Abstract
Background
Proton pump inhibitors (PPIs) may reduce the risk of esophageal adenocarcinoma (EAC) in patients with Barrett’s esophagus. PPIs are prescribed for virtually all patients with Barrett’s esophagus, irrespective of the presence of reflux symptoms, and represent a de facto chemopreventive agent in this population. However, long-term PPI use has been associated with several adverse effects, and the cost-effectiveness of chemoprevention with PPIs has not been evaluated.
Aim
The purpose of this study was to assess the cost-effectiveness of PPIs for the prevention of EAC in Barrett’s esophagus without reflux.
Methods
We designed a state-transition Markov micro-simulation model of a hypothetical cohort of 50-year-old white men with Barrett’s esophagus. We modeled chemoprevention with PPIs or no chemoprevention, with endoscopic surveillance for all treatment arms. Outcome measures were life-years, quality-adjusted life years (QALYs), incident EAC cases and deaths, costs, and incremental cost-effectiveness ratios.
Results
Assuming 50 % reduction in EAC, chemoprevention with PPIs was a cost-effective strategy compared to no chemoprevention. In our model, administration of PPIs cost $23,000 per patient and resulted in a gain of 0.32 QALYs for an incremental cost-effectiveness ratio of $12,000/QALY. In sensitivity analyses, PPIs would be cost-effective at $50,000/QALY if they reduce EAC risk by at least 19 %.
Conclusions
Chemoprevention with PPIs in patients with Barrett’s esophagus without reflux is cost-effective if PPIs reduce EAC by a minimum of 19 %. The identification of subgroups of Barrett’s esophagus patients at increased risk for progression would lead to more cost-effective strategies for the prevention of esophageal adenocarcinoma.
Keywords: Cost-effectiveness, Proton pump inhibitors, Barrett’s esophagus, Esophageal adenocarcinoma, Clostridium difficile infection, Pharmacoepidemiology, Chemoprevention
Introduction
The incidence of esophageal adenocarcinoma (EAC) has risen dramatically over the past four decades in western countries [1, 2]. The prognosis of this cancer remains extremely poor, with a 5-year survival rate of 16 % in the United States [3]. Barrett’s esophagus (BE) is the established precursor to EAC, and the rate of progression of BE to adenocarcinoma is 0.1–0.5 % per year [4–7]. One of the mainstays of BE management is regular endoscopic surveillance [8]. The aim of surveillance is to identify patients at a preclinical or asymptomatic early stage of cancer and initiate treatment leading to improved long-term outcomes. However, it is unclear whether surveillance alone leads to reduced mortality from EAC [9].
In light of the poor outcomes associated with EAC, combined with the presence of a readily identifiable precursor lesion, Barrett’s esophagus represents an attractive target for chemoprevention. Because the absolute risk of EAC is very low even in BE patients [4–6], a viable chemoprevention strategy would have to be safe, inexpensive, and effective. Gastroesophageal reflux (GERD) is a primary risk factor for EAC [10], and several epidemiologic studies suggest that gastric acid suppression with proton pump inhibitors (PPIs) has chemopreventive effects in patients with BE [11–14]. A recent meta-analysis of these studies reported that PPI use in BE patients was associated with a 71 % reduced risk of progression to high-grade dysplasia (HGD) or EAC [15]. While clinical guidelines do not formally recommend gastric acid suppression as a means of cancer risk reduction for patients with BE, in clinical practice, PPIs have become a de facto chemopreventive agent [16]. Currently, between 95 and 98 % of patients with BE are prescribed PPIs [4, 17]. However, 30–50 % of BE patients do not have regular reflux symptoms yet are still prescribed PPIs [18–22]. Furthermore, novel, less invasive diagnostic techniques for BE such as a cytological sponge or transnasal endoscopy have the potential to increase the proportion of asymptomatic BE patients [23, 24].
Proton pump inhibitors (PPIs) have historically been considered safe medications. However, recent observational data suggest that chronic PPI use is associated with increased risks of bone fractures and of Clostridium difficile infection [25–29]. Based on these data, the Food and Drug Administration has issued warnings regarding long-term PPIs and bone fracture and PPIs and C. difficile infection [30, 31]. Despite these concerns, practitioners continue to prescribe PPIs to virtually all BE patients. To date, no formal quantitative analysis has been published to support the use of PPIs for the prevention of EAC. We therefore constructed a decision-analytic model to weigh the benefit of PPIs against their adverse effects and to evaluate the cost-effectiveness of PPIs as chemoprevention for EAC in BE patients without GERD. Using this model, we determined the threshold for the efficacy of PPIs to be cost-effective at common cost-effectiveness benchmarks and, assuming 50 % efficacy in reducing progression of BE, the incremental cost-effectiveness ratio for PPIs.
Methods
Patient Population and Time Frame
We modeled a hypothetical cohort of 250,000 50-year-old white men newly diagnosed with non-dysplastic BE at baseline until they reached age 80 or died, whichever occurred first. This cohort was chosen because white men of this age range represent the demographic most at risk for EAC [1]. Non-dysplastic BE was defined by both the American College of Gastroenterology and the American Gastroenterological Association definitions of endoscopically suspected Barrett’s esophagus combined with the presence of intestinal metaplasia on esophageal biopsies [8, 32]. This study was approved by the institutional review board of Columbia University.
Strategies
We modeled two different strategies: no chemoprevention (comparator) and chemoprevention with PPIs. Endoscopic surveillance represents the current standard of care for BE [8] and was incorporated into all of the strategies. All patients in the PPI chemoprevention arm received a oncedaily dose of PPI. While a recent meta-analysis reported that PPI use is associated with a 71 % reduction in the risk of HGD or EAC in BE patients [15], we chose a more conservative 50 % risk reduction for EAC and varied this estimate widely (0–100 %) in the sensitivity analysis.
In our analysis, we assumed that patients without dysplasia would undergo endoscopic surveillance every three years and patients with low-grade dysplasia every year until no dysplasia was detected [8]. Patients with highgrade dysplasia underwent radiofrequency ablation, now endorsed as a preferred management strategy [32]. Successful ablation was followed by an endoscopy four times a year in the first year, twice in the following year, and then yearly thereafter. This schedule was derived from recent guidelines [32, 33] as well as from the post-ablation protocol from the AIM-Dysplasia Trial of radiofrequency ablation for BE with low- and high-grade dysplasia [34]. For all patients, endoscopic surveillance continued until reaching 80 years of age or death. If EAC was diagnosed, patients were considered for surgical resection; we assumed that 15 % of these cases would be unresectable [35, 36] and that patients would then undergo palliative therapy.
Markov Model
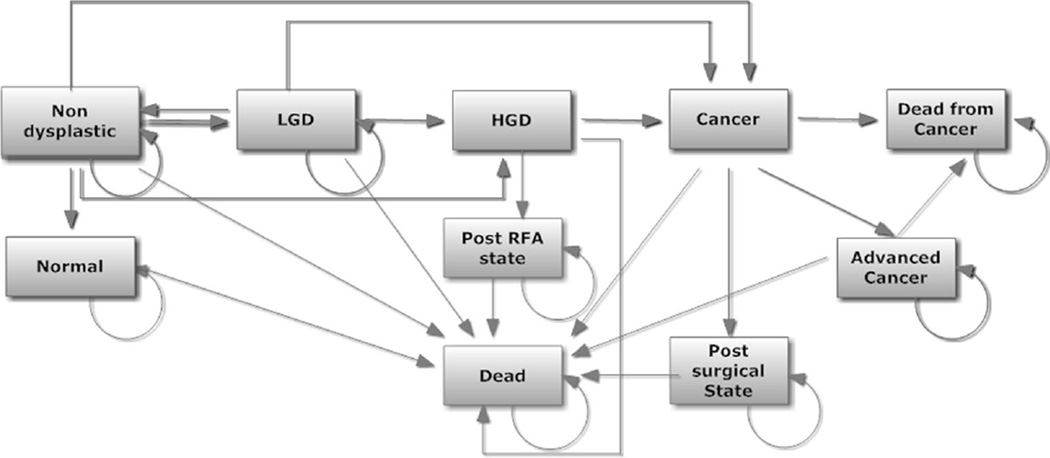
A state-transition Markov microsimulation model (TreeAge Pro 2011, Williamstown, MA) was constructed to simulate the disease progression, outcomes, and costs incurred under each strategy. At the end of each annual cycle, each simulated patient faced a probability of transition to another state (Fig. 1). All patients started in the BE without dysplasia state at baseline. Each state was assigned a state-specific cost and utility per year. The model also included events such as surgical mortality, morbidity from both radiofrequency ablation (RFA) and surgery, and risk of recurrent cancer.
Fig. 1.
Markov model, with the various health and disease states, each associated with a different set of utilities
Transition probabilities, cost, and utility weights were obtained from the published literature (Table 1). In our base case, a 50 % reduction in the risk of EAC was assumed for patients who received PPIs. The model included the two primary adverse effects of PPI use: increased risk of bone fractures and increased risk of C. difficile infection. In the PPI strategy, the rate of bone fracture was assumed to increase after 2 years of use; increase in C. difficile infection, on the other hand, was assumed to occur from the outset. The model also considered that a proportion of community-acquired C. difficile cases would result in hospitalization [37, 38]. Mortality from EAC and from other causes was derived from the Surveillance, Epidemiology, and End Results (SEER) database, adjusted for age and sex [39].
Table 1.
Estimates of modeled variables
| Description | Base | Low | High |
|---|---|---|---|
| Cost (2010 US$) | |||
| Cost of cancer | $49,523 | $10,522 | $52,620 |
| Cost of surveillance endoscopy | $932 | $350 | $1,100 |
| Cost of surgical morbidity | $35,870 | $17,230 | $70,934 |
| Cost of cancer palliation | $1,652 | $1,000 | $5,066 |
| Cost of RFAa | $22,818 | $10,638 | $45,600 |
| Cost of esophagectomy | $24,994 | $10,000 | $40,000 |
| Cost of generic PPI (annually) | $360 | $76 | $700 |
| Cost of bone fracture | $11,000 | $7,800 | $19,000 |
| Cost of C. difficile treatmentb | $120 | $60 | $1,200 |
| Cost of complicated C. difficile | $10,000 | $5,000 | $26,338 |
| Discount rate | 0.03 | 0.00 | 0.05 |
| Transition rates | |||
| ND BE to LGD | 0.03 | 0.01 | 0.08 |
| ND BE to HGD | 0.0055 | 0.0028 | 0.07 |
| ND BE to cancer | 0.0035 | 0.002 | 0.01 |
| LGD to ND BE | 0.50 | 0.45 | 0.80 |
| LGD to HGD | 0.1 | 0.01 | 0.2 |
| LGD to cancer | 0.015 | 0.005 | 0.09 |
| HGD to cancerc | 0.06 | 0.05 | 1.0 |
| HGD to ND BE post-RFA | 0.94 | 0.88 | 0.97 |
| Mortality in unresectable cancer | 0.9 | 0.8 | 1 |
| Mortality from other causes | Varies with age | ||
| Efficacy (proportion of EAC cases prevented) | |||
| PPIs | 0.50 | 0 | 100 |
| Complications of therapy | |||
| Mortality from EGD | 0.000021 | 0 | 0.00005 |
| Mortality from esophagectomy | 0.05 | 0.025 | 0.1 |
| Morbidity from esophagectomy | 0.15 | 0.05 | 0.4 |
| Morbidity from esophagectomy after perforation | 0.2 | 0.1 | 0.5 |
| Perforation with RFA | 0.0005 | 0.0001 | 0.001 |
| Stricture with RFA | 0.025 | 0.01 | 0.05 |
| Rate of fractures in PPI users | 0.00014 | 0.0001 | 0.0009 |
| Rate of fractures in nonusers | 0.0001 | 0.00005 | 0.00015 |
| Rate of C. difficile in PPI users | 0.00018 | 0.0001 | 0.00038 |
| Rate of C. difficile in nonusers | 0.00008 | 0.00005 | 0.0001 |
| Proportion of complicated C difficile infectionsd | 0.05 | 0.025 | 0.1 |
| Utilities | |||
| Utility of BE without dysplasia | 1 | 0.79 | 1 |
| Utility of LGD | 1 | 0.8 | 1 |
| Utility of HGD | 0.9 | 0.6 | 1 |
| Utility after RFA | 0.95 | 0.6 | 1 |
| Utility after esophagectomy | 0.8 | 0 | 1 |
| Utility of cancer | 0.5 | 0 | 1 |
| Utility of fracture | 0.79 | 0.7 | 0.95 |
| Utility of C. difficile | 0.998 | 0.997 | 0.999 |
| Utility of complicated C. difficile | 0.88 | 0.8 | 0.95 |
Represents cost of three RFA sessions
14-day course of metronidazole and/or vancomycin
Base rate for HGD to cancer based on the progression rate of non-responders to RFA
Proportion of community-acquired C. difficile cases resulting in hospitalization
Costs were analyzed from the third-party payers’ perspective. Only direct medical costs were considered, and all costs were expressed in 2011 US dollars [40]. Procedure costs were estimated based on the 2011 national average Medicare reimbursement rate [41] and included the cost of the procedure as well as professional and facility fees [41, 42]. Medication costs were derived from the Pharmacy Redbook and Internet retail sources, based on the average Redbook pricing for generic omeprazole 20 mg daily [43]. Treatment costs for fracture and C. difficile management were taken from the published literature [44, 45]. Utility weights for health states and events were directed from the literature, ranging from 1 (perfect health) to 0 (death). These weights were aggregated to estimate quality-adjusted life years (QALYs) for each modeled patient under different clinical strategies [46].
Cost-Effectiveness Analysis
The primary outcome of the study was the incremental cost per QALY gained, also known as the incremental cost-effectiveness ratio (ICER). The ICER is calculated by dividing the difference in costs by the difference in average QALY between the two strategies. Both costs and QALYs were discounted at an annual rate of 3 % [46]. We evaluated the ICER on two often-cited benchmark willingness-to-pay levels: $50,000/QALY and $100,000/QALY.
Sensitivity Analysis
Sensitivity analyses were performed to investigate the effects of parameter uncertainties on the resulting cost and effectiveness outcomes [46]. Probabilistic sensitivity analysis was performed by simultaneously and probabilistically varying costs, probabilities, quality of life weights, and discount rates. Ranges were based on published 95 % confidence intervals (CIs); in their absence, we varied the parameter from 50 to 200 % of its base-case value.
Results
Base-Case Results
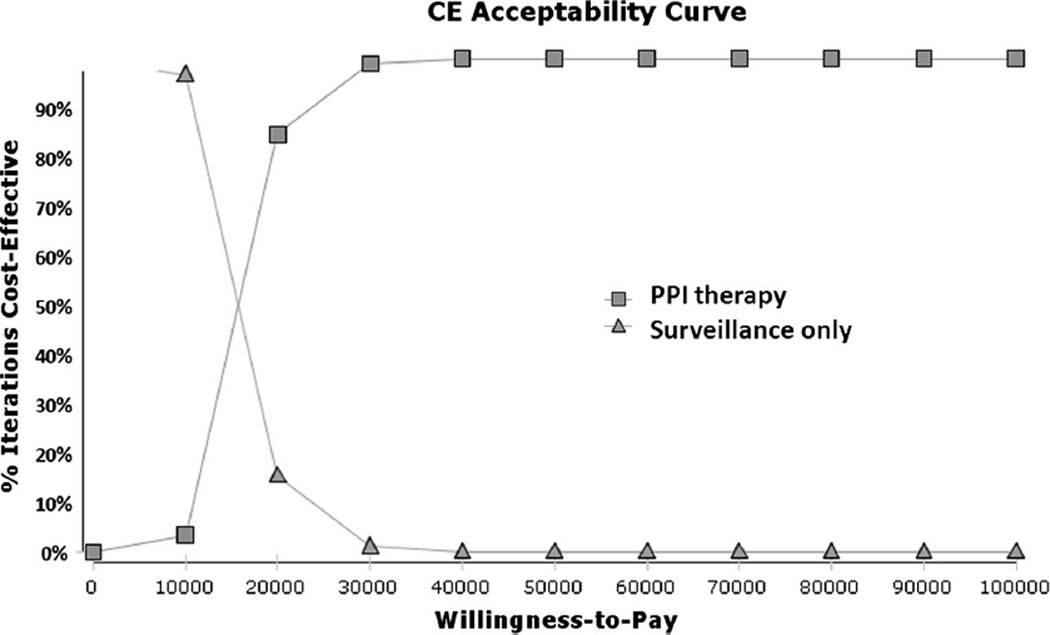
Consistent with previously published estimates [47, 48], our model estimated a 5.6 % lifetime risk of EAC for this non-dysplastic BE cohort with an average life expectancy of 19.5 years. In the absence of chemoprevention, 14,000 esophageal adenocarcinomas were expected to develop among the simulated 250,000 50-year-old patients with non-dysplastic BE who received the current standard of care with endoscopic surveillance. Use of PPIs proved to be a cost-effective strategy. Administration of PPIs as chemoprevention resulted in a gain of 0.32 QALYs at a total cost of $23,495 per patient, with an ICER of $11,760 per QALY (Table 2). Greater than 95 % of the simulations showed that the strategy that involved PPIs was the most cost-effective at $50,000/QALY (Fig. 2).
Table 2.
Baseline analysis. Endoscopic surveillance is performed in both scenarios, and proton pump inhibitors are assumed to reduce the risk of esophageal adenocarcinoma by 50 %
| Strategy | Cost ($) |
Effectiveness (dQALY) |
Incremental cost ($) |
Incremental effectiveness (dQALY) |
ICER ($/dQALY) |
Cancers per 100 NDBE |
|---|---|---|---|---|---|---|
| No chemoprevention | 19,789 | 19.02 | 0 | 0 | 0 | 5.6 |
| PPIs | 23,495 | 19.33 | 3,706 | 0.32 | 11,760 | 2.3 |
Fig. 2.
Monte Carlo simulation with the optimal strategy stratified by WTP in patients with NDBE. The lines illustrate the proportion of trials in which each strategy was calculated to comprise the optimal strategy, defined as the strategy associated with the greatest QALYS obtainable with a corresponding WTP
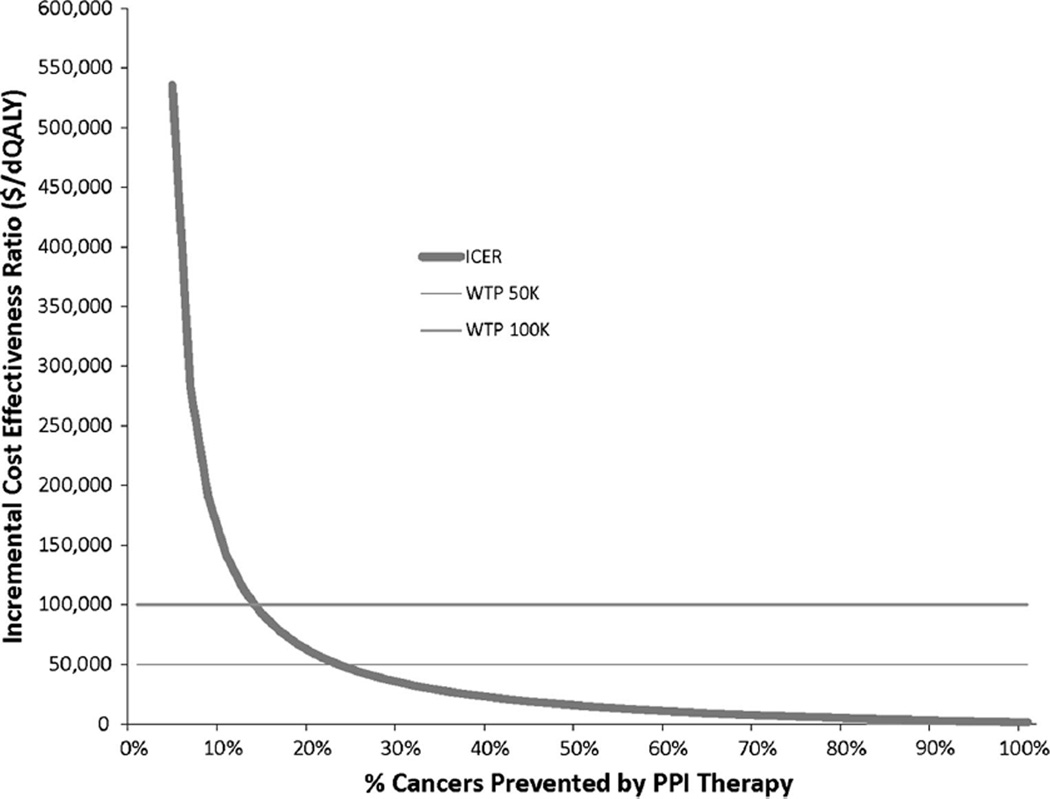
Minimum Cost-Effective Chemopreventive Effect
Proton pump inhibitors (PPIs) remained cost-effective if they reduced EAC risk by ≥ 19 % at $50,000/QALY and by ≥11 % at $100,000/QALY (Fig. 3). For lower effectiveness of PPIs in preventing EAC, surveillance alone represented the dominant strategy. Under our base-case assumptions, PPIs remained cost-effective up to an annual cost of $940 at $50,000/QALY and $1,660 for $100,000/ QALY (Supplemental Figure 1).
Fig. 3.
One-way sensitivity analysis with risk reduction in chemoprevention with proton pump inhibitors
Discussion
Our study shows that chemoprevention with proton pump inhibitors for patients with Barrett’s esophagus without GERD could provide significant risk reduction in esophageal adenocarcinoma with acceptable costs. The incidence of EAC has been rising rapidly for the past four decades [1, 2], and interventions to lower the risk of EAC have the potential for a major impact at the population level. Our Markov model’s base case assumed a 50 % reduction in EAC risk for patients who received PPIs. This assumption correlated to an absolute 3.3 % reduction in lifetime EAC risk among patients with non-dysplastic BE. Although the absolute risk of neoplastic progression in BE is low [4–6, 49–51], our model indicates that PPIs are cost-effective in asymptomatic BE patients as long as the drugs remain inexpensive and current estimates of PPI-related risks remain unchanged. Our results lend support to the current practice of prescribing PPI therapy to all patients with BE, irrespective of the presence of acid reflux symptoms.
In order to appropriately interpret the significance of these findings, one must consider the variable clinical presentation of patients with BE. Many patients with BE have chronic reflux symptoms; PPIs have obvious therapeutic value in this group and therefore have benefits beyond potential EAC risk reduction. However, in two studies aimed at determining BE prevalence, 44–54 % of Barrett’s patients denied a history of regular reflux symptoms [21, 22], and the results of a meta-analysis found no association between GERD and short-segment BE [52]. Despite this, recent cohort studies report that 95–98 % of BE patients under surveillance are prescribed PPIs [4, 53]. This discrepancy is due to the belief among physicians that acid suppression may reduce the risk of progression to EAC [54].
What is the evidence that acid suppression with PPIs prevents EAC? While clinical trial data are lacking, epi-demiologic studies suggest that PPI use in patients with BE has chemopreventive effects [12–14, 55]. In a study by El-Serag et al. [13] of veterans with BE, PPI use (compared to histamine-2 antagonists and no acid suppression) had a lower risk of progression to dysplasia (HR 0.25, 95 %CI 0.13–0.47). In a case–control study from the Netherlands, patients with EAC were less likely to have used PPIs (OR 0.1, 95 % CI 0.05–0.2) compared to BE patients with no dysplasia or low-grade dysplasia [14]. A recent meta-analysis including data from 2,813 patients with BE concluded that PPI use was associated with a 71 % decreased risk of progression to EAC or high-grade dysplasia [15]. Laboratory data also suggest that gastric acid reflux promotes cancer in BE via increased cellular proliferation and decreased apoptosis [56–58].
Our model demonstrates that PPIs must prevent 19 % of EACs in order to remain cost-effective. This moderately low threshold for efficacy is driven largely by the low cost of generic PPIs. Prior to the FDA approval of generic omeprazole in 2002, the cost of brand PPIs was roughly $1,000–1,500 per year [59]. PPIs at this cost would have needed to prevent 60–80 % of EACs to remain a cost-effective strategy. Therefore, market cost is a key factor in the evaluation of any chemopreventive drug in BE, including PPIs. Other potential chemopreventive agents that have been studied or are currently under investigation for BE include celecoxib, aspirin, difluoromethylornithine, green tea extract, cholestyramine, and a gastrin-receptor inhibitor [60–63]. A new drug on patent will likely be associated with significant costs and will need to be extremely effective at preventing EAC to achieve standard benchmarks of cost-effectiveness.
The decision-analytic model in the present study was sensitive to both PPI efficacy and costs, both of drug and of adverse effects. The model included the two adverse effects of PPI use for which the best evidence exists: increased risks of bone fractures and of C. difficile infection. The association between PPIs and incident C. difficile infection is well established [64, 65] while the association between PPIs and bone fracture is more controversial [25, 66]. When the analysis was performed without incorporation of adverse effects, PPIs were cost-effective for risk reductions as low as 2 % (data not shown). Should additional adverse effects of long-term PPI use be discovered, then the minimum chemopreventive efficacy required for PPIs to remain cost-effective will increase.
Currently, all patients with non-dysplastic BE are treated with a “one-size-fits-all” approach. However, the risk of developing EAC is extremely low, which limits the cost-effectiveness of any strategy aimed at improving outcomes in this population. If the transition rates in the model are markedly increased, for example through the identification of a BE subgroup at high risk for progression, then the minimum required chemopreventive efficacy is substantially reduced. In the future, chemoprevention targeted to high-risk subgroups in which a particular agent provides maximal benefit will likely represent the optimal management strategy. Conversely, patients at lower risk to progress would derive the least benefit from chemoprevention.
Prior studies have examined the cost-effectiveness of chemoprevention in Barrett’s esophagus. All models must rely upon contemporaneous data, and transitional probabilities for progression of BE have decreased over time while drug cost estimates have simultaneously fallen. Sonnenberg and Fennerty [67] used Markov modeling to show that NSAID use without endoscopic surveillance was a cost-effective strategy. For patients with non-dys-plastic BE, the disease states consisted of BE, EAC, post-esophagectomy, or death, with dysplasia and endoscopic therapy not included in the model. When progression rates were reduced below 0.5 % per year in sensitivity analyses, chemoprophylaxis became prohibitively expensive. This is relevant given recent cohort studies suggesting that the rate of progression from BE to EAC may be closer to 0.1–0.3 % per year [4–6, 49–51], rather than the traditionally cited 0.5 % per year [7]. Hur et al. evaluated the cost-effectiveness of aspirin for the prevention of EAC [47]. This model included dysplastic states, and all patients with high-grade dysplasia were considered for esophagectomy. Aspirin was assigned a base efficacy of 50 % EAC risk reduction, and hemor-rhagic complications of aspirin were included in the model. The analyses showed that aspirin with and without endoscopic surveillance represented cost-effective strategies. Choi et al. [68] also recently demonstrated that chemoprevention with aspirin could be a cost-effective strategy when added to endoscopic surveillance for non-dysplastic BE, with a base-case assumption of 50 % reduction in EAC incidence with aspirin. Finally, comprehensive cost-effectiveness analyses of radiofrequency ablation show that RFA is a cost-effective strategy in dysplastic BE, although the strategy of RFA for non-dysplastic BE remains controversial [48, 69].
Our study has several strengths. Our model included the two primary major adverse effects of PPIs: increased risks of bone fractures and of C. difficile infection. These adverse effects play a key role in the clinical decision to prescribe long-term PPIs for the purpose of chemoprevention in BE. Because endoscopic surveillance currently represents the standard of care in BE, surveillance was incorporated into all arms of the study unlike prior models. Endoscopic therapy has been endorsed as the preferred management strategy for high-grade dysplasia [32] and was made a standard element in our model. Our model expands on prior work, incorporates recent data on progression rates for non-dysplastic BE, and uses recently published post-RFA utility data. Finally, our model uses sensitivity analyses to determine optimal cutoffs within categories and utility estimates based on the observed data among BE patients rather than on physician estimates. Although our model requires assumptions regarding the efficacy of chemoprevention with PPIs, our study is the first of its kind to provide evidence supporting the clinical practice of prescribing PPIs to patients with non-dysplastic BE without reflux.
The current analyses have some limitations. Our model data are drawn primarily from US sources, and our model may not be generalizable to other populations. In the construction of the model, a 50 % EAC risk reduction was assigned for PPIs. However, there is no clinical trial data on which to base this estimate, and epidemiologic data suffer from potential confounding by indication as well as by variable compliance rates. This underlying uncertainty was accounted for by varying the effect from 0 to 100 % in the sensitivity analyses. The model did not include disease regression (other than LGD to no dysplasia) or misdiagnosis because these factors would impact all study arms in a comparable fashion. Furthermore, the impact of these factors would likely have been relatively small, and our model produced lifetime incidence rates of EAC comparable to other models and to estimates in humans [4, 48].
Our study shows that the use of PPIs in patients with Barrett’s esophagus without reflux symptoms represents a cost-effective strategy for the prevention of esophageal adenocarcinoma and demonstrates that PPIs remain cost-effective at $50,000/QALY provided that they attain a minimum efficacy in preventing progression to EAC of 19 %. Our model was sensitive to rates of progression, efficacy of PPIs, and costs associated with adverse effects of PPIs. Clinical trial data are needed to better estimate the efficacy of PPIs or other chemopreventive agents in patients with Barrett’s esophagus. Future studies should identify subgroups of Barrett’s esophagus patients at increased risk for progression to facilitate risk-stratified management strategies including chemoprevention.
Supplementary Material
Acknowledgments
Dr. Sharaiha was supported by a training grant from the National Cancer Institute (T32 CA009529). Dr. Freedberg is supported in part by a training grant from the National Institute of Diabetes and Digestive and Kidney Diseases (T32 DK083256-04). Dr. Abrams was supported in part by a Career Development Award from the National Cancer Institute (K07 CA132892). This publication was also supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1 RR024156.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10620-014-3186-3) contains supplementary material, which is available to authorized users.
Conflict of interest None.
Contributor Information
Reem Z. Sharaiha, Email: rzs9001@med.cornell.edu, Division of Gastroenterology and Hepatology, Department of Medicine, Weill Cornell Medical College, 1305 York Avenue, 4th Floor, New York, NY 10021, USA.
Daniel E. Freedberg, Email: def2004@cumc.columbia.edu, Division of Digestive and Liver Diseases, Columbia University Medical Center, 630 West 168th Street, PH Building, 7th Floor, New York, NY 10032, USA.
Julian A. Abrams, Email: ja660@cumc.columbia.edu, Division of Digestive and Liver Diseases, Columbia University Medical Center, 630 West 168th Street, PH Building, 7th Floor, New York, NY 10032, USA.
Y. Claire Wang, Email: ycw2102@columbia.edu, Department of Health Policy and Management, Mailman School of Public Health, 722 West 168th Street, New York, NY 10032, USA.
References
- 1.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–1187. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrams JA, Sharaiha RZ, Gonsalves L, et al. Dating the rise of esophageal adenocarcinoma: analysis of Connecticut Tumor Registry data, 1940–2007. Cancer Epidemiol Biomarkers Prev. 2011;20:183–186. doi: 10.1158/1055-9965.EPI-10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Wani S, Falk G, Hall M, et al. Patients with nondysplastic Barrett’s esophagus have low risks for developing dysplasia or esophageal adenocarcinoma. Clin Gastroenterol Hepatol. 2011;9:220–227. doi: 10.1016/j.cgh.2010.11.008. quiz e26. [DOI] [PubMed] [Google Scholar]
- 5.Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375–1383. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 6.Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103:1049–1057. doi: 10.1093/jnci/djr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaheen NJ, Crosby MA, Bozymski EM, et al. Is there publication bias in the reporting of cancer risk in Barrett’s esophagus? Gastroenterology. 2000;119:333–338. doi: 10.1053/gast.2000.9302. [DOI] [PubMed] [Google Scholar]
- 8.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 9.Corley DA, Mehtani K, Quesenberry C, et al. Impact of endo-scopic surveillance on mortality from Barrett’s esophagus-associated esophageal adenocarcinomas. Gastroenterology. 2013;145 doi: 10.1053/j.gastro.2013.05.004. 312 e1–319 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lagergren J, Bergstrom R, Lindgren A, et al. Symptomatic gas-troesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 11.Jung KW, Talley NJ, Romero Y, et al. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett’s esophagus: a population-based study. Am J Gas-troenterol. 2011;106:1447–1455. doi: 10.1038/ajg.2011.130. quiz 1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillman LC, Chiragakis L, Shadbolt B, et al. Effect of proton pump inhibitors on markers of risk for high-grade dysplasia and oesophageal cancer in Barrett’s oesophagus. Aliment Pharmacol Ther. 2008;27:321–326. doi: 10.1111/j.1365-2036.2007.03579.x. [DOI] [PubMed] [Google Scholar]
- 13.El-Serag HB, Aguirre TV, Davis S, et al. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett’s esophagus. Am J Gastroenterol. 2004;99:1877–1883. doi: 10.1111/j.1572-0241.2004.30228.x. [DOI] [PubMed] [Google Scholar]
- 14.de Jonge PJ, Steyerberg EW, Kuipers EJ, et al. Risk factors for the development of esophageal adenocarcinoma in Barrett’s esophagus. Am J Gastroenterol. 2006;101:1421–1429. doi: 10.1111/j.1572-0241.2006.00626.x. [DOI] [PubMed] [Google Scholar]
- 15.Singh S, Garg SK, Singh PP, et al. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta-analysis. Gut. 2013 doi: 10.1136/gutjnl-2013-305997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spechler SJ. Barrett esophagus and risk of esophageal cancer: a clinical review. JAMA. 2013;310:627–636. doi: 10.1001/jama.2013.226450. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen DM, Richardson P, El-Serag HB. Medications (NSAIDs, statins, proton pump inhibitors) and the risk of esophageal ade-nocarcinoma in patients with Barrett’s esophagus. Gastroenterology. 2010;138:2260–2266. doi: 10.1053/j.gastro.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veldhuyzen van Zanten SJ, Thomson AB, Barkun AN, et al. The prevalence of Barrett’s oesophagus in a cohort of 1040 Canadian primary care patients with uninvestigated dyspepsia undergoing prompt endoscopy. Aliment Pharmacol Ther. 2006;23:595–599. doi: 10.1111/j.1365-2036.2006.02813.x. [DOI] [PubMed] [Google Scholar]
- 19.Voutilainen M, Sipponen P, Mecklin JP, et al. Gastroesophageal reflux disease: prevalence, clinical, endoscopic and histopathological findings in 1,128 consecutive patients referred for endos-copy due to dyspeptic and reflux symptoms. Digestion. 2000;61:6–13. doi: 10.1159/000007730. [DOI] [PubMed] [Google Scholar]
- 20.Abrams JA, Fields S, Lightdale CJ, et al. Racial and ethnic disparities in the prevalence of Barrett’s esophagus among patients who undergo upper endoscopy. Clin Gastroenterol Hepatol. 2008;6:30–34. doi: 10.1016/j.cgh.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gas-troenterology. 2005;129:1825–1831. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 22.Rex DK, Cummings OW, Shaw M, et al. Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology. 2003;125:1670–1677. doi: 10.1053/j.gastro.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 23.Peery AF, Hoppo T, Garman KS, et al. Feasibility, safety, acceptability, and yield of office-based, screening transnasal esophagoscopy (with video) Gastrointest Endosc. 2012;75:945–953. doi: 10.1016/j.gie.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadri SR, Lao-Sirieix P, O’Donovan M, et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. BMJ. 2010;341:c4372. doi: 10.1136/bmj.c4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang YX, Lewis JD, Epstein S, et al. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947–2953. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 26.Dial S, Delaney JA, Barkun AN, et al. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294:2989–2995. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 27.Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmeno-pausal women: results from the Women’s Health Initiative. Arch Intern Med. 2010;170:765–771. doi: 10.1001/archinternmed.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corley DA, Kubo A, Zhao W, et al. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology. 2010;139:93–101. doi: 10.1053/j.gastro.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365:1693–1703. doi: 10.1056/NEJMoa1012413. [DOI] [PubMed] [Google Scholar]
- 30.Food and Drug Administration. [Accessed October 15, 2013];U.S. Department of Health and Human Services. http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm213206.htm.
- 31.Food and Drug Administration. [Accessed October 15, 2013];U.S. Department of Health and Human Services. http://www.fda.gov/drugs/drugsafety/ucm290510.htm.
- 32.Spechler SJ, Sharma P, Souza RF, et al. American Gastroentero-logical Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084–1091. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 33.Spechler SJ, Sharma P, Souza RF, et al. American Gastroenter-ological Association technical review on the management of Barrett’s esophagus. Gastroenterology. 2011;140:e18–e52. doi: 10.1053/j.gastro.2011.01.031. (quiz e13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 35.Streitz JM, Jr, Andrews CW, Jr, Ellis FH., Jr Endoscopic surveillance of Barrett’s esophagus Does it help? J Thorac Cardiovasc Surg. 1993;105:383–387. [PubMed] [Google Scholar]
- 36.Corley DA, Levin TR, Habel LA, et al. Surveillance and survival in Barrett’s adenocarcinomas: a population-based study. Gastroenterology. 2002;122:633–640. doi: 10.1053/gast.2002.31879. [DOI] [PubMed] [Google Scholar]
- 37.Khanna S, Pardi DS, Aronson SL, et al. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol. 2012;107:89–95. doi: 10.1038/ajg.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173:1359–1367. doi: 10.1001/jamainternmed.2013.7056. [DOI] [PubMed] [Google Scholar]
- 39.National Cancer Institute. [Accessed October 15, 2013];Surveillance Epidemiology and End Results. http://seer.cancer.gov/
- 40.Gold M. Panel on cost-effectiveness in health and medicine. Med Care. 1996;34:197–199. [PubMed] [Google Scholar]
- 41.Centers for Medicare and Medicaid Services. [Accessed October 15, 2013];Physician Fee Schedule Search. http://www.cms.gov/apps/physician-fee-schedule/
- 42.Physician Fee Schedule Look-up. [Accessed September 1, 2011]; Available from: https://www.cms.gov/
- 43.Roberts KJ, Harper E, Alderson D, et al. Long-term survival and cost analysis of an annual Barrett’s surveillance programme. Eur J Gastroenterol Hepatol. 2010;22:399–403. doi: 10.1097/MEG.0b013e328331fc9c. [DOI] [PubMed] [Google Scholar]
- 44.Liu H, Michaud K, Nayak S, et al. The cost-effectiveness of therapy with teriparatide and alendronate in women with severe osteoporosis. Arch Intern Med. 2006;166:1209–1217. doi: 10.1001/archinte.166.11.1209. [DOI] [PubMed] [Google Scholar]
- 45.Lee BY, Popovich MJ, Tian Y, et al. The potential value of Clostridium difficile vaccine: an economic computer simulation model. Vaccine. 2010;28:5245–5253. doi: 10.1016/j.vaccine.2010.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hunink M, Glasziou P. Decision Making in Health and Medicine: Integrating Evidence and Values. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- 47.Hur C, Nishioka NS, Gazelle GS. Cost-effectiveness of aspirin chemoprevention for Barrett’s esophagus. J Natl Cancer Inst. 2004;96:316–325. doi: 10.1093/jnci/djh039. [DOI] [PubMed] [Google Scholar]
- 48.Inadomi JM, Somsouk M, Madanick RD, et al. A cost-utility analysis of ablative therapy for Barrett’s esophagus. Gastroen-terology. 2009;136 doi: 10.1053/j.gastro.2009.02.062. 2101 e1–6–2114 e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon LG, Mayne GC, Hirst NG, et al. Cost-effectiveness of endoscopic surveillance of non-dysplastic Barrett’s esophagus. Gastrointest Endosc. 2013;79:242–256. doi: 10.1016/j.gie.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 50.Desai TK, Krishnan K, Samala N, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett’s oesophagus: a meta-analysis. Gut. 2012;61:970–976. doi: 10.1136/gutjnl-2011-300730. [DOI] [PubMed] [Google Scholar]
- 51.Kastelein F, Spaander MC, Steyerberg EW, et al. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2013;11:382–388. doi: 10.1016/j.cgh.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 52.Taylor JB, Rubenstein JH. Meta-analyses of the effect of symptoms of gastroesophageal reflux on the risk of Barrett’s esophagus. Am J Gastroenterol. 2010;105:1729. doi: 10.1038/ajg.2010.194. 1730–7; quiz 1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laroui H, Dalmasso G, Nguyen HT, et al. Drug-loaded nano-particles targeted to the colon with polysaccharide hydrogel reduce colitis in a mouse model. Gastroenterology. 2010;138 doi: 10.1053/j.gastro.2009.11.003. 843e1-2-853e1-2. [DOI] [PubMed] [Google Scholar]
- 54.Chey WD, Inadomi JM, Booher AM, et al. Primary-care physicians’ perceptions and practices on the management of GERD: results of a national survey. Am J Gastroenterol. 2005;100:1237–1242. doi: 10.1111/j.1572-0241.2005.41364.x. [DOI] [PubMed] [Google Scholar]
- 55.Hillman LC, Chiragakis L, Shadbolt B, et al. Proton-pump inhibitor therapy and the development of dysplasia in patients with Barrett’s oesophagus. Med J Aust. 2004;180:387–391. doi: 10.5694/j.1326-5377.2004.tb05991.x. [DOI] [PubMed] [Google Scholar]
- 56.Fitzgerald RC, Omary MB, Triadafilopoulos G. Dynamic effects of acid on Barrett’s esophagus An ex vivo proliferation and differentiation model. J Clin Invest. 1996;98:2120–2128. doi: 10.1172/JCI119018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Souza RF, Shewmake K, Terada LS, et al. Acid exposure activates the mitogen-activated protein kinase pathways in Barrett’s esophagus. Gastroenterology. 2002;122:299–307. doi: 10.1053/gast.2002.30993. [DOI] [PubMed] [Google Scholar]
- 58.Souza RF, Shewmake K, Pearson S, et al. Acid increases proliferation via ERK and p38 MAPK-mediated increases in cyclooxygenase-2 in Barrett’s adenocarcinoma cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G743–G748. doi: 10.1152/ajpgi.00144.2004. [DOI] [PubMed] [Google Scholar]
- 59.Drug Topics. 2001 Red Book. Montvale, NJ: PDR Network, LLC; 2001. [Google Scholar]
- 60.Heath EI, Canto MI, Piantadosi S, et al. Secondary chemoprevention of Barrett’s esophagus with celecoxib: results of a randomized trial. J Natl Cancer Inst. 2007;99:545–557. doi: 10.1093/jnci/djk112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abrams JA. Chemoprevention of esophageal adenocarcinoma. Therap Adv Gastroenterol. 2008;1:7–18. doi: 10.1177/1756283X08093568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sinicrope FA, Broaddus R, Joshi N, et al. Evaluation of difluo-romethylornithine for the chemoprevention of Barrett’s esophagus and mucosal dysplasia. Cancer Prev Res (Phila) 2011;4:829–839. doi: 10.1158/1940-6207.CAPR-10-0243. [DOI] [PubMed] [Google Scholar]
- 63.Jankowski J, Moayyedi P. Re: cost-effectiveness of aspirin che-moprevention for Barrett’s esophagus. J Natl Cancer Inst. 2004;96:885–887. doi: 10.1093/jnci/djh171. (author reply 887) [DOI] [PubMed] [Google Scholar]
- 64.Kwok CS, Arthur AK, Anibueze CI, et al. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107:1011–1019. doi: 10.1038/ajg.2012.108. [DOI] [PubMed] [Google Scholar]
- 65.Janarthanan S, Ditah I, Adler DG, et al. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107:1001–1010. doi: 10.1038/ajg.2012.179. [DOI] [PubMed] [Google Scholar]
- 66.Targownik LE, Leslie WD, Davison KS, et al. The relationship between proton pump inhibitor use and longitudinal change in bone mineral density: a population-based study [corrected] from the Canadian Multicentre Osteoporosis Study (CaMos) Am J Gastroenterol. 2012;107:1361–1369. doi: 10.1038/ajg.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sonnenberg A, Fennerty MB. Medical decision analysis of chemoprevention against esophageal adenocarcinoma. Gastroenterology. 2003;124:1758–1766. doi: 10.1016/s0016-5085(03)00393-7. [DOI] [PubMed] [Google Scholar]
- 68.Choi SE, Perzan K, Tramontano AC, et al. Statins and aspirin for chemoprevention in Barrett’s esophagus: results of a cost-effectiveness analysis. Cancer Prev Res (Phila) 2014;7:341–350. doi: 10.1158/1940-6207.CAPR-13-0191-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hur C, Choi SE, Rubenstein JH, et al. The cost effectiveness of radiofrequency ablation for Barrett’s esophagus. Gastroenterology. 2012;143:567–575. doi: 10.1053/j.gastro.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.