Abstract
Clinical trials are examining the efficacy of viral vector-mediated gene delivery for treating Parkinson’s disease (PD). While viral vector strategies have been successful in preclinical studies, to date clinical trials have disappointed. This may be due to the fact that preclinical studies fail to account for aging. Aging is the single greatest risk factor for developing PD and age alters cellular processes utilized by viral vectors. We hypothesized that the aged brain would be relatively resistant to transduction when compared to the young adult. We examined recombinant adeno-associated virus 2/5 mediated green fluorescent protein (rAAV2/5 GFP) expression in the young adult and aged rat nigrostriatal system. GFP overexpression was produced in both age groups. However, following rAAV2/5 GFP injection to the substantia nigra (SN) aged rats displayed 40-60% less GFP protein in the striatum, regardless of rat strain or duration of expression. Further, aged rats exhibited 40% fewer cells expressing GFP and 4-fold less GFP mRNA. rAAV2/5-mediated gene transfer is compromised in the aged rat midbrain, with deficiencies in early steps of transduction leading to significantly less mRNA and protein expression.
1. Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease and currently impacts approximately 4.6 million individuals worldwide, with the prevalence expected to increase in the coming decades with longer life expectancies (Dorsey et al, 2007). The cardinal symptoms of PD are motor deficits resulting from the degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and the accompanying loss of dopamine neurotransmission within the striatum. While it is still unclear what causes PD, aging is known to be a primary risk factor for this disease, with the vast majority of idiopathic cases occurring in patients over the age of 65 (Collier, Kanaan, and Kordower, 2011). Currently, there are no therapies that halt, slow, or reverse the progression of neurodegeneration in PD (Gombash et al, 2014). However, viral vector-mediated gene therapy is a promising therapeutic avenue due to its ability to continuously replenish diminished proteins or overexpress neuroprotective factors that alleviate symptoms or alter disease progression.
Preclinical studies using viral vector-mediated gene transfer have been successful in ameliorating symptoms and rescuing nigral dopamine neuron loss in PD models (Emborg et al, 2009; Gash et al, 1996; Gasmi et al, 2007; Gombash et al, 2012; Herzog et al, 2008; Kordower et al, 2000), yet human clinical trials have not experienced similar success (Bartus et al, 2011; ClinicalTrials.gov, 2013; Marks et al, 2010; Stocchi and Olanow, 2013). This discrepancy may be, in part, attributable to the almost exclusive use of young adult animals in preclinical studies that fail to recapitulate the aged host brain environment typical of most PD patients. For example, previous studies demonstrated that fetal dopamine neuron grafts completely ameliorated motor impairments in young rats yet produced no improvement in aged rats due to dramatically decreased survival and neurite extension in the aged host (Collier et al, 1999; Sortwell et al, 2001). Thus, experimental results derived using young adult animals may have contributed to the overly optimistic expectations of clinical efficacy.
Several groups have reported successful viral vector-mediated gene transfer to the aged brain of rodents and non-human primates (Bartus et al, 2011; Emborg et al, 2009; Klein et al, 2010; Kordower et al, 2000; Wu et al, 2004). However, only two of these reports directly compared transduction efficiency between aged and young animals, with contradicting results (Klein et al, 2010; Wu et al, 2004). Klein et al. (2010) reported equally efficient gene transfer in aged and young adult rat brains utilizing recombinant adeno-associated virus serotype 2/9 (rAAV2/9), while the Wu et al. (2004) concluded that serotype 2/2 (rAAV2/2) was less efficient in the aged brain as compared to the young adult brain. These results were secondary to the primary findings of the study and, consequently, additional experiments comparing viral vector efficiency between young adult and aged brains were not conducted.
It is well established that certain cellular processes that are normally altered in the aged brain (D’Angelo et al, 2009; Gao et al, 2013; Lee, Weindruch, and Prolla, 2000;Ryazanov and Nefsky, 2002; Smith, Sun, and Sokoloff, 1995) are also utilized by viral vectors for transduction (Schultz and Chamberlain, 2008). The present study sought to determine whether viral vector-mediated transgene expression was reduced in the aged rat nigrostriatal system as compared to the young adult. We utilized the rAAV2/5 serotype to examine this question because of its efficient transduction of nigral neurons (Burger et al, 2004; Gombash et al, 2013). Intranigral injections of rAAV serotype 2/5 expressing green fluorescent protein (rAAV2/5 GFP) resulted in significantly reduced exogenous protein expression in the aged rat brain as compared to the young adult brain. Significantly fewer cells throughout the aged rat midbrain expressed the transgene delivered by rAAV2/5 GFP, in addition to producing significantly less GFP protein and GFP mRNA. Collectively, our results indicate that rAAV2/5-mediated gene transfer is compromised in the aged rat brain environment.
2. Methods
2.1 Experimental overview
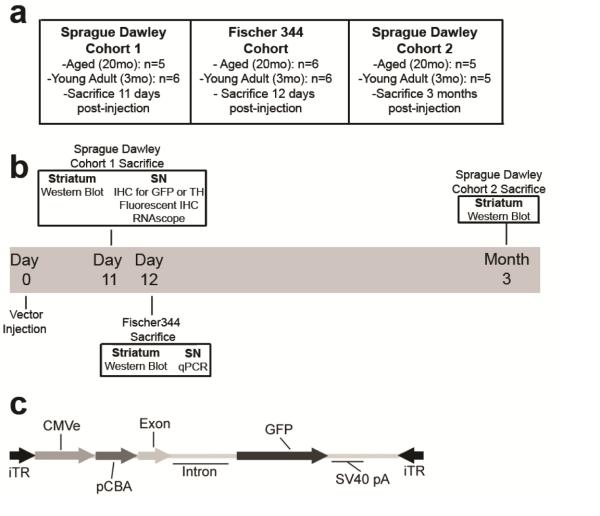
A cohort of twenty-one male Sprague Dawley (SD) rats, eleven young adult (3 months old) and ten aged (20 months old) were unilaterally injected with rAAV2/5 expressing GFP into the substantia nigra (SN). Six young and five aged SD rats were sacrificed eleven days post-injection and five young and five aged SD rats were sacrificed three months post-injection. Similarly, a cohort of twelve male Fischer344 (F344) rats, six young adult and six aged, were unilaterally injected with rAAV2/5 expressing GFP in the SN and sacrificed twelve days post-injection. The striatum was used for western blot analysis from SD rats sacrificed 11 days and three months post-injection and the SN tissue was used for immunohistochemical staining and in situ hybridization. The striatum from F344 rats sacrificed 12 days post-injection was used for western blot analyses and the SN tissue was used for qPCR. The overall experimental design is illustrated in Figure 1.
Figure 1. Schematic of experimental design and rAAV2/5 viral vector construct.
(a-b) Overview and timeline of the three cohorts of rats used in these studies. Young adult (3 months) and aged (20 months) Sprague Dawley and Fischer344 rats were all injected in the substantia nigra (SN) with the rAAV2/5 GFP viral vector and were sacrificed at indicated time points to harvest tissue for various outcome measures. (c) Recombinant adeno-associated virus (rAAV) genomic map of rAAV2/5 expressing GFP used in all experiments.
2.2 Animals
Male, Sprague Dawley rats (Harlan, Indianapolis, IN) 3 months of age (n = 11) and 20 months of age (n = 10) and male, Fischer344 rats (National Institute on Aging, Bethesda, MD) 3 months of age (n = 6) and 20 months of age (n = 6) were used in this study. All animals were given food and water ad libitum and housed in 12h reverse light-dark cycle conditions in the Van Andel Research Institute vivarium, which is fully AAALAC approved. All procedures were conducted in accordance with guidelines set by the Institutional Animal Care and Use Committee (IACUC) of Michigan State University.
2.3 Viral vectors
Plasmid and rAAV vector production were completed as previously described (Gombash et al, 2014). In short, humanized green fluorescent protein (GFP) was inserted into an AAV plasmid backbone. Expression of the transgene was driven by the chicken beta actin/cytomegalovirus enhancer (CβA/CMV) promoter hybrid. The vector contained AAV2 inverted terminal repeats (ITRs) and was packaged into AAV5 capsids via co-transfection with a plasmid containing rep and cap genes and adenovirus helper functions. Particles were purified using iodixanol gradients and Q-sepharose chromatography, and dotblot was used to determine vector titer (Zolotukhin et al, 1999). The vector titer used in this study was 5.88 × 1013 genomes/mL (Gombash et al, 2013). In order to preserve the viral stability and titer, viral preparations were stored at 4°C and never frozen. All surfaces (syringes, pipettes, and microcentrifuge tubes) were coated in SigmaCote (Sigma-Aldrich, St. Louis, MO SL2) prior to coming in contact with the virus to minimize binding of viral particles.
2.4 rAAV2/5 GFP injections
All surgical procedures were performed under isofluorane anesthesia (5% in O2 for induction and 2% in O2 for maintenance). Rats were placed in a stereotaxic frame and two 2μL injections of rAAV2/5 were injected in the left SN at coordinates (from dura) AP −5.3mm, ML +2.0mm, DV −7.2mm, and AP −6.0mm, ML +2.0mm, and DV −7.2mm as previously described (Gombash et al, 2013). A Hamilton syringe fitted with a glass capillary needle (Hamilton Gas Tight syringe 80,000, 26s/2” needle; Hamilton, Reno, NV; coated in SigmaCote) was used for injection. The glass needle was lowered to the site and vector injection began immediately at a rate of 0.5 μl/minute and remained in place after the injection for an additional 5 minutes before retraction.
2.5 Sacrifice and tissue preparation
Six young and five aged SD rats were sacrificed 11 days post-injection, all F344 rats were sacrificed 12 days post-injection, and five young and five aged SD rats were sacrificed 3 months post-injection. All rats were deeply anesthetized (60mg/kg, pentobarbital, i.p.) and perfused intracardially with 0.9% saline containing 1ml/10,000 USP heparin, followed by ice cold 0.9% saline. SD rat brains were immediately removed and hemisected in the coronal plane at approximately AP −2.64mm. The caudal tissue from SD rats was processed for immunohistochemistry, whereas rostral tissue from SD and whole brains from F344 were processed for microdissections.
2.6 Microdissections
After brain removal, whole brains and rostral brains were flash frozen in 2-methylbutane on dry ice.
SD Striatal Tissue for Protein Analyses
The rostral section of the brain was frozen at −18°C for at least one hour before striatal dissections. 1-2mm coronal slabs were blocked from each brain utilizing an aluminum brain blocker (Zivic, Pittsburg, PA) and striatal tissue from both hemispheres was microdissected with a small tissue punch while being held at a constant −12°C on a modified cold plate (Teca, Chicago, IL). Frozen dissected structures were stored at −80°C until analysis.
F344 Striatal Tissue for Protein Analyses and SN Tissue for RNA Analyses
All surfaces and instruments were sprayed with RNase Away (Invitrogen, Carlsbad, CA). Brains were moved from −80°C to −20°C before use. Brains were kept on dry ice an d mounted onto metal chucks with Tissue-Tek O.C.T. (VWR, Vatavia, IL) before being moved to a −20°C cryostat for sectioning. Tissue was sectioned until the structures of interest became visible. 2mm sections of the striatum were removed from both hemispheres of SD brains with a small tissue punch, and 2mm sections of striatum and SN were removed from both hemispheres of the F344 brains with a small tissue punch. Punches were taken from the middle of the brain structures to ensure precision. Striatal punches were placed in separate pre-frozen RNase free microcentrifuge tubes and immediately placed back on dry ice. SN punches were placed in separate pre-frozen RNase free microcentrifuge tubes containing TRIzol Reagent (Invitrogen, Grand Island, NY) and tissue was homogenized with a pestle before storage. Samples were stored at −80°C until time of assay.
2.7 Immunohistochemistry
The caudal portion of the brains was post-fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in 0.1mol PO4 buffer for seven days. After this period they were transferred to 30% sucrose in 0.1mol PO4 buffer until sinking. Brains were frozen on dry ice and sectioned at a 40μm thickness using a sliding microtome. Every sixth section was processed for immunohistochemistry using the free floating method.
Immunohistochemistry
Tissue sections were rinsed in Tris buffer and quenched in 0.3% H2O2 for 15 minutes, blocked in 10% normal goat serum for 1 hour, and incubated in primary antisera (TH: Millipore MAB318, mouse anti-TH, 1:4000; GFP: Abcam Ab290, rabbit anti-GFP, 1:100,000) overnight at 4°C. Following primary incubation, TH-labeled se ctions were incubated in secondary antisera against mouse IgG (Vector BA-2001, biotinylated horse anti-mouse IgG, rat absorbed, 1:1000) and GFP-labeled sections were incubated in secondary antisera against rabbit IgG (Millipore AP132b, goat anti-rabbit IgG, 1:500) for 2 hours at room temperature, followed by Vector ABC detection kit using horseradish peroxidase (Vector Laboratories, Burlingame, CA). Antibody labeling was visualized by exposure to 0.5mg/ml 3,3’ diaminobenzidine and 0.03% H2O2 in Tris buffer. Sections were mounted on subbed slides and coverslipped with Cytoseal. Images were taken on a Nikon Eclipse 90i microscope with a QICAM camera (QImaging, Surrey, British Colombia, Canada). Figures were produced in Photoshop 7.0 (San Jose, CA), with brightness, saturation, and sharpness adjusted only as needed to best replicate the immunostaining as viewed directly under the microscope.
TH and GFP Double Label Immunofluorescence
Sections of rat tissue were blocked in 10% normal donkey serum for 1 hour and subsequently transferred to the primary antisera (TH: Millipore Ab152, rabbit anti-TH, 1:4000) to incubate overnight at 4°C. Following primary incubation, tissue was incubated in the dark in secondary antisera against rabbit IgG (Invitrogen A10042, Alexa Fluor 568 donkey anti-rabbit, 1:500) for 1 hour at room temperature. Tissue was then blocked in 10% normal goat serum for 20 minutes at room temperature before being incubated in the primary antisera against GFP (Abcam Ab290, rabbit anti-GFP, 1:100,000) overnight at room temperature in the dark. Following primary incubation, tissue was incubated in secondary antisera against chicken IgG (Life Technologies A11039, Alex Fluor 488 goat anti-chicken IgG, 1:400) for 3 hours at room temperature. Sections were mounted on subbed slides and coverslipped with Vectashield Hardset Mounting Medium (Vector Laboratories H1400, Burlingame, CA). Images were taken on a Nikon 90i fluorescence microscope with a Nikon DS-Ri1 camera. Figures were produced in Photoshop 7.0 (San Jose, CA). Brightness, saturation, and sharpness were adjusted only as necessary to best replicate the immunostaining as viewed directly under the microscope.
2.8 Western blot
Striatal punch samples were homogenized on ice in 2% SDS. Total protein concentration was determined by the Bradford protein assay. Samples were prepared at 25ng total protein samples. Western blot protocol was completed as previously described (Piltonen et al, 2009). Samples were run using SDS-PAGE and transferred to Immobilon-FL membranes (Millipore, Bedford, MA). Membranes were incubated in primary GFP antisera (AbCam, Cambridge, MA; rabbit polyclonal IgG, Ab290, 1:1,000) and β-tubulin antisera (Cell Signaling, Danvers, MA; mouse monoclonal IgG, 4466, 1:1,000) overnight. IRDye800 conjugated goat anti-rabbit (LI-COR Biosciences; 926-32211, 1:15,000) and IRDye680 conjugated goat anti-mouse (LI-COR Biosciences, 926-68020, 1:15,000) were used as secondary antibodies. All antibody dilutions were made in LI-COR specific blocking buffer (LI-COR Biosciences, 927-40000). Multiplexed signal intensities were imaged with both 700 and 800 nm channels in a single scan with a resolution of 169μm using the Odyssey infrared image system (LI-COR Biosciences). Reported integrated intensity measurements of GFP expression were normalized according to the corresponding β-tubulin densitometry measurements. The representative image was produced in Photoshop 7.0 (San Jose, CA).
2.9 RNA isolation and cDNA synthesis
RNA was isolated using the QIAshredder (Quiagen, Valencia, CA) and RNeasy Plus Mini kit (Quiagen, Valencia, CA). The Quiagen protocol for purification of total RNA from animal tissue was used. RNA from tissue was then converted into cDNA using SuperScript VILO Master Mix (Life Technologies, Grand Island, NY). The RNA was assumed to be converted 100% to cDNA.
2.10 GFP qPCR
The PCR reactions were carried out with 1x TaqMan Universal Mastermix (ABI, Carlsbad, CA), and a customized TaqMan gene expression assay kit for GFP (ABI, Carlsbad, CA). This kit includes 6,000 pmoles of the TaqMan MGB-fluorescent probe and 10,000 pmoles of each manufactured primer. Forward (R) and reverse (L) primers, as well as the probe (P) sequence were synthesized by ABI. The sequences were as follows: R: AGACCATATGAAGCAGCATGACTTTT, L: GTCTTGTAGTTCCCGTCATCTTTGA, P: 5’6FAM CTCCTGCACATAGCCC MGBNFQ. A total of 20ng of cDNA was added to each 50μl reaction mixture which also contained 150nM forward and 150nM reverse primers, a 150nM probe sequence, and TaqMan master mix. qPCR reactions to control for cDNA quantities were run using GAPDH (Life Technologies 4352338E, Grand Island, NY) as an endogenous control. The qPCR reactions were run on an ABI 7500 real-time thermocycler using the following setup: Step1: Incubation at 50°C for 2 mi nutes. Step 2: Incubation at 95°C for 10 minutes. Step 3: Denaturation at 95°C for 15 seconds followe d by annealing-elongation at 60°C for 1 minute followed by data collection. Step 3 was cycled 40 times for the qPCR run. Cycle thresholds were chosen during the linear phase of amplification using the AutoCT function. All samples were run on the same plate along with a no-template control. Analysis was first carried out using the 2-ΔCT method and Relative Expression Software Tool (REST-XL) method (Pfaffl, Horgan, and Dempfle, 2002; Schmittgen and Livak, 2008).
2.11 RNAscope in situ hybridization for GFP mRNA
Tissue sections at the level of the SN were incubated in Pretreat 1 from the RNAscope Pretreatment Kit (310020, Advanced Cell Diagnostics, Hayward, CA) for 1 hour. Tissue was then incubated for 10 minutes in Pretreat 2 at 99°C befo re being mounted on Histabond slides and incubated on a plate warmer set to 60°C overnight. After Pretreat 2, slides were dipped in 100% ethanol and incubated with Pretreat 3 in a hybridization oven at 40°C. Slides were dried and the VS Probe for GFP (409016, Advanced Cell Diagnostics, Hayward, CA) was added for a 2 hour incubation in the hybridization oven. Six amplification steps with the amplification buffers (320600, Advanced Cell Diagnostics, Hayward, CA) were then performed in alternating 30 and 15 minute incubation intervals in the hybridization oven. Tissue was developed using the supplied DAB reagent (320600, Advanced Cell Diagnostics, Hayward, CA). Slides were rinsed and coverslipped with Cytoseal. Images were taken on a Nikon Eclipse 90i microscope with a QICAM camera (QImaging, Surrey, British Colombia, Canada). Figures were produced in Photoshop 7.0 (San Jose, CA), with brightness, saturation, and sharpness adjusted only as needed to best replicate the mRNA labeling as viewed directly under the microscope.
2.12 Stereology
Quantification of TH immunoreactive (THir) neurons in the SN pars compacta (SNpc) or total number of GFP immunoreactive (GFPir) cells was completed as previously described (Gombash et al, 2012). Briefly, stereology was performed using a Nikon Eclipse 80i microscope (Nikon), StereoInvestigator software (Microbrightfield Bioscience, Williston, VT) and Retiga 4000R camera (Qimaging, Surrey, BC Canada). Using the optical fractionator probe, THir neurons in the vector injected and control hemisphere in every sixth section of the entire SN were counted at 60x magnification. Similarly, GFPir cells were counted at 60x magnification in the injected hemisphere in every sixth section of the brain where GFP staining was visible. A coefficient of error <0.10 was accepted. THir data are reported as total estimates of THir neurons in each hemisphere.
2.13 Counts of GFP and TH immunoreactive cells
Tiled images at 10x magnification were taken on three SD brain sections per animal that were stained for GFP and TH immunofluorescence. These sections contained the medial terminal nucleus of the accessory optic tract (MTN) and were approximately −5.04mm, −5.28mm, and −5.52mm relative to bregma (Gombash et al., 2014). Images were taken with a Nikon 90i fluorescence microscope with a Nikon DS-Ri1. All GFP immunoreactive (GFPir) cells that were in focus with a clear nucleus were manually counted to determine the total number of transduced cells. All TH immunoreactive (THir) neurons that were in focus with a visible nucleus and were also GFPir were manually counted to determine the number of THir cells expressing GFP. The percentage of THir neurons that were transduced was determined by dividing the total number of GFPir cells by the number of neurons that were immunoreactive for both TH and GFP. The remaining GFPir cells that did not stain for TH were designated as non-TH transduced cells. Data are reported as the average percent of GFPir/THir and GFPir/non-THir cells.
2.14 Volume of GFPir Mesencephalic Expression
Neurolucida v.7, Neurolucida Explorer (Microbrightfield), a Nikon Eclipse 80i microscope (Nikon), and a Retiga 4000R camera (Qimaging) were used to outline the area of GFPir expression and calculate the volume of this area. The entire mesencephalic section and area of transgene expression were manually traced approximately every sixth serial section along the anterior-posterior (coronally sectioned) axis. Tracings began at −3.18mm from bregma and were terminated at −6.78mm from bregma. The 40μm section thickness and distance between each section (~240μm) allowed Neurolucida to construct a 3D model of the mesencephalon and area of transgene expression from 2D tracings. Using the model, characteristic volumes of these outlined areas were calculated by Neurolucida Explorer. These tracings and calculations were performed on a representative young adult and aged Sprague Dawley rat at 11 days post-injection with rAAV2/5 GFP as depicted in Figure 3.
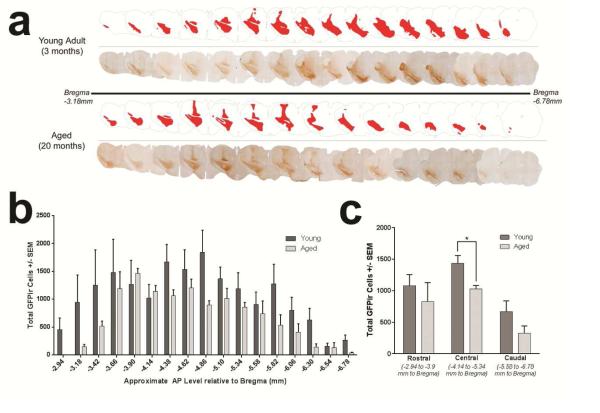
Figure 3. Decreases in exogenous transgene expression in the aged brain span the anterior-posterior (AP) mesencephalic axis.
(a) Representative montage of GFP immunoreactivity within individual midbrain sections 11 days following rAAV2/5 GFP injection to either young adult or aged rats. GFP immunoreactive cells are generally observed spanning the entire AP axis in both young adult and aged rats, with aged rats generally exhibiting smaller areas of transgene expression within individual coronal sections. The total volume of transgene expression in the young adult brain was 205,524,000μm3, or 10.47% of the mesencephalon, whereas the total volume of transgene expression in the aged brain was 135,517,000μm3, or 6.62% of the mesencephalon. (b) Stereological estimates of the number of GFP immunoreactive cells per individual mesencephalic coronal section indicate a general trend of fewer GFP immunoreactive cells within each aged brain section as compared to the comparable section in the young adult. (c) Stereological estimates of the number of GFP immunoreactive cells within the rostral, central, and caudal regions of the area displaying transgene expression. Aged rats possess a trend for fewer GFP immunoreactive cells in the rostral and caudal regions with significantly fewer GFP immunoreactive cells in the central region (*p<0.05) than young adult rats. Values are expressed as the mean ± SEM for each group (Young Adult, n=6; Aged, n=5).
2.15 Statistical analyses
All statistical tests were completed using IBM SPSS statistics software (version 22.0, IBM, Armonk, NY). Graphs were created using GraphPad Prism software (version 6, GraphPad, La Jolla, CA). All studies utilized independent samples t-tests to assess differences between the young adult and aged animals. The THir quantification results were analyzed with two-way repeated measures ANOVA with two treatment factors, age and hemisphere. The GFPir/THir and GFPir/non-THir quantification results were analyzed with two-way repeated measures ANOVA with a single treatment factor. Tukey post hoc analyses were used on all ANOVA tests to determine significance between individual groups using the harmonic mean of the group sizes to account for unequal sample sizes. For the qPCR gene expression study, fold changes for the GFP gene were normalized to the GAPDH gene expression and calculated before being subjected to t-test analysis using the REST-XL.
3 Results
3.1 Aging and rAAV2/5 GFP injection do not impact SN pars compacta (SNpc) tyrosine hydroxylase immunoreactive (THir) neuron number
Sprague Dawley (SD) rats are classified as young adult from the time of sexual maturity (P35) until 6 months, middle aged between 6 and 18 months, old (aged) after 18 months, and senile after 24 months with an average lifespan of 26-32 months (Alemán et al, 1998; Gao et al, 2011). Importantly, after 22-24 months, SD rats exhibit a 13% reduction in SN dopamine neurons (Gao et al, 2011). In the present study, we used 3-month-old young adult rats and 20-month-old aged rats (Figure 1). In order to verify that no differences existed in the quantity of SNpc THir neurons due to aging (at 20 months) or rAAV2/5 GFP injection, we used unbiased stereology to estimate the number of SNpc THir neurons in both the uninjected and rAAV2/5 GFP injected hemispheres at 11 days post-injection (Figure 2a). Aged rats possessed equivalent SNpc THir neurons compared to young adult rats in both hemispheres (F(1,9 = 0.025, p > 0.05). In addition, there were no significant differences between THir neuron counts in the SNpc ipsilateral to the rAAV2/5 GFP injection as compared to the SNpc contralateral to the injection, indicating that at 11 days post-injection the viral vector was not toxic to the SNpc neurons (F(1,9 = 2.546, p > 0.05).
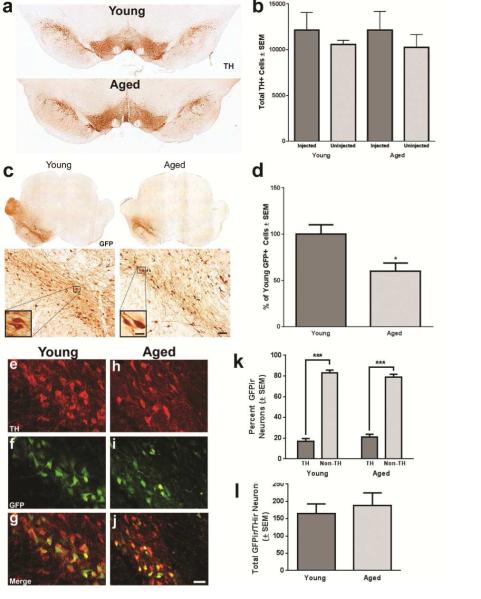
Figure 2. GFP immunoreactivity reveals fewer transduced cells in aged rats following rAAV2/5 GFP injection.
(a) Representative image of TH immunoreactive (THir) neurons in the substantia nigra pars compacta (SNpc) of young adult (Young) and aged (Aged) SD brains at 11 days post-injection. TH immunoreactivity appears equal between hemispheres and age groups. Scale bar = 1000 μm. (b) Stereological quantification of THir neurons in young adult and aged SD rats in the rAAV2/5 GFP injected and uninjected SNpc. No significant reductions in tyrosine hydroxylase immunoreactive (THir) neurons are observed in aged (n=5) as compared to young adult (n=6) rats or in the injected as compared to the uninjected hemisphere 11 days following rAAV2/5 GFP injection. (c) Representative samples of GFP immunoreactivity in the Young and Aged SD rat brain 11 days post-injection. GFP immunoreactivity is observed throughout the midbrain ipsilateral to the injection site (left) with dense cellular staining observed at higher magnfications. GFP immunoreactivity in the midbrain of aged rats is visibly less abundant than that of young adult rats. Scale bars = 1000μm in top panels, 100μm in lower panels and 25μm in the high magnification inserts. (d) Stereological quantification of the total number of GFP immunoreactive cells for young adult (n=6) and aged (n=5) rats revealed a statistically significant (*p<0.05) 40% decrease in GFP immunoreactive cells in the aged brain as compared to the young adult brain. (e-j) GFP co-expression within THir SN neurons in young adult (e-g) and aged (h-j). Numerous THir neurons and non-THir cells expressing GFP are apparent in both age groups. Scale bar = 50μm. (k) Quantification of the number of THir neurons expressing GFP in three sections containing a readily identifiable SNpc in young adult and aged rats. No significant differences were observed in the number of GFPir/THir neurons at these levels of the mesencephalon. (l) Quantification of the percentage THir and non-THir cells that colocalize with GFP expression. No significant differences were observed between young adult and aged rats in the percent of THir neurons or the percent of non-THir cells expressing GFP, although both age groups exhibited significantly more GFPir non-THir cells than GFPir/THir neurons (Aged, n=5; Young Adult, n=6). All values are expressed as the mean ± SEM for each group (Aged, n=5; Young Adult, n=6).
3.2 Aged rats exhibit decreased numbers of transduced cells as compared to young adult rats
We examined the total number of transduced cells in the SD mesencephalon at 11 days post-injection with rAAV2/5 GFP. Both young adult and aged SD rats exhibited GFP immunoreactivity in the midbrain that included the SNpc and numerous cells in the neighboring mesencephalic parenchyma (Figures 2b, Figure 3). However, the young adult midbrain appeared to possess a greater number of GFP immunoreactive (GFPir) cells as compared to the aged midbrain. Stereological quantification of the number of cells displaying GFP immunoreactivity indicated that the aged rat had significantly fewer (approximately 40%) total transduced cells as compared to the young adult rat (p = 0.038; Figure 2c). To determine whether THir neurons of the SNpc in aged rats specifically were resistant to gene transfer we determined the number of THir cells expressing GFP and the percentage of THir versus non-THir transduced cells in both age groups within sections in which the THir neurons of the SNpc could be definitively delineated from THir neurons of the ventral tegmental area. Dual immunofluorescence revealed successful transduction of THir and non-THir neurons with rAAV2/5 GFP in both young and aged rats (Figures 2e-j). Unlike total cell counts resulting from unbiased stereology, no age-related differences were observed in the number of GFPir cells within this subregion of the mesencephalon (Figure 3b) and, similarly, no difference in the number of GFPir/THir cells was observed between young and aged rats (p>0.05, Figure 2k). Further, there were no significant differences in the percentage of THir and non-THir cells expressing GFP between the young adult and aged SD rat at 11 days post-injection (F(1,7) = 1.161, p > 0.05, Figure 2l), although there were significantly more (~80% more) non-THir cells than THir neurons expressing GFP in both age groups (F(1,7) = 257.78, p < 0.001). Together, these results suggest that identical rAAV2/5 vector injections result in significantly fewer total cells expressing the rAAV2/5 transgene in the aged SN as compared to the young adult SN, and that age-related deficiencies in gene transfer are not specific to SNpc THir neurons.
3.3 Reduced exogenous transgene expression in the aged brain spans the entire rostral-caudal mesencephalic axis
We next investigated the pattern of transduction to determine whether reduced vector spread in the aged brain drives the observed age-related decrease in the number of cells expressing the GFP transgene. The number of GFPir cells per section was quantified within sixteen coronal sections spanning the extent of the visibly transduced area in the young adult and aged rat mesencephalon. Qualitatively the anterior-posterior (AP) axis of transgene expression appeared to be approximately equal, with differences occasionally observed in both dorsal-ventral (DV) and medial-lateral (ML) spread (Figure 3a). Quantification of the number of GFPir cells per section indicated that the aged mesencephalon possessed fewer GFPir cells within the overwhelming majority of sections (13 of 16). Within individual mesencephalic sections, reductions in GFPir cells in the aged brain did not achieve significance (p > 0.05, Figure 3b). However, when all sections were combined for stereological assessment of the total number of transgene-expressing cells, the aged brain exhibited significantly fewer GFPir cells (Figure 2c). Furthermore, when sections were grouped into rostral, central, and caudal regions of transgene expression, there is a trend for decreased GFPir neurons in the rostral and caudal regions with a significant decrease (p = 0.014) in total GFPir neurons in the central region of transgene expression. The lack of significant differences at the level of the individual sections suggests that a difference in the diffusivity of the vector does not underlie the age-related decrease in transgene expression.
3.4 Aged rats display decreased GFP protein expression in the striatum as compared to young adult rats
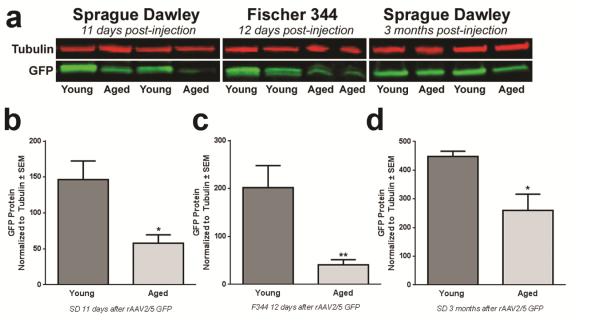
rAAV2/5-mediated protein expression in young adult rats is not maximal until 1 month after injection (Gombash et al, 2012), and it is possible that the aged brain may require more time to achieve maximal GFP expression. Therefore, we utilized protein immunoblotting to more accurately quantify GFP protein levels at varying time points after rAAV2/5 GFP injection. We examined the levels of striatal GFP protein expression induced by rAAV2/5 GFP in young adult and aged rats from two different rat strains at three different time points post-injection (SD at 11 days, F344 at 12 days, and SD at 3 months, Figure 4a-d). Aged rats consistently displayed lower levels of GFP protein (approximately 40-60% less) as compared to their young adult rat counterparts, regardless of the rat strain or the duration of gene expression (SD 11 days, p = 0.030; F344 12 days, p = 0.008; SD 3 months, p = 0.014). These results indicate that although GFP protein increases with duration of rAAV2/5 GFP expression, aged rats consistently display deficiencies in GFP protein expression in the midbrain as compared to young adult rats, regardless of rat strain.
Figure 4. rAAV2/5-mediated GFP protein expression is diminished in the aged striatum.
(a) Representative western blot of GFP immunodetection in striatal samples of young adult and aged Sprague Dawley (SD) rats 11 days post-injection and Fischer344 (F344) rats 12 days post-injection. Young adult rats consistently express more GFP protein than aged rats. (b) Quantification of striatal GFP protein levels in young adult (n=6) and aged (n=5) SD rats 11 days post-injection. Aged rats exhibited significantly lower levels of GFP protein (*p<0.05) than young adult rats. (c) Quantification of striatal GFP protein levels in young adult (n=6) and aged (n=6) F344 rats at 12 days post-injection; aged rats display significantly less GFP protein (**p<0.01) than young adult rats. (d) Quantification of GFP protein levels in the striatum of young adult (n=5) and aged (n=5) SD rats at 3 months post-injection. Aged rats exhibit significantly less GFP protein (*p<0.05) than young adult rats. Values are expressed as the mean optical density scores, normalized to tubulin controls ± SEM for each group. rAAV, recombinant adeno-associated virus.
3.5 Aged rats exhibit decreased levels of GFP mRNA expression as compared to young adult rats
To determine whether reduced GFP protein expression in the aged brain was the result of aging-related decreases in protein synthesis (Ryazanov and Nefsky, 2002; Smith, Sun, and Sokoloff, 1995), GFP mRNA expression in the SN was examined using the complementary approaches of qPCR and RNAscope in situ hybridization (Wang et al, 2012). qPCR analyses revealed significantly lower (p < 0.001) GFP mRNA levels in the aged SN as compared to the young adult SN, with approximately 4-fold lower levels of mRNA expression in the aged rat (Figure 5a). Furthermore, GFP mRNA expression visualized using RNAscope in situ hybridization confirmed our qPCR results. GFP mRNA expression in the mesencephalon of both young adult and aged rats was observed, with a large areas of expression containing dense staining in the midbrain ipsilateral to the injection site (Figure 5b). Qualitatively, when compared to GFP mRNA expression in the young adult, the aged rat midbrain displayed a smaller area of cells expressing GFP with less dense staining within this area. Collectively, these findings indicate that rAAV2/5 transduction of the aged rat midbrain as compared to the young adult rat midbrain results in significantly less GFP mRNA expression, further leading to significant deficiencies in GFP protein levels. This signifies that steps prior to translation are deficient in the process of viral transduction in the aged midbrain.
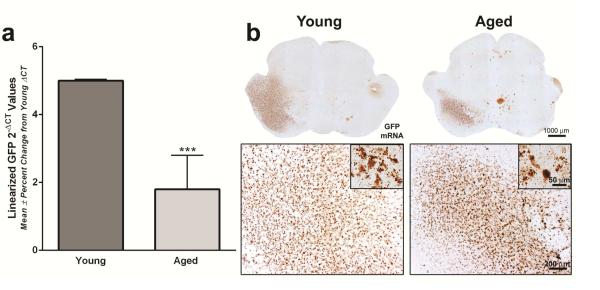
Figure 5. rAAV2/5-mediated GFP mRNA expression is diminished in the aged SN.
(a) GFP mRNA quantitation using qPCR of the Fischer344 (F344) substantia nigra (SN). GFP mRNA expression in the aged F344 SN was significantly lower (approximately 4-fold less) than expression levels in the young adult SN (***p<0.001) 12 days following rAAV2/5 vector injection. Values are displayed as the mean linearized GFP 2-ΔCT values ± percent change from young adult ΔCT values (Young Adult, n=6; Aged, n=6). (b) Representative images using RNAscope in situ hybridization for GFP in the Sprague Dawley (SD) midbrain 11 days following rAAV2/5 vector injection. GFP mRNA expression in the young adult rat is observed throughout the midbrain ipsilateral to the injection site, with dense staining observed at higher magnifications. Scale bar = 1000μm in top panel of lower magnification images, 200μm in lower panel images and 50μm in lower panel inserts. GFP mRNA expression in the midbrain of aged rats is visually less abundant than that of young adult rats with robust staining observed at higher magnifications.
4 Discussion
In the present study, we investigated the ability of rAAV2/5 to transduce neurons of the aged rat brain as compared to the young adult rat brain. We first verified, as demonstrated previously, that our rAAV2/5 expressing GFP is not toxic to nigral dopaminergic neurons of the SNpc (Gombash et al, 2013). Age-related reductions in total nigral dopaminergic neurons are not a factor in our present results as equivalent numbers of nigral THir neurons are present in the SD rat at 20 months as compared to 3 months of age. Although we achieved rAAV2/5-mediated GFP overexpression in the nigrostriatal system of both young adult and aged rats, aged rats consistently displayed lower levels of exogenous transgene expression than young adult rats. Aged rats exhibited approximately 40% fewer total GFPir cells throughout the midbrain. Our data suggests that both THir and non-THir neurons are transduced in the young adult and aged brain, and that a lack of transgene expression in dopaminergic cells is not the primary cause of the observed discrepancy as both age groups exhibit approximately equal percentages of THir neurons expressing GFP. Furthermore, the decrease in GFPir neurons in the aged brain does not appear to be related to differences in vector spread, because although the aged brain displayed fewer total numbers of GFPir neurons, the spread of transgene expression was observed in approximately the same number of sections through the rostral-caudal axis of the midbrain in both age groups and the rostral and caudal regions of transgene expression in the aged brain did not display significantly fewer GFPir neurons than the young adult brain. We observed decreases in GFP protein (western blot) in the aged rat striatum as well as the aged SN (immunohistochemistry), suggesting that the reduction in transgene expression in the aged striatum is a result of the deficient transgene expression in aged SN. These decreases in GFP protein expression were generalizable across different rat strains (SD and F344) and different expression durations (11 days to 3 months). Furthermore, we observed the most pronounced deficits in levels of GFP mRNA in the aged SN, demonstrating that the transduction deficiencies occur prior to translation and are not primarily the result of decreased protein synthesis with age (Ryazanov and Nefsky, 2002; Smith, Sun, and Sokoloff, 1995).
Although previous studies have shown successful gene transfer in the aged brain (Bartus et al, 2011; Emborg et al, 2009; Klein et al, 2010; Kordower et al, 2000; Wu et al, 2004), to our knowledge only two studies have previously conducted a direct comparison between the transduction efficiencies in young adult and aged animals (Klein et al, 2010; Wu et al, 2004). Wu et al. (2004) found transduction deficiencies in the aged rat when using rAAV2/2 to overexpress either GFP or nerve growth factor (NGF) in septal neurons in aged F344/Brown Norway rats. A modest and non-significant decrease in the total number of transduced cells in the aged septum was reported as compared to young adult rats. Due to the lighter immunostaining and decreased NGF protein levels in the aged as compared to the young adult rat, this group concluded that age-related deficits in protein expression within individual cells drove the observed transduction deficits. Klein et al. (2010) compared transduction efficiency of rAAV2/9 GFP or human wild-type tau in the SN of young adult and aged SD rats and reported no deficiencies in the aged rat brain. However, the sole comparative measure of transduction efficiency in this study was a western blot to quantify GFP and normalization to a loading control protein was not reported (Klein et al, 2010). In contrast, our results examining viral vector-mediated gene transfer utilizing numerous methodologies to evaluate vector-driven GFP protein and mRNA levels demonstrate that robust deficits in transgene expression that are associated with aging in the midbrain, and that specifically, rAAV2/5-mediated gene transfer results in decreases in both transgene mRNA and protein in aged as compared to young adult rats.
Due to the overlap between the steps of rAAV transduction (Schultz and Chamberlain, 2008) and the cellular processes affected by age (Gao et al, 2013; Lee, Weindruch, and Prolla, 2000; Ryazanov and Nefsky, 2002; Smith, Sun, and Sokoloff, 1995), it is reasonable to suggest that the aging brain environment alters the ability of rAAV2/5 to transduce neurons (Summarized in Figure 6). Specifically, in order to transduce a cell, rAAV2/5 must first bind to the N-linked sialic acid glycan receptor (Walters et al, 2001), platelet derived growth factor receptor (PDGFR) co-receptor (Di Pasquale et al, 2003), and possibly other receptors on the cell surface to initiate clathrin-mediated endocytosis of the rAAV5 capsid (Figure 6 step 1;Meier and Greber, 2004). Previous work has shown that N-linked sialic acid receptors are present in large quantities in the SN (Takahashi et al, 1995), and that these receptors are necessary for rAAV5 transduction whereby blocking these receptors will prevent rAAV5 from entering and transducing cells (Walters et al, 2001). Furthermore, age has been shown to impact levels of N-linked sialic acid receptors in various brain regions, with decreases reported in the aged rat hippocampus (Sato et al, 2001) and cerebellum (Sasaki et al, 2002) as compared to the young adult rats. Therefore, it is plausible that decreases in N-linked sialic acid receptors also exist in the aged rat SN, causing a decrease in the number of vector capsids that can bind the cells to initiate endocytosis and, consequently, a decrease in overall transduction in the aged brain.
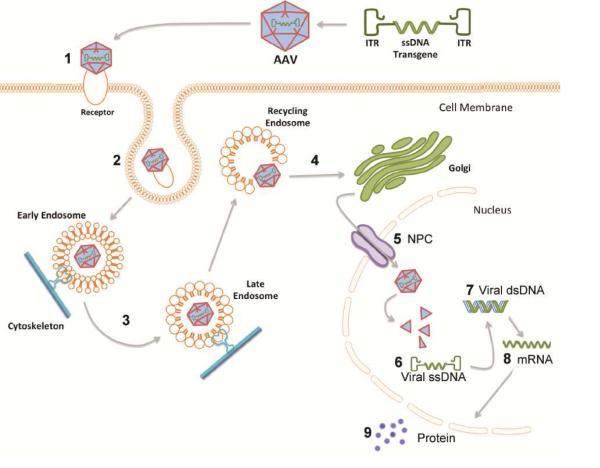
Figure 6. Steps of recombinant adeno-associated virus (rAAV) transduction and potential impact of the aged brain environment.
In viral transduction a single stranded DNA (ssDNA) expression cassette of interest is flanked by inverted terminal repeats (ITRs) to make up the recombinant genome that is packaged into rAAV capsid. 1) Injected rAAV capsids bind to designated cell surface receptor and co-receptors; rAAV2/5 binds with high affinity to 2,3-linked sialic acid glycan receptor (Walters et al, 2001) and platelet derived growth factor receptor (PDGFR) co-receptor (Di Pasquale et al, 2003). 2) Endocytosis of the viral particle occurs through clathrin coated pits or calveolar endocytosis; rAAV2/5 enters cell through clathrin-mediated endocytosis (Meier and Greber, 2004). 3) The rAAV capsid is trafficked through the cytoskeleton by dynamin in early, late, and recycling endosomes where acidification processes within the endosomes initiate capsid breakdown and genome release (Ding et al, 2005). 4) The viral particle is released from the endosome into the cytoplasm for golgi-mediated capsid processing (Bantel-Schall, Hub, and Kartenbeck, 2002). 5) The viral particle is internalized into the nucleus purportedly through the nuclear pore complex (NPC) (Hansen, Qing, and Srivastava, 2001). 6) The ssDNA genome is released from the viral capsid (Bartlett, Wilcher, and Samulski, 2000; Johnson and Samulski, 2009). 7) Viral ssDNA is converted to double stranded DNA (dsDNA) either by strand annealing or by second strand synthesis. 8) Viral dsDNA is converted into mRNA encoded by the transgene. 9) Transgene mRNA is translated into the protein encoded by the transgene. Numbers represent steps where viral transduction could be impeded by age-related cellular deficiencies (Bender et al, 2006; Blanpied, Scott, and Ehlers, 2003; D’Angelo et al, 2009; Gao et al, 2013; Kraytsberg et al, 2006; Park et al, 2001; Ryazanov and Nefsky, 2002; Sato et al, 2001; Sasaki et al, 2002; Smith, Sun, and Sokoloff, 1995). In the present set of experiments we demonstrate that robust deficits in exogenous transgene expression are associated with aging and that deficiencies leading to 4-fold less mRNA encoded by the transgene are primarily responsible for the diminished protein expression observed in the aged rats.
Endocytosis of rAAV2/5 then occurs through clathrin coated pits (Figure 6 step 2; Meier and Greber, 2004), a process which is thought to be less efficient in aged neurons (Blanpied, Scott, and Ehlers, 2003; Park et al, 2001). After endocytosis the viral particle is trafficked through the cytoskeleton by dynamin in early, late, and recycling endosomes where it undergoes acidification to initiate capsid breakdown and genome release (Figure 6 step 3; Ding et al, 2005). Cytoskeletal trafficking is highly energy-dependent, and decreased mitochondrial functioning with age (Kraytsberg et al, 2006; Bender et al, 2006) could decrease energy levels available for use in trafficking, leading to a buildup of viral particles in the cytoplasm that are unable to continue through the transduction process. Viral particles that are successfully trafficked then undergo golgi-mediated capsid processing (Figure 6 step 4; Bantel-Schall, Hub, and Kartenbeck, 2002) and enter the nucleus purportedly through the nuclear pore complex (NPC) (Figure 6 step 5; Hansen, Qing, and Srivastava, 2001). Aging neurons have demonstrated compromised NPC function (D’Angelo et al, 2009) which could decrease the ability of rAAV2/5 to enter the nucleus. However, once in the nucleus, the single stranded DNA (ssDNA) genome is released from the viral capsid (Figure 6 step 6; Bartlett, Wilcher, and Samulski, 2000; Johnson and Samulski, 2009) and is converted into double stranded DNA (dsDNA) by strand annealing or second strand synthesis (Figure 6 step 7). As age increases the permeability of the NPC (D’Angelo et al, 2009), aged nuclei have an influx of large, obtrusive proteins that could interfere with virion uncoating or conversion of ssDNA to dsDNA.
Aging is also known to impact transcription levels in the rodent SN (Gao et al, 2013), which would lead to decreased levels of viral genome transcription into mRNA (Figure 6 step 8). Thus, changes in transcription with age could potentially alter levels of other enzymes or proteins that are required for earlier stages of transduction, such as endocytosis or transport to the nucleus. This would hamper viral vector transduction before the level of transcription, reducing the levels of the viral transgene mRNA and, further downstream, the levels of viral protein produced (Figure 6 step 9). Moreover, protein synthesis is known to be modestly decreased (17% lower) in the aged rat brain and the SN in particular (Ryazanov and Nefsky, 2002; Smith, Sun, and Sokoloff, 1995). This could, in turn, affect viral vector transduction efficiency by decreasing the rate or amount of translation of the viral transgene into its protein products. We investigated this possibility by examining levels of protein expression of the viral transgene. While we did see a decrease in the viral protein product in the aged brain, the substantial (~50%) decrease that we observe cannot be completely accounted for by the previously reported modest decreases in SN protein synthesis with age (Smith, Sun, and Sokoloff, 1995). Furthermore, examination of transgene mRNA levels support this conclusion by revealing a more marked, 4-fold, decrease in transgene mRNA expression in the aged as compared to the young adult rat brain. Future studies will be required to accurately pinpoint which step(s) of transduction are deficient in aged nigral neurons that result in reduced transgene mRNA.
Our results do not address whether the reduced transgene expression that we observe with rAAV2/5 in the aged rat midbrain can be generalized to other vector constructs, other brain regions, or other species. Additional experiments are required to make these determinations, determinations that will be critical to the success of clinical trials using gene therapy in neurodegenerative diseases of aging. In summary, there are no current therapies that can halt or alter disease progression in age-related neurodegenerative diseases, such as PD. If the promise of gene therapy to supply the nigrostriatal system with trophic factors is to be realized in a population of aged patients, characteristic of PD, clinical success will depend on adequate transgene transfer to the aged brain. It is possible that the diminished transgene expression in the aged midbrain will result in sufficient expression to provide the desired biological effect. Yet despite displaying remarkable efficacy in preclinical trials in young animal models of PD, clinical trials with rAAV2-neurturin have yielded disappointing results (Bartus et al, 2011; Marks et al, 2010). Post-mortem analyses from human subjects that received rAAV2-neurturin indicated that transgene levels were remarkably low (Bartus et al, 2011). Although a multitude of explanations have been put forth, we argue that age may contribute to this discrepancy between preclinical and clinical trials. Moreover, a gene therapy trial using rAAV to express glial cell line-derived neurotrophic factor (GDNF) is ongoing (ClinicalTrials.gov, 2013). Aging-related deficits in exogenous transgene expression have the potential to impact these and other gene therapy trials in the future. Thus, future investigations examining transduction efficiency of various vector constructs and the mechanisms responsible for reductions in exogenous transgene expression in the aged brain are needed to identify approaches that will allow efficient transduction of the aged human brain.
Gene therapy is presently being utilized as a treatment strategy for Parkinson’s disease.
rAAV2/5 expressing GFP was injected into the substantia nigra of young and aged rats.
Aged rats demonstrated significantly fewer transduced cells, less GFP protein and less GFP mRNA.
rAAV2/5-mediated gene transfer is less efficient to the aged rat mesencephalon.
Acknowledgements
This research was supported by the Michael J. Fox Foundation (CES), the Graduate School of Michigan State University (NKP), Mercy Health Saint Mary’s (FPM) and the Morris K. Udall Center of Excellence for Parkinson’s Disease Research at Michigan State University NS058830 (TJC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
Authors have no conflicts of interest to report.
References
- Alemán CL, Más RM, Rodeiro I, Noa M, Hernández C, Menéndez R, Gámez R. Reference database of the main physiological parameters in Sprague-Dawley rats from 6-32 months. Lab Anim. 1998;32(4):457–466. doi: 10.1258/002367798780599802. [DOI] [PubMed] [Google Scholar]
- Bantel-Schall U, Hub B, Kartenbeck J. Endocytosis of adeno-associated virus type 5 leads to accumulation of virus particles in the Golgi compartment. J Virol. 2002;76:2340–2349. doi: 10.1128/jvi.76.5.2340-2349.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JS, Wilcher R, Samulski RJ. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J Virol. 2000;74:2777–2785. doi: 10.1128/jvi.74.6.2777-2785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Herzog CD, Chu Y, Wilson A, Brown L, Siffert J, Johnson EM, Jr., Olanow CW, Mufson EJ, Kordower JH. Bioactivity of AAV2-neurturin gene therapy (CERE-120): differences between Parkinson’s disease and nonhuman primate brains. Mov Disord. 2011;28(1):27–36. doi: 10.1002/mds.23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson’s disease. Nat Genet. 2006;38(5):515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Scott DB, Ehlers MD. Age-related regulation of dendritic endocytosis associated with altered clathrin dynamics. Neurobiol Ageing. 2003;24(8):1095–1104. doi: 10.1016/j.neurobiolaging.2003.04.004. [DOI] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzczka M. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1,2, and 5 display differential efficacy and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10(2):302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov. AAV2-GDNF for advanced Parkinson’s disease. 2013 http://www.clinicaltrials.gov/ct2/show/NCT01621581.
- Collier TJ, Sortwell CE, Daley BF. Diminished viability, growth, and behavioral efficacy of fetal dopamine neuron grafts in aging rats with long-term dopamine depletion: an argument for neurotrophic supplementation. J Neurosci. 1999;19(13):5563–5573. doi: 10.1523/JNEUROSCI.19-13-05563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier TJ, Kanaan NM, Kordower JH. Ageing as a primary risk factor for Parkinson’s disease: evidence from studies of non-human primates. Nat Rev Neurosci. 2011;12(6):359–366. doi: 10.1038/nrn3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo MA, Raices M, Panowski SH, Hetzer MW. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 2009;136:284–295. doi: 10.1016/j.cell.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale G, Davidson BL, Stein CS, Martins I, Scudiero D, Monks A, Chiorini JA. Identification of PDGFR as a receptor for AAV-5 transduction. Nat Med. 2003;9:1306–1312. doi: 10.1038/nm929. [DOI] [PubMed] [Google Scholar]
- Ding W, Zhang L, Yan Z, Engelhardt JF. Intracellular trafficking of adeno-associated viral vectors. Gene Ther. 2005;12:873–880. doi: 10.1038/sj.gt.3302527. [DOI] [PubMed] [Google Scholar]
- Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, Tanner CM. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- Emborg ME, Miorano J, Raschke J, Bondarenko V, Zufferey R, Peng S, Ebert AD, Joers V, Roitberg B, Holden JE, Koprich J, Lipton J, Kordower JH, Aebischer P. Response of aged parkinsonian monkeys to in vivo gene transfer of GDNF. Neurobiol Dis. 2009;36(2):303–311. doi: 10.1016/j.nbd.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Miao H, Xiao CH, Sun Y, Du X, Yuan HH, Yu HL, Gao DS. Influence of aging on the dopaminergic neurons in the substantia nigra pars compacta of rats. Curr Aging Sci. 2011;4(1):19–24. doi: 10.2174/1874609811104010019. [DOI] [PubMed] [Google Scholar]
- Gao L, Hidalgo-Figueroa M, Escudero LM, Diaz-Martin J, Lopez-Barneo J, Pascual A. Age-mediated transcriptomic changes in adult mouse substantia nigra. PlosOne. 2013;8(4):e62456. doi: 10.1371/journal.pone.0062456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D, Lapchak PA, Collins F, Hoffer BJ, Gerhardt GA. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- Gasmi M, Brandon EP, Herzog CD, Wilson A, Bishop KM, Hofer EK, Cunningham JJ, Printz MA, Kordower JH, Bartus RT. AAV2-mediated delivery of human neurturin to the rat nigrostriatal system: Long-term efficacy and tolerability of CERE-120 for Parkinson's disease. Neurobiol Dis. 2007;27:67–76. doi: 10.1016/j.nbd.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Gombash SE, Manfredsson FP, Mandel RJ, Collier TJ, Fischer DL, Kemp CJ, Kuhn NM, Wohlgenant SL, Fleming SM, Sortwell CE. Striatonigral pleiotrophin overexpression provides superior protection compared to overexpression in both striatonigral and nigrostriatal pathways. Gene Ther. 2014 doi: 10.1038/gt.2014.42. doi: 10.1038/gt.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombash SE, Manfredsson FP, Kemp CJ, Kuhn NC, Fleming SM, Egan AE, Grant LM, Ciucci MR, MacKeigan JP, Sortwell CE. Morphological and behavioral impact of AAV2/5-mediated overexpression of human wildtype alpha-synuclein in the rat nigrostriatal system. PLoS One. 2013;8(11):e81426. doi: 10.1371/journal.pone.0081426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombash SE, Lipton JW, Collier TJ, Madhavan L, Steece-Collier K, Cole-Strauss A, Terpstra BT, Spieles-Engemann AL, Daley BF, Wohlgenant SL, Thompson VB, Manfredsson FP, Mandel RJ, Sortwell CE. Striatal pleiotrophin overexpression provides functional and morphological neuroprotection in the 6-hydroxydopamine model. Mol Ther. 2012;20(3):544–554. doi: 10.1038/mt.2011.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Qing K, Srivastava A. Infection of purified nuclei by adeno-associated virus 2. Mol Ther. 2001;4(4):289–296. doi: 10.1006/mthe.2001.0457. [DOI] [PubMed] [Google Scholar]
- Herzog CD, Brown L, Gammon D, Kruegel B, Lin R, Wilson A, Bolton A, Printz M, Gasmi M, Bishop KM, Kordower KH, Bartus RT. Expression, bioactivity, and safety 1 year after adeno-associated viral vector type 2-mediated delivery of neurtrin to the monkey nigrostriatal system support CERE-120 for Parkinson’s disease. Neurosurgery. 2008;64(6):602–613. doi: 10.1227/01.NEU.0000340682.06068.01. [DOI] [PubMed] [Google Scholar]
- Johnson J, Samulski R. Enhancement of adeno-associated virus infection by mobilizing capsids into and out of the nucleolus. J Virol. 2009;83:2632–2644. doi: 10.1128/JVI.02309-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Dayton RD, Diaczynsky CG, Wang DB. Pronounced microgliosis and neurodegeneration in aged rats after tau gene transfer. Neurobiol Aging. 2010;31(12):2091–2102. doi: 10.1016/j.neurobiolaging.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Déglon N, Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko N. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet. 2006;38(5):518–520. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- Lee C, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25:294–297. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- Marks WJ, Jr, Bartus RT, Siffert J, Davis CS, Lozano A, Boulis N, Vitek J, Stacy M, Turner D, Verhagen L, Bakay R, Watts R, Guthrie B, Jankovic J, Sompson R, Tagliati M, Alterman R, Stern M, Baltuch G, Starr PA, Larson PS, Ostrem JL, Nutt J, Kieburtz K, Kordower JH, Olanow CW. Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind randomized, controlled trial. Lancet. 2010;9:1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- Meier O, Greber UF. Adenovirus endocytosis. J Gene Med. 2004;6(Suppl 1):S152–S163. doi: 10.1002/jgm.553. [DOI] [PubMed] [Google Scholar]
- Park J, Park W, Cho K, Kim D, Jhun B, Kim S, Park SC. Down-regulation of amphiphysin-1 is responsible for reduced receptor-mediated endocytosis in the senescent cells. FASEB J. 2001;15(9):1625–1627. doi: 10.1096/fj.00-0723fje. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST©) for groupwise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Research. 2002;30(9):e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piltonen M, Bespalov MM, Ervasti D, Matilainen T, Sidorova YA, Rauvala H, Saarma M, Mannisto PT. Heparin-binding determinants of GDNF reduce its tissue distribution but are beneficial for the protection of nigral dopaminergic neurons. Exp Neurol. 2009;219(2):499–506. doi: 10.1016/j.expneurol.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Ryazanov AG, Nefsky BS. Protein turnover plays a key role in aging. Mec Ageing Dev. 2002;123:207–213. doi: 10.1016/s0047-6374(01)00337-2. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Akimoto Y, Sato Y, Kawakami H, Hirano H, Endo T. Distribution of sialoglycoconjugates in the rat cerebellum and its change with age. J Histochem Cytochem. 2002;50(9):1179–1186. doi: 10.1177/002215540205000904. [DOI] [PubMed] [Google Scholar]
- Sato Y, Akimoto Y, Kawakami H, Hirano H, Endo T. Location of sialoglycoconjugates containing sia(alpha)2-3Gal and Sia(alpha)2-6Gal groups in the rat hippocampus and the effect of aging on their expression. J Histochem Cytochem. 2001;49(10):1311–1319. doi: 10.1177/002215540104901014. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schultz BR, Chamberlain JS. Recombinant adeno-associated virus transduction and integration. Mol Ther. 2008;16(7):119–1199. doi: 10.1038/mt.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CB, Sun Y, Sokoloff L. Effects of aging on regional rates of cerebral protein synthesis in the Sprague-Dawley rat: examination of the influence of recycling of amino acids derived from protein degradation into the precursor pool. Neurochem. 1995;27(4/5):407–416. doi: 10.1016/0197-0186(95)00022-z. [DOI] [PubMed] [Google Scholar]
- Sortwell CE, Camargo MD, Pitzer MR, Gyawali S, Collier TJ. Diminished survival of mesencephalic dopamine neurons grafted into aged hosts occurs during the immediate postgrafting interval. Exp Neurol. 2001;169(1):23–29. doi: 10.1006/exnr.2001.7644. [DOI] [PubMed] [Google Scholar]
- Stocchi F, Olanow CW. Obstacles to the development of a neuroprotective therapy for Parkinsons’s disease. Mov Disord. 2013;28(1):3–7. doi: 10.1002/mds.25337. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Yamada T, Nakajima S, Yamamoto T, Okada H. The substantia nigra is a major target for neurovirulent influenza A virus. J Exp Med. 1995;181(6):2161–2169. doi: 10.1084/jem.181.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RW, Yi SM, Keshavjee S, Brown KE, Welsh MJ, Chiorini JA, Zabner J. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J Biol Chem. 2001;276:20610–20616. doi: 10.1074/jbc.M101559200. [DOI] [PubMed] [Google Scholar]
- Wang F, Flanagan J, Su N, Wang L, Bui S, Nielson A, Wu X, Vo HT, Ma XJ, Luo Y. RNAscope: A novel in situ RNA platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14(1):22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Meyers CA, Guerra NK, King MA, Meyer EM. The effects of rAAV2-mediated NGF gene delivery in adult and aged rats. Mol Ther. 2004;9(2):262–269. doi: 10.1016/j.ymthe.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, Byme BJ, Mason E, Zolotukhin E, Potter M, Chesnut K, Summerford C, Samulski RJ, Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]