Abstract
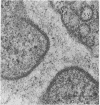
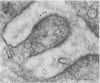
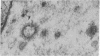
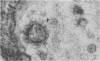
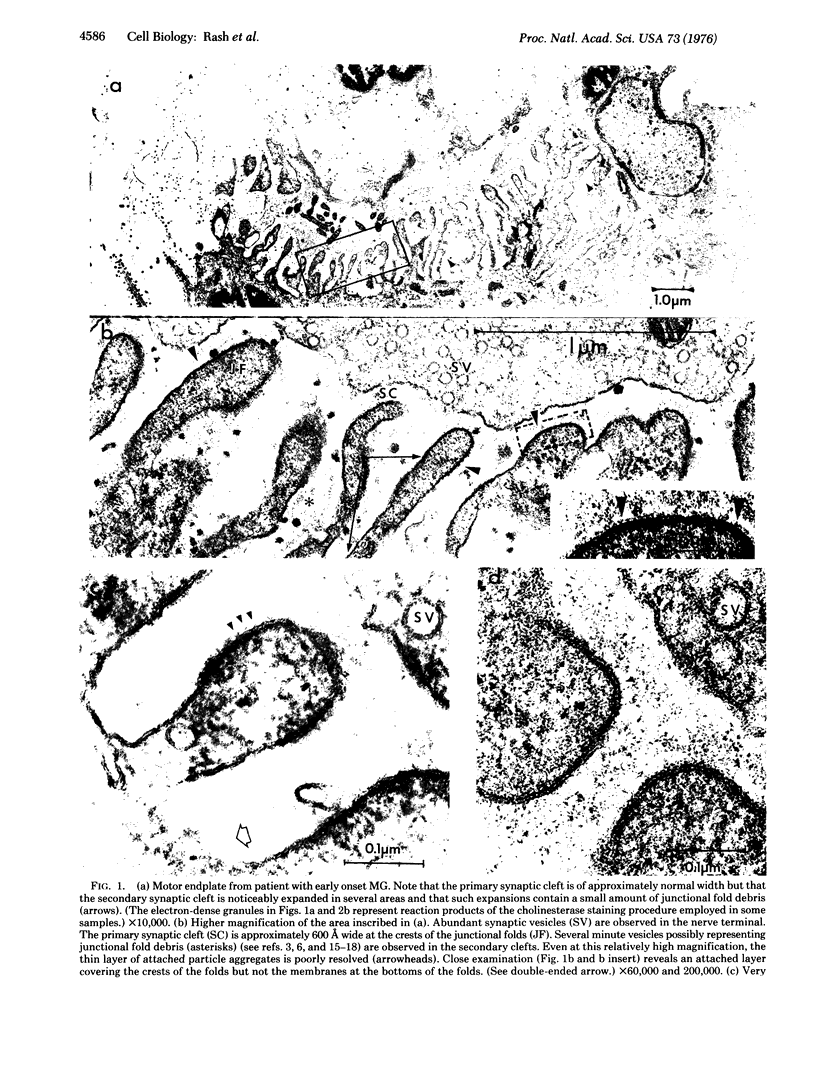
Neuromuscular junctions from patients with early onset and chronic myasthenia gravis were examined by electrophysiological and ultrastructural techniques. Acetylcholine (AcCh) sensitivities were reduced by 34-63% in early onset myasthenia and 60-80% in chronic myasthenia. Ultrastructural analysis revealed that virtually all junctional folds of the early onset patients were intact but that the AcCh-receptor-rich crests of these folds were uniformly covered by an attached layer of 30 X 70 A particles arranged in small tufts or rosettes. In chronic myasthenic endplates, however, junctional fold crests were destroyed, apparently being replaced by vesicular membrane debris similarly labeled by tufts of 30 X 70 A particles. Thus, the initial reduction in junctional AcCh sensitivity observed in early onset myasthenia gravis may be attributed at least in part to in situ masking or inactivation of AcCh receptors, whereas the marked decrease in AcCh sensitivity observed in the chronic myasthenic patient may represent a combination of two factors: (a) in situ masking of AcCh receptors and (b) destruction of the receptor-containing crests of the junctional folds. These observations are compatible with an autoimmune etiology of myasthenia gravis initially involving an apparent antibody attachment to one or more components of the functional AcCh receptor complex, followed by systematic destruction and removal of junctional folds by both humoral and cell-mediated autoimmune responses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramsky O., Aharonov A., Webb C., Fuchs S. Cellular immune response to acetylcholine receptor-rich fraction, in patients with myasthenia gravis. Clin Exp Immunol. 1975 Jan;19(1):11–16. [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Barnard E. A., Porter C. W., Warnick J. E. The density of acetylcholine receptors and their sensitivity in the postsynaptic membrane of muscle endplates. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2818–2822. doi: 10.1073/pnas.71.7.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Lebeda F. J., Appel S. H., Almon R., Kauffman F. C., Mayer R. F., Narahashi T., Yeh J. Z. Effects of normal and myasthenic serum factors on innervated and chronically denervated mammalian muscles. Ann N Y Acad Sci. 1976;274:475–492. doi: 10.1111/j.1749-6632.1976.tb47709.x. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Rash J. E., Mayer R. F., Satterfield J. R. An electrophysiological and morphological study of the neuromuscular junction in patients with myasthenia gravis. Exp Neurol. 1976 Jun;51(3):536–563. doi: 10.1016/0014-4886(76)90179-5. [DOI] [PubMed] [Google Scholar]
- Almon R. R., Andrew C. G., Appel S. H. Serum globulin in myasthenia gravis: inhibition of alpha-bungarotoxin binding to acetylcholine receptors. Science. 1974 Oct 4;186(4158):55–57. doi: 10.1126/science.186.4158.55. [DOI] [PubMed] [Google Scholar]
- Bender A. N., Ringel S. P., Engel W. K., Vogel Z., Daniels M. P. Immunoperoxidase localization of alpha bungarotoxin: a new approach to myasthenia gravis. Ann N Y Acad Sci. 1976;274:20–30. doi: 10.1111/j.1749-6632.1976.tb47673.x. [DOI] [PubMed] [Google Scholar]
- Bergman R. A., Johns R. J. Ultrastructural alterations in muscle from patients with myasthenia gravis and Eaton-Lumbert syndrome. Ann N Y Acad Sci. 1971 Sep 15;183:88–122. doi: 10.1111/j.1749-6632.1971.tb30744.x. [DOI] [PubMed] [Google Scholar]
- Drachman D. B., Kao I., Pestronk A., Toyka K. V. Myasthenia gravis as a receptor disorder. Ann N Y Acad Sci. 1976;274:226–234. doi: 10.1111/j.1749-6632.1976.tb47688.x. [DOI] [PubMed] [Google Scholar]
- Ellisman M. H., Rash J. E., Staehelin L. A., Porter K. R. Studies of excitable membranes. II. A comparison of specializations at neuromuscular junctions and nonjunctional sarcolemmas of mammalian fast and slow twitch muscle fibers. J Cell Biol. 1976 Mar;68(3):752–774. doi: 10.1083/jcb.68.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A. G., Lambert E. H., Santa T. Study of long-term anticholinesterase therapy. Effects on neuromuscular transmission and on motor end-plate fine structure. Neurology. 1973 Dec;23(12):1273–1281. doi: 10.1212/wnl.23.12.1273. [DOI] [PubMed] [Google Scholar]
- Engel A. G., Tsujihata M., Lindstrom J. M., Lennon V. A. The motor end plate in myasthenia gravis and in experimental autoimmune myasthenia gravis. A quantitative ultrastructural study. Ann N Y Acad Sci. 1976;274:60–79. doi: 10.1111/j.1749-6632.1976.tb47676.x. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M., Drachman D. B., Satyamurti S. Neuromuscular junction in myasthenia gravis: decreased acetylcholine receptors. Science. 1973 Oct 19;182(4109):293–295. doi: 10.1126/science.182.4109.293. [DOI] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. Localization of acetylcholine receptor by 125I-labeled alpha-bungarotoxin binding at mouse motor endplates. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1376–1378. doi: 10.1073/pnas.71.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. Quantitation of junctional and extrajunctional acetylcholine receptors by electron microscope autoradiography after 125I-alpha-bungarotoxin binding at mouse neuromuscular junctions. J Cell Biol. 1976 Apr;69(1):144–158. doi: 10.1083/jcb.69.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampar B., Hsu K. C., Martos L. M., Walker J. L. Serologic evidence that a herpes-type virus is the etiologic agent of heterophile-positive infectious mononucleosis. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1407–1411. doi: 10.1073/pnas.68.7.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon V. A., Lindstrom J. M., Seybold M. E. Experimental autoimmune myasthenia gravis: cellular and humoral immune responses. Ann N Y Acad Sci. 1976;274:283–299. doi: 10.1111/j.1749-6632.1976.tb47693.x. [DOI] [PubMed] [Google Scholar]
- NASTUK W. L., PLESCIA O. J., OSSERMAN K. E. Changes in serum complement activity in patients with myasthenia gravis. Proc Soc Exp Biol Med. 1960 Oct;105:177–184. doi: 10.3181/00379727-105-26050. [DOI] [PubMed] [Google Scholar]
- Patrick J., Lindstrom J. Autoimmune response to acetylcholine receptor. Science. 1973 May 25;180(4088):871–872. doi: 10.1126/science.180.4088.871. [DOI] [PubMed] [Google Scholar]
- Penn A. S., Chang H. W., Lovelace R. E., Niemi W., Miranda A. Antibodies to acetylcholine receptors in rabbits: immunochemical and electrophysiological studies. Ann N Y Acad Sci. 1976;274:354–376. doi: 10.1111/j.1749-6632.1976.tb47697.x. [DOI] [PubMed] [Google Scholar]
- Rash J. E., Ellisman M. H. Studies of excitable membranes. I. Macromolecular specializations of the neuromuscular junction and the nonjunctional sarcolemma. J Cell Biol. 1974 Nov;63(2 Pt 1):567–586. doi: 10.1083/jcb.63.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D. B., Schleifer L. S., Eldefrawi M. E., Norcross N. L., Cobb E. E. An immunologically induced defect of neuromuscular transmission in rats and rabbits. Ann N Y Acad Sci. 1976;274:319–336. doi: 10.1111/j.1749-6632.1976.tb47695.x. [DOI] [PubMed] [Google Scholar]
- Santa T., Engel A. G., Lambert E. H. Histometric study of neuromuscular junction ultrastructure. I. Myasthenia gravis. Neurology. 1972 Jan;22(1):71–82. doi: 10.1212/wnl.22.1.71. [DOI] [PubMed] [Google Scholar]
- Tarrab-Hazdai R., Aharonov A., Silman I., Fuchs S., Abramsky O. Experimental autoimmune myasthenia induced in monkeys by purified acetylcholine receptor. Nature. 1975 Jul 10;256(5513):128–130. doi: 10.1038/256128a0. [DOI] [PubMed] [Google Scholar]
- Tarrab-Hazdi R., Aharonov A., Abramsky O., Yaar I., Fuchs S. Passive transfer of experimental autoimmune myasthenia by lymph node cells in inbred guinea pigs. J Exp Med. 1975 Sep 1;142(3):785–789. doi: 10.1084/jem.142.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine R. C., Green N. M. Electron microscopy of an antibody-hapten complex. J Mol Biol. 1967 Aug 14;27(3):615–617. doi: 10.1016/0022-2836(67)90063-0. [DOI] [PubMed] [Google Scholar]
- Zacks S. I., Shields D. R., Steinberg S. A. A myasthenic syndrome in the dog: a case report with electron microscopic observations on motor end plates and comparisons with the fine structure of end plates in myasthenia gravis. Ann N Y Acad Sci. 1966 Jan 26;135(1):79–97. doi: 10.1111/j.1749-6632.1966.tb45465.x. [DOI] [PubMed] [Google Scholar]