Abstract
Aims
To examine the relationship of anxiety and depression symptoms with treatment outcomes (treatment discontinuation, rates of ongoing use of illicit drugs, and likelihood of preterm delivery) in opioid-dependent pregnant women and describe their use of psychotropic medications.
Design and setting
Secondary data analysis from a randomized controlled trial of treatment for opioid dependence during pregnancy.
Participants
175 opioid-dependent pregnant women, of whom 131 completed treatment.
Measurements
Symptoms of anxiety and depression were captured with the 15-item Mini International Neuropsychiatric Interview (MINI) screen. Use of illicit drugs was measured by urine drug screening. Preterm delivery was defined as delivery prior to 37 weeks gestation. Self-reported use of concomitant psychotropic medication at any point during the study was recorded.
Findings
Women reporting only anxiety symptoms at study entry were more likely to discontinue treatment (adjusted OR = 4.56, 95% CI = 1.91–13.26, P = 0.012) while those reporting only depression symptoms were less likely to discontinue treatment (adjusted OR = 0.14, 95% CI = 0.20 – 0.88, P = 0.036) compared to women who reported neither depression nor anxiety symptoms. No statistically significant between group differences were observed for ongoing illicit drug use or preterm delivery. A majority (61.4%) of women reported use of concomitant psychotropic medication at some point during study participation.
Conclusions
Opioid-agonist-treated pregnant patients with co-occurring symptoms of anxiety require additional clinical resources to prevent premature discontinuation.
Keywords: tbc
INTRODUCTION
Opioid dependence occurring during pregnancy is a significant public health challenge resulting in potential risks to mother and fetus and enormous healthcare costs [1,2]. Co-occurring psychiatric symptoms complicate clinical management of an already vulnerable patient population [3]. Rates of co-occurring anxiety and depression in clinical samples of opioid-dependent pregnant women range from 65 to 73% [3,4] compared with only about 20% in epidemiologic samples of non-pregnant drug-dependent individuals [5]. Rates of depression during pregnancy, not complicated by drug-dependence, range from 7 to 16% [6].
An extensive literature on the relationship of co-morbid anxiety and depression disorders with substance abuse treatment outcomes among non-pregnant individuals reveals mixed findings regarding outcomes of addiction treatment [7–9]. The difficulty of differentiating anxiety and depression symptoms from complications of drug use, withdrawal, and protracted abstinence [10] to arrive at valid diagnoses along with the possibility that addiction treatment approaches for different drugs of abuse may indirectly exacerbate or mitigate symptoms of anxiety and depression make the findings of such outcome studies particularly challenging to interpret.
The effect of co-occurring psychiatric conditions in opioid-dependent pregnant women, has been less well studied and conclusions are even less clear as pregnancy itself presents a particularly vulnerable period for many women. A single study has examined the relationship of co-occurring anxiety and mood disorders to treatment outcomes in opioid-dependent pregnant women. Women in methadone-maintenance treatment with depression were more likely to discontinue comprehensive treatment while those with anxiety were more likely to remain in treatment longer and to attend more treatment hours/day [4]. Further analysis of the same cohort found that infants born to opioid-dependent women with depression compared to those without depression had an increased length of stay in the neonatal intensive care unit [11]. To our knowledge, no study has examined the relationship of anxiety or depression symptoms to treatment outcomes for buprenorphine-maintained pregnant women.
The goal of the current study was to examine the relationship of co-occurring symptoms of anxiety and/or depression identified at entry into a randomized controlled trial of methadone and buprenorphine with treatment outcomes in pregnant women with opioid dependence. We hypothesized that co-occurring symptoms of anxiety and/or depression would be associated with differing rates of treatment discontinuation, lower rates of abstinence from illicit drug use, and a greater likelihood of preterm delivery. We focused on anxiety and depression symptoms because of their prevalence in the sample and the availability of a wide-range of evidence-based treatment options for these conditions including psychotherapy and pharmacotherapy. In addition, we described the patterns of prescribed concomitant psychotropic medications in this group of participants.
METHODS
Additional details regarding the study protocol can be found in Jones, et al. (Addiction, this issue [12]) and prior publications [1,3,12,13].
Study Design
This is a secondary analysis of data collected during the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study. The MOTHER study was an international multi-site double-blind, double-dummy, randomized controlled trial of the safety and efficacy of buprenorphine and methadone for treatment of opioid-dependence during pregnancy that enrolled patients between May 4, 2005 and October 31, 2008. All sites received approval from local Institutional Review Boards. Oversight was provided by a Data and Safety Monitoring Board.
Participants
Opioid-dependent women between 18 and 41 years of age, carrying a single fetus with estimated gestational age of 6 to 30 weeks, were eligible for screening. Of 1074 women screened for participation, 438 (40.8%) were eligible and available for further screening. Of those, 208 (47.5%) consented to participate. Thirty-eight of the 438 (8.7%) women were excluded due to psychiatric illness with severity resulting in significant safety concerns. Of 175 women randomized to receive study medication, 131 (74.9%) completed the study (remaining in the study until delivery of a live infant). Prior to initiation of study drug, participants were stabilized as inpatients with immediate release morphine sulfate to facilitate induction to study medication.
Assessments
Structured Clinical Interview for DSM Disorders, Substance Use Disorders Module (SCID-E)
The SCID-E [14] was administered during screening to assess current and lifetime substance use disorders. Study participants were required to meet criteria for current opioid-dependence criteria and were excluded for current alcohol- or benzodiazepine-abuse or dependence due to potential medication interaction effects and known effects of these agents on neonatal outcomes.
Mini International Neuropsychiatric Interview (MINI)
To assess symptoms of other co-occurring psychiatric disorders, the screening version of the MINI [15] was administered at study entry. The MINI is a valid and reliable tool for diagnosis of co-occurring psychiatric conditions in opioid-dependent individuals [16,17]. The shorter 15-item screening tool was used in the current study to reduce response burden. The MINI-screen items are listed in our earlier work [13]. Individual symptoms were collapsed into categories based on symptom clusters. Items 1–4 described symptoms of major depression disorder, dysthymia, and suicidal thinking and were included in a depression symptom cluster. Items 7–13 and 15 described symptoms of panic, agoraphobia, social phobia, obsessive compulsive disorder, post-traumatic stress disorder, and generalized anxiety disorder and were included in an anxiety symptom cluster. An affirmative answer to any of the items in the cluster resulted in a positive screen for that cluster. Participants were then grouped into four categories based on their responses: neither symptom reported, anxiety symptoms only reported, depression symptoms only reported, or both symptoms reported.
Addiction Severity Index (ASI)
The ASI is an interview-based rating of severity of impairment in seven domains: alcohol use, drug use, employment, legal, family/social, medical, and psychological [18] administered at study entry. It does not distinguish quality of symptoms or psychiatric diagnoses that contribute to impairment severity. Interviewers were trained and periodically screened for reliable use of the instrument. This study focused on the psychological composite score and examined whether this variable was associated with treatment outcomes.
Use of concomitant medications
Participants were asked at study entry and weekly about use of any other medications in addition to the study medication. Concomitant medications were prescribed by a clinician of the participant’s choosing, not necessarily study staff. The study protocol included a list of acceptable concomitant medications; however, participants may have received non-approved medications from physicians unrelated to the study. Such use was noted as a protocol deviation, but was not cause for termination of participation in the study. Current abuse or dependence on benzodiazepines (including physiological dependence related to prescribed use) or positive urine drug screen for benzodiazepines at study entry was exclusionary. Medications were grouped into categories based on typical indication rather than mechanism of action. Anxiolytic medications reported by participants included buspirone, hydroxyzine, passedan-tropfen (Austria), and zolpidem as well as benzodiazepines (clonazepam, diazepam, lormetazepam, midazolam, and oxazepam). Antidepressants included selective serotonin reuptake inhibitors (citalopram, escitalopram oxalate, fluoxetine, paroxetine, sertraline), mixed neurotransmitter reuptake inhibitors (buproprion, trazodone), and a tricyclic antidepressant, doxepin. Antipsychotics included olanzapine and quetiapine. Mood stabilizers included lithium and lamotrigine. Self-reported concomitant medication data were available for 166 (94.8%) participants. Report of concomitant medication use at any point during the study was coded as positive use.
Treatment Provided during the Study
The study protocol ensured that comprehensive care was provided for all participants at each site. In addition to daily on-site administration of study medication, study participants received individual and/or group counseling, additional psychiatric treatment as needed, and obstetric and medical care. While access to psychiatric treatment was available, such care was not always provided by a clinician affiliated with the study. Data regarding the frequency and specific nature of any adjunct services pursued by participants in this multi-site study were not collected. Contingency management was implemented to encourage abstinence from alcohol and other concomitant illicit drug use during the study. Patients earned monetary vouchers for providing drug-negative urine samples [12].
Treatment outcomes
Treatment discontinuation
The primary outcome of this secondary dataanalysis was discontinuation of study participation prior to delivery of a live infant. Women who discontinued participation were referred to local resources for ongoing treatment. Great effort was made to maintain women in treatment in the study, including considerable outreach efforts and rescheduling appointments for the convenience of each woman. Transportation to daily medication visits was provided when needed. At study entry, each participant provided contact information for three individuals who could be contacted in the event of a missed appointment to help encourage adherence to treatment. In spite of these efforts, 44 (25.1%) participants discontinued treatment prior to delivery. The reason for treatment discontinuation was voluntary for 36 participants (dissatisfaction with medication, missing more than 5 consecutive dosing days, or other reason for withdrawal), and involuntary for 8 participants (administrative discharge, loss of pregnancy, incarceration, other medical reason) [1].
Urine drug screening
Participants were asked to provide urine samples three times weekly throughout their participation in the study. Urine drug screening included tests for cocaine, benzodiazepines, marijuana, and opioids (excluding methadone and buprenorphine in order to protect randomization blinding). A positive urine drug screening test for any drug at any point during the study was considered as a positive result for this outcome variable. Refusal to provide a urine sample was coded as a positive screen. Participants received vouchers for negative screens. One participant in the group of women who reported no symptoms had missing urine drug screen data and was not included, resulting in a sample size of 130 for this analysis.
Preterm delivery
Preterm was defined as delivery before 37 weeks of gestation.
Data Analysis
Statistical analyses were performed using SPSS 18.0 and Stata 10. Descriptive statistics were used to summarize sample characteristics and psychiatric symptoms. Univariate differences in baseline characteristics among the psychiatric symptom groups were assessed using likelihood ratio chi-square tests of independence for discrete data distributions and analysis of variance for continuous data. Characteristics found to differ among the psychiatric symptom groups were not associated with any of the outcome variables. Hierarchical multiple logistic regression analysis was used to generate adjusted associations of the presence of anxiety and/or depression symptoms with treatment discontinuation. Replicating the approach used in the primary study, this hierarchical analysis controlled for site of participation (U.S. urban vs. U.S. rural vs. European) and randomized medication assignment, age, education, and race. An α of 0.05 was used for determining statistical significance.
RESULTS
Sample Characteristics
A description of participants can be found in the primary outcome paper for the MOTHER study [1]. A majority of participants (62.3%) reported some symptoms of anxiety or depression at study entry, with 19.4% reporting only anxiety symptoms, 10.9% reporting only depression symptoms, and 32.0% reporting both anxiety and depression symptoms. Table 1 describes characteristics of the participants in each symptom group at screening. Participants who reported no symptoms were younger (P = 0.018) and had completed fewer years of education (P = 0.002) than those in the other groups. There were no statistically significant differences between groups for the other characteristics summarized. As expected, the ASI composite score rating was associated with the presence of self-reported anxiety and/or depression symptoms (P < 0.001).
Table 1.
Characteristics of participants at study entry by psychiatric symptom group (n=175)
| Characteristics | Reported Symptoms at Screening n (%) | P | |||
|---|---|---|---|---|---|
| Neither | Anxiety | Depression | Both | ||
| 66 (37.7) | 34 (19.4) | 19 (10.9) | 56 (32.0) | ||
| Maternal age, years M(SD) | 25.5 (5.4) | 28.6 (5.5) | 28.5 (6.6) | 28.2 (6.0) | 0.018 |
| EGA, weeks M(SD) | 18.4 (6.7) | 19.2 (7.0) | 19.2 (5.8) | 18.5 (5.7) | 0.902 |
| Maternal education, years M(SD) | 10.1 (1.9) | 12.0 (1.4) | 11.0 (1.6) | 11.7 (1.9) | 0.002 |
| Non-White n (%) | 6 (9.1) | 6 (17.6) | 7 (36.8) | 10 (17.9) | 0.053 |
| Married n (%) | 10 (15.2) | 7 (20.6) | 2 (10.5) | 4 (7.1) | 0.277 |
| Employed n (%) | 11 (16.7) | 3 (8.8) | 3 (15.8) | 6 (10.7) | 0.636 |
| ASI Psychological Composite n (%) | < 0.001 | ||||
| No problem | 42 (63.6) | 8 (24.2) | 7 (36.8) | 6 (10.7) | |
| Slight problem | 10 (15.2) | 7 (21.2) | 4 (21.1) | 7 (12.5) | |
| Moderate problem | 9 (13.6) | 11 (33.3) | 4 (21.1) | 20 (35.7) | |
| Considerable/extreme problem | 5 (7.6) | 7 (21.2) | 4 (21.1) | 23 (41.1) | |
Note: EGA: estimated gestational age
Anxiety and depression symptoms and retention in treatment
The rate of study discontinuation for women in the group reporting no symptoms was 18.2% compared with 47.1% in the group reporting only anxiety symptoms, 10.5% in the group reporting only depression symptoms, and 25.0% in the group reporting symptoms in both clusters (P < 0.01, Figure 1). We used logistic regression to further characterize the relationship of anxiety and depression symptoms with treatment discontinuation adjusting for study site, randomized medication assignment, age, race, and years of education. Compared to women reporting no anxiety or depression symptoms, women reporting symptoms of anxiety at study entry, had a 4.6 fold increase in likelihood of study discontinuation (adjusted OR = 4.56, 95% CI = 1.39–14.95, P = 0.001, see Table 2). In contrast, women reporting symptoms of depression were considerably less likely to discontinue the study than women with no symptoms (adjusted OR = 0.14, 95% CI = 0.02–0.88, P = 0.036). There was no statistically significant difference in the probability for discontinuation in the group with both depression and anxiety symptoms compared to those reporting no such symptoms. No statistically significant association was found between the overall ASI psychological composite and treatment discontinuation (P = 0.472).
Figure 1. Treatment discontinuation by symptom groups (n = 175).
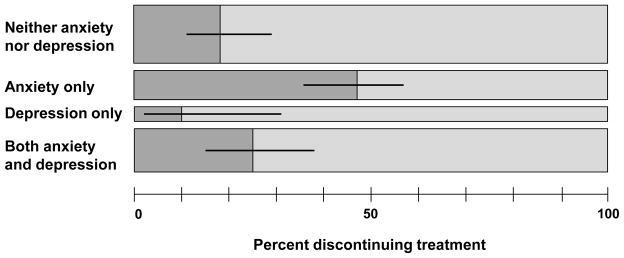
Opioid-dependent pregnant women reporting anxiety only were more likely and those reporting depression only were less likely to discontinue treatment prior to delivering an infant than women reporting symptoms in neither category. Symptoms of anxiety and depression were determined at study entry using the 15-item MINI screen. Width of each bar is proportional to the number of participants in each group. Error bars represent 95% confidence interval.
Table 2.
Associations of baseline depression and anxiety with treatment discontinuation (n = 175)
| Psychiatric symptoms reported | Adjusted OR (95% CI) | P |
|---|---|---|
| Anxiety only | 4.56 (1.91–13.26) | 0.012 |
| Depression only | 0.14 (0.20–0.88) | 0.036 |
| Anxiety and Depression | 0.68 (0.12–2.18) | 0.680 |
Note: Association analyzed by hierarchical logistic regression with neither anxiety nor depression group as referent category. Covariates included site, randomized medication assignment, age, race, and years of education.
Psychiatric symptoms and illicit drug use
The proportion of women with positive urine drug screens at any point during the study was 46.9% for the group as a whole and 35.8% for women with no anxiety or depression symptoms, 38.9% for women with only anxiety symptoms, 52.9% for women with only depression symptoms, and 61.9% for women with symptoms of both anxiety and depression, but these differences did not reach the threshold for statistical significance (P = 0.07).
Psychiatric symptoms and preterm delivery
We observed no statistically significant association between the presence of symptoms of anxiety or depression at study entry and the likelihood of preterm birth (delivery prior to 37 weeks). The number (proportion) of preterm deliveries was 18 (13.7%) for the group as a whole and 3 (5.6%) for women with no anxiety or depression symptoms, 4 (22.2%) for women with only anxiety symptoms, 4 (23.5%) for women with only depression symptoms, and 7 (16.7%) for women with symptoms of both anxiety and depression (P = 0.10).
Prescription medications for anxiety and depression
Data regarding self-reported use of prescribed concomitant medication were available for 166 (94.9%) randomized participants. A majority of women (61.4%) reported taking psychotropic medication at some point during the study; however, not all of those taking medication had reported symptoms of anxiety or depression at study entry (Table 3). Women who reported symptoms of anxiety or depression were significantly more likely to report use of psychotropic medication (P < 0.001). A large proportion (40.3%) of women who reported no symptoms of anxiety or depression at study entry reported using one or more psychotropic medication at some point during the study. Anxiolytic medications were most frequently reported for all groups of participants except those who reported only symptoms of depression, for whom antidepressants were most frequently reported. Antipsychotic medication use was reported by 12 (7.2%) participants. The rate of reported use of mood stabilizers (lamotrogine or lithium) was 4.8%. Of women who reported symptoms of both anxiety and depression at study entry, 23.6% reported that they had taken no additional prescribed psychotropic medications throughout the entire study duration.
Table 3.
Prescribed psychiatric medications by self-report corresponding to reported anxiety and depression symptoms
| Reported Symptoms at Screening | |||||
|---|---|---|---|---|---|
| Neither (n = 62) | Anxiety (n = 31) | Depression (n = 18) | Both (n =55) | All Cases (n = 166) | |
| Any medication* | 25 (40.2) | 21 (67.7) | 14 (77.8) | 42 (76.4) | 102 (61.4) |
| Medication class a | |||||
| Anxiolytic | 19 (30.6) | 17 (54.8) | 7 (38.9) | 26 (47.3) | 69 (41.6) |
| Antidepressant | 10 (16.1) | 8 (25.8) | 8 (44.4) | 23 (41.8) | 49 (29.5) |
| Antipsychotic | 4 (6.5) | 1 (3.2) | 2 (11.1) | 5 (9.1) | 12 (7.2) |
| Mood stabilizer | 1 (1.6) | 1 (3.2) | 0 (0.0) | 6 (10.9) | 8 (4.8) |
Note:
P < 0.001,
Participants may have been prescribed more than one type of medication; therefore, the column frequencies (percentages) are not mutually exclusive.
DISCUSSION
The major findings of this secondary analysis of MOTHER study data are that symptoms of anxiety and depression were associated with significant differences in treatment discontinuation in opioid-dependent pregnant women participating in a clinical trial of methadone and buprenorphine. Differences in rates of ongoing use of illicit drugs and preterm delivery did not meet the threshold for statistical significance. Women reporting anxiety symptoms at study initiation were significantly more likely to discontinue treatment prior to delivery while women reporting depression symptoms were less likely to discontinue treatment prematurely. Women reporting both anxiety and depression symptoms had no appreciable difference in treatment retention compared with women reporting no symptoms.
These findings contradict those of a previous study in a similar population treated with methadone in which anxiety was associated with improved treatment retention, and depression with poorer retention [4]. Several methodological differences between the two studies may explain the contradictory findings. First, the current study was a randomized clinical trial of methadone and buprenorphine while the prior report studied women enrolled in a methadone-maintenance program. Second, the effects of buprenorphine on symptoms of anxiety and depression are not well understood and treatment with this medication may have contributed to treatment discontinuation for women with anxiety. Third, symptoms of anxiety may have contributed to a greater difficulty tolerating treatment in a randomized controlled trial setting in which participants did not know which medication they were receiving.
It is important to note that the threshold for anxiety and depression symptoms used in this study was considerably lower than would be required for formal diagnosis of an anxiety disorder or a depression disorder. Women who endorsed just one relevant screening item on the MINI at study entry were categorized as having symptoms of anxiety or depression. Thus, the current study may underestimate the influence of such symptoms on successful treatment of opioid dependence during pregnancy. This clinical trial was not designed to make a formal diagnosis of anxiety or depression or to measure severity of such symptoms; therefore, we were unable to examine the relationship between anxiety or depression symptom severity and treatment outcomes.
In this clinical sample, ASI psychological composite score at study entry was associated with reported MINI symptoms, but did not predict treatment retention, perhaps due to the opposing relationships of anxiety and depression. The ASI is helpful in providing an overview of the severity of psychological and other impairment, but it does not provide information regarding psychiatric causes of distress, thus the addition of another qualitative tool like the MINI screen adds clinically useful information that allows for a clearer understanding of individual needs of patients.
Women who reported depression symptoms were more likely, and women who reported anxiety symptoms were less likely to produce urine samples that were positive for illicit drugs compared to women who reported no symptoms at study entry, but these differences did not meet our threshold for statistical significance. These results are complicated by the differing rates of study discontinuation, EGA at time of study entry, and duration of pregnancy at delivery--women who remained in the study longer had a greater number of opportunities to provide urine samples for drug screening. Further investigation of the relationship of anxiety and depression symptoms with ongoing illicit drug use during treatment with opioid replacement during pregnancy is warranted.
The current study reveals interesting information about medication prescribing by physicians to opioid-dependent patients during pregnancy. First, nearly 25% of women who reported symptoms of anxiety or depression at study entry received no medication other than study drug throughout the study duration. On the other hand, many of the women to whom medications for anxiety and depression were ultimately prescribed had not reported such symptoms at study entry. For these women, symptoms may have been present, but well managed with medication and therefore not reported at study entry, symptoms may not have been captured by responses to the MINI questions, or these women may have developed symptoms later during the course of their pregnancy and treatment for opioiddependence. Symptoms of anxiety and depression can be challenging to diagnose during pregnancy and change over time making ongoing assessment of co-occurring symptoms of anxiety and depression essential in this vulnerable population. The design of the current study did not allow for further assessment of the relationship of concomitant psychotropic medication to psychiatric diagnosis, opioid treatment retention, or pregnancy outcomes.
There are several limitations of this secondary analysis of data collected as part of a randomized controlled trial. First, due to the need to reduce response burden, this study employed only a brief screening tool for psychiatric symptoms. Use of the screening tool did not allow for confirmative diagnosis of psychiatric disorders or for designation of a primary disorder when symptoms of both depression and anxiety were present. Second, screening for psychiatric symptoms was performed only upon study entry without a longitudinal measure of how such symptoms changed over the course of pregnancy and treatment. Third, while each site employed contingency management to enhance treatment adherence and minimize other substance use, the study protocol for this multi-site medication trial did not standardize psychosocial treatments provided at each site. Therefore, adjunctive treatments may have varied between sites. Because details regarding which adjunctive services were provided to individuals in the study were not collected, the effects of psychosocial interventions on outcomes could not be evaluated. The analysis of birth outcomes was possible only for study completers, limiting the ability to detect any relationship with psychiatric symptoms. Finally, because the current study is a secondary analysis of data collected from a randomized controlled trial, statistical power to address the current research questions is limited and results should be considered as exploratory.
In spite of these limitations, this study reveals a statistically significant association between self-reported co-occurring symptoms of anxiety on entry into this clinical trial and likelihood of premature treatment discontinuation, emphasizing the importance of early identification of such symptoms to the provision of optimal care for such patients. Further work is needed to better understand the impact that treatment of depression and anxiety may have on obstetric, neonatal and drug related treatment outcomes. Future studies should examine the specific psychopharmacologic effects of methadone and buprenorphine in women with and without co-morbid depression and anxiety. Another important question is the timing of study discontinuation to determine at what point women are most vulnerable to discontinue treatment allowing for additional services to increase treatment adherence. Also, additional research is needed to better understand psychosocial factors influencing treatment adherence in this particularly vulnerable population. Finally, study of both pharmacologic and non-pharmacologic approaches to treatment of depression and anxiety symptoms in this population is needed to attain the ultimate goal of improving outcomes for the opioid-dependent mother and her newborn.
Acknowledgments
The work presented here was supported by the following grants from the National Institute on Drug Abuse: Brown University (R01 DA015778); Johns Hopkins University (R01 DA015764); Thomas Jefferson University (R01 DA015738); University of Vermont (R01 DA018410 and M01 RR109); University of Vienna (R01 DA018417); Vanderbilt University (R01 DA 017513, K12 DA000357) and; and Wayne State University (R01 DA15832). Additional support was provided by CTSA grant 1 UL1 RR024975 to Vanderbilt from the National Center for Research Resources, National Institutes of Health.
Footnotes
Declaration of Interest:
This study was funded by the National Institute on Drug Abuse and National Center for Research Resources, National Institutes of Health.
The clinical trial was registered with ClinicalTrials.gov (Identifier: NCT00271219; Title: RCT Comparing Methadone and Buprenorphine in Pregnant Women).
H.J. discloses that she has received reimbursement for time and travel from Reckitt Benckiser.
G.F. discloses that she has received financial support and honoraria for presentations from Reckitt Benckiser, as well as financial support and honoraria for presentations from Schering Plough.
KEO’G discloses that he has received reimbursement for time from Reckitt Benckiser
Contributor Information
Margaret M. Benningfield, Departments of Psychiatry and Pediatrics, Addiction Center, Vanderbilt University
Mary S. Dietrich, Departments of Biostatistics and Psychiatry, Addiction Center, Vanderbilt University
Hendrée E. Jones, Departments of Psychiatry and Behavioral Sciences and Obstetrics and Gynecology, Johns Hopkins University; RTI International
Karol Kaltenbach, Departments of Pediatrics and Psychiatry and Human Behavior, Thomas Jefferson University
Sarah H. Heil, Departments of Psychiatry and Psychology, University of Vermont
Susan M. Stine, Department of Psychiatry and Behavioral Neurosciences, Wayne State University
Mara G. Coyle, Department of Pediatrics, The Warren Alpert Medical School of Brown University
Amelia M. Arria, Center for Young Adult Health and Development, University of Maryland, College Park
Kevin E. O’Grady, Department of Psychology, University of Maryland, College Park
Gabriele Fischer, Department of Psychiatry and Psychotherapy, Addiction Clinic, Medical University of Vienna
Peter R. Martin, Addiction Center, Departments of Psychiatry and Pharmacology, Vanderbilt University
References
- 1.Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, et al. Neonatal Abstinence Syndrome after Methadone or Buprenorphine Exposure. N Eng J Med. 2010;363:2320–31. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones HE, Martin PR, Heil SH, Kaltenbach K, Selby P, Coyle MG, et al. Treatment of opioid-dependent pregnant women: clinical and research issues. J Subst Abuse Treat. 2008;35:245–59. doi: 10.1016/j.jsat.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benningfield MM, Arria AM, Kaltenbach K, Heil SH, Stine SM, Coyle MG, et al. Co-occurring psychiatric symptoms are associated with increased psychological, social, and medical impairment in opioid dependent pregnant women. Am J Addict. 2010;19:416–21. doi: 10.1111/j.1521-0391.2010.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzsimons HE, Tuten M, Vaidya V, Jones HE. Mood disorders affect drug treatment success of drug-dependent pregnant women. J Subst Abuse Treat. 2007;32:19–25. doi: 10.1016/j.jsat.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Mcgonagle KA, Zhao SY, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-Month Prevalence of DSM-III-R Psychiatric-Disorders in the United-States - Results from the National-Comorbidity-Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 6.Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 2004;103:698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- 7.Curran GM, Kirchner JE, Worley M, Rookey C, Booth BM. Depressive symptomatology and early attrition from intensive outpatient substance use treatment. J Behav Health Serv Res. 2002;29:138–43. doi: 10.1007/BF02287700. [DOI] [PubMed] [Google Scholar]
- 8.Kleinman PH, Kang SY, Lipton DS, Woody GE, Kemp J, Millman RB. Retention of cocaine abusers in outpatient psychotherapy. Am J Drug Alcohol Abuse. 1992;18:29–43. doi: 10.3109/00952999209001609. [DOI] [PubMed] [Google Scholar]
- 9.Gerra G, Leonardi C, D’Amore A, Strepparola G, Fagetti R, Assi C, et al. Buprenorphine treatment outcome in dually diagnosed heroin dependent patients: A retrospective study. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:265–72. doi: 10.1016/j.pnpbp.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Alegria AA, Hasin DS, Nunes EV, Liu SM, Davies C, Grant BF, et al. Comorbidity of Generalized Anxiety Disorder and Substance Use Disorders: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2010;71:1187–95. doi: 10.4088/JCP.09m05328gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuten M, Heil SH, O’Grady KE, Fitzsimons H, Chisolm MS, Jones HE. The impact of mood disorders on the delivery and neonatal outcomes of methadone-maintained pregnant patients. Am J Drug Alcohol Abuse. 2009;35:358–63. doi: 10.1080/00952990903108231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones HE, Fischer G, Heil SH, Kaltenbach K, Martin PR, Coyle MG, et al. Maternal Opioid Treatment: Human Experimental Research (MOTHER): Approach, issues, and lessons learned. Addiction. 2011 doi: 10.1111/j.1360-0443.2012.04036.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin PR, Arria AM, Fischer G, Kaltenbach K, Heil SH, Stine SM, et al. Psychopharmacologic management of opioid-dependent women during pregnancy. Am J Addict. 2009;18:148–56. doi: 10.1080/10550490902772975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–9. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 15.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 16.Hides L, Lubman DI, Devlin H, Cotton S, Aitken C, Gibbie T, et al. Reliability and validity of the Kessler 10 and Patient Health Questionnaire among injecting drug users. Austr NZ J of Psychiatry. 2007;41:166–8. doi: 10.1080/00048670601109949. [DOI] [PubMed] [Google Scholar]
- 17.Chiang SC, Chan HY, Chang YY, Sun HJ, Chen WJ, Chen CK. Psychiatric comorbidity and gender difference among treatment-seeking heroin abusers in Taiwan. Psych Clin Neurosci. 2007;61:105–11. doi: 10.1111/j.1440-1819.2007.01618.x. [DOI] [PubMed] [Google Scholar]
- 18.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The Fifth Edition of the Addiction Severity Index. Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]


