [Google Scholar]
- 51.Berna MJ, Seiz O, Nast JF, et al. CCK1 and CCK2 receptors are expressed on pancreatic stellate cells and induce collagen production. J Biol Chem. 2010;285:38905–38914. doi: 10.1074/jbc.M110.125534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phillips PA, Yang L, Shulkes A, et al. Pancreatic stellate cells produce acetylcholine and may play a role in pancreatic exocrine secretion. Proc Natl Acad Sci U S A. 2010;107:17397–17402. doi: 10.1073/pnas.1000359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tashiro M, Ernst SA, Edwards J, et al. Hyperthermia induces multiple pancreatic heat shock proteins and protects against subsequent arginine-induced acute pancreatitis in rats. Digestion. 2002;65:118–126. doi: 10.1159/000057713. [DOI] [PubMed] [Google Scholar]
- 54.Akimoto T, Nakama K, Katsuta Y, et al. Characterization of a novel congenic strain of diabetic fatty (WBN/Kob-Lepr(fa)) rat. Biochem Biophys Res Commun. 2008;366:556–562. doi: 10.1016/j.bbrc.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Ohashi K, Kim JH, Hara H, et al. WBN/Kob rats. A new spontaneously occurring model of chronic pancreatitis. Int J Pancreatol. 1990;6:231–247. [PubMed] [Google Scholar]
- 56.Nakamura T, Ito T, Oono T, et al. Bacterial DNA promotes proliferation of rat pancreatic stellate cells thorough toll-like receptor 9: potential mechanisms for bacterially induced fibrosis. Pancreas. 2011;40:823–831. doi: 10.1097/MPA.0b013e318224a501. [DOI] [PubMed] [Google Scholar]
- 57.Nakamura T, Ito T, Igarashi H, et al. Cytosolic double-stranded DNA as a damage-associated molecular pattern induces the inflammatory response in rat pancreatic stellate cells: a plausible mechanism for tissue injury-associated pancreatitis. Int J Inflam. 2012:504128. doi: 10.1155/2012/504128. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White GE, Tan TC, John AE, et al. Fractalkine has anti-apoptotic and proliferative effects on human vascular smooth muscle cells via epidermal growth factor receptor signalling. Cardiovasc Res. 2010;85:825–835. doi: 10.1093/cvr/cvp341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.KAMADA K, MASHIMA H, GOTO T, et al. MCP-1 INHIBITS DNA SYNTHESIS IN RAT PANCREATIC STELLATE CELLS. Akita journal of medicine. 2009;36:185–194. [Google Scholar]
- 60.Marra F. Renaming cytokines: MCP-1, major chemokine in pancreatitis. Gut. 2005;54:1679–1681. doi: 10.1136/gut.2005.068593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhatia M, Ramnath RD, Chevali L, et al. Treatment with bindarit, a blocker of MCP-1 synthesis, protects mice against acute pancreatitis. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2005;288:G1259–G1265. doi: 10.1152/ajpgi.00435.2004. [DOI] [PubMed] [Google Scholar]
- 62.Cavestro GM, Zuppardo RA, Bertolini S, et al. Connections between genetics and clinical data: Role of mcp-1, cftr, and spink-1 in the setting of acute, acute recurrent, and chronic pancreatitis. Am J Gastroenterol. 2010;105:199–206. doi: 10.1038/ajg.2009.611. [DOI] [PubMed] [Google Scholar]
- 63.Ishibashi T, Zhao H, Kawabe K, et al. Blocking of monocyte chemoattractant protein-1 (MCP-1) activity attenuates the severity of acute pancreatitis in rats. J Gastroenterol. 2008;43:79–85. doi: 10.1007/s00535-007-2126-9. [DOI] [PubMed] [Google Scholar]
- 64.Bhatia M, Brady M, Zagorski J, et al. Treatment with neutralising antibody against cytokine induced neutrophil chemoattractant (CINC) protects rats against acute pancreatitis associated lung injury. Gut. 2000;47:838–844. doi: 10.1136/gut.47.6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sugita H, Yamaguchi Y, Ikei S, et al. Effects of propentofylline on tumor necrosis factor-α and cytokine-induced neutrophil chemoattractant production in rats with cerulein-induced pancreatitis and endotoxemia. Pancreas. 1997;14:267–275. doi: 10.1097/00006676-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 66.Sugita H, Yamaguchi Y, Ikei S, et al. Enhanced expression of cytokine-induced neutrophil chemoattractant (CINC) by bronchoalveolar macrophages in cerulein-induced pancreatitis rats. Dig Dis Sci. 1997;42:154–160. doi: 10.1023/a:1018809810561. [DOI] [PubMed] [Google Scholar]
- 67.Yamaguchi Y, Okabe K, Liang J, et al. The novel carboxamide derivative IS-741 reduces neutrophil chemoattractant production by bronchoalveolar macrophages in rats with cerulein-induced pancreatitis complicated by sepsis. Digestion. 1999;60:52–56. doi: 10.1159/000051454. [DOI] [PubMed] [Google Scholar]
- 68.Meiser A, Mueller A, Wise EL, et al. The chemokine receptor CXCR3 is degraded following internalization and is replenished at the cell surface by de novo synthesis of receptor. Journal of Immunology. 2008;180:6713–6724. doi: 10.4049/jimmunol.180.10.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Y, Foudi A, Geay J-, et al. Intracellular localization and constitutive endocytosis of CXCR4 in human CD34+ hematopoietic progenitor cells. Stem Cells. 2004;22:1015–1029. doi: 10.1634/stemcells.22-6-1015. [DOI] [PubMed] [Google Scholar]
- 70.Abe N, Watanabe T, Suzuki Y, et al. An increased high-mobility group A2 expression level is associated with malignant phenotype in pancreatic exocrine tissue. Br J Cancer. 2003;89:2104–2109. doi: 10.1038/sj.bjc.6601391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geismann C, Morscheck M, Koch D, et al. Up-regulation of L1CAM in pancreatic duct cells is transforming growth factor beta1- and slug-dependent: role in malignant transformation of pancreatic cancer. Cancer Res. 2009;69:4517–4526. doi: 10.1158/0008-5472.CAN-08-3493. [DOI] [PubMed] [Google Scholar]
- 72.Miyatsuka T, Kaneto H, Shiraiwa T, et al. Persistent expression of PDX-1 in the pancreas causes acinar-to-ductal metaplasia through Stat3 activation. Genes Dev. 2006;20:1435–1440. doi: 10.1101/gad.1412806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schussler MH, Skoudy A, Ramaekers F, et al. Intermediate filaments as differentiation markers of normal pancreas and pancreas cancer. Am J Pathol. 1992;140:559–568. [PMC free article] [PubMed] [Google Scholar]
- 74.Strobel O, Dor Y, Alsina J, et al. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology. 2007;133:1999–2009. doi: 10.1053/j.gastro.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neuschwander-Tetri BA, Bridle KR, Wells LD, et al. Repetitive acute pancreatic injury in the mouse induces procollagen alpha1(I) expression colocalized to pancreatic stellate cells. Lab Invest. 2000;80:143–150. doi: 10.1038/labinvest.3780018. [DOI] [PubMed] [Google Scholar]
- 76.Gonzalez AM, Garcia T, Samper E, et al. Assessment of the protective effects of oral tocotrienols in arginine chronic-like pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2011;301:G846–G855. doi: 10.1152/ajpgi.00485.2010. [DOI] [PubMed] [Google Scholar]
- 77.Shimizu K, Kobayashi M, Tahara J, et al. Cytokines and peroxisome proliferator-activated receptor gamma ligand regulate phagocytosis by pancreatic stellate cells. Gastroenterology. 2005;128:2105–2118. doi: 10.1053/j.gastro.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 78.Kuno A, Yamada T, Masuda K, et al. Angiotensin-converting enzyme inhibitor attenuates pancreatic inflammation and fibrosis in male Wistar Bonn/Kobori rats. Gastroenterology. 2003;124:1010–1019. doi: 10.1053/gast.2003.50147. [DOI] [PubMed] [Google Scholar]
- 79.Borroni EM, Mantovani A, Locati M, et al. Chemokine receptors intracellular trafficking. Pharmacology and Therapeutics. 2010;127:1–8. doi: 10.1016/j.pharmthera.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 80.Wynne AM, Henry CJ, Huang Y, et al. Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav Immun. 2010;24:1190–1201. doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klonowski-Stumpe H, Reinehr R, Fischer R, et al. Production and effects of endothelin-1 in rat pancreatic stellate cells. Pancreas. 2003;27:67–74. doi: 10.1097/00006676-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 82.Masamune A, Kikuta K, Satoh M, et al. Protease-activated receptor-2-mediated proliferation and collagen production of rat pancreatic stellate cells. J Pharmacol Exp Ther. 2005;312:651–658. doi: 10.1124/jpet.104.076232. [DOI] [PubMed] [Google Scholar]
- 83.White GE, Tan TCC, John AE, et al. Fractalkine has anti-apoptotic and proliferative effects on human vascular smooth muscle cells via epidermal growth factor receptor signalling. Cardiovasc Res. 2010;85:825–835. doi: 10.1093/cvr/cvp341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park J, Song KH, Ha H. Fractalkine increases mesangial cell proliferation through reactive oxygen species and mitogen-activated protein kinases. Transplant Proc. 2012;44:1026–1028. doi: 10.1016/j.transproceed.2012.03.045. [DOI] [PubMed] [Google Scholar]
- 85.Volin MV, Huynh N, Klosowska K, et al. Fractalkine-induced endothelial cell migration requires MAP kinase signaling. Pathobiology. 2010;77:7–16. doi: 10.1159/000272949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meucci O, Fatatis A, Simen AA, et al. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Popovic M, Laumonnier Y, Burysek L, et al. Thrombin-induced expression of endothelial CX3CL1 potentiates monocyte CCL2 production and transendothelial migration. J Leukoc Biol. 2008;84:215–223. doi: 10.1189/jlb.0907652. [DOI] [PubMed] [Google Scholar]
- 88.Maciejewski-Lenoir D, Chen S, Feng L, et al. Characterization of fractalkine in rat brain cells: migratory and activation signals for CX3CR-1-expressing microglia. J Immunol. 1999;163:1628–1635. [PubMed] [Google Scholar]
- 89.Hatori K, Nagai A, Heisel R, et al. Fractalkine and fractalkine receptors in human neurons and glial cells. J Neurosci Res. 2002;69:418–426. doi: 10.1002/jnr.10304. [DOI] [PubMed] [Google Scholar]
- 90.Guo X, Pan Y, Xiao C, et al. Fractalkine stimulates cell growth and increases its expression via NF-kappaB pathway in RA-FLS. Int J Rheum Dis. 2012;15:322–329. doi: 10.1111/j.1756-185X.2012.01721.x. [DOI] [PubMed] [Google Scholar]
- 91.Gaudin F, Nasreddine S, Donnadieu AC, et al. Identification of the chemokine CX3CL1 as a new regulator of malignant cell proliferation in epithelial ovarian cancer. PLoS One. 2011;6:e21546. doi: 10.1371/journal.pone.0021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sakurai T, Kudo M, Fukuta N, et al. Involvement of angiotensin II and reactive oxygen species in pancreatic fibrosis. Pancreatology. 2011;11(Suppl 2):7–13. doi: 10.1159/000323478. [DOI] [PubMed] [Google Scholar]
- 93.Masamune A, Satoh M, Kikuta K, et al. Endothelin-1 stimulates contraction and migration of rat pancreatic stellate cells. World J Gastroenterol. 2005;11:6144–6151. doi: 10.3748/wjg.v11.i39.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee YS, Morinaga H, Kim JJ, et al. The fractalkine/CX3CR1 system regulates beta cell function and insulin secretion. Cell. 2013;153:413–425. doi: 10.1016/j.cell.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kikuta K, Masamune A, Hamada S, et al. Pancreatic stellate cells reduce insulin expression and induce apoptosis in pancreatic beta-cells. Biochem Biophys Res Commun. 2013;433:292–297. doi: 10.1016/j.bbrc.2013.02.095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.