Abstract
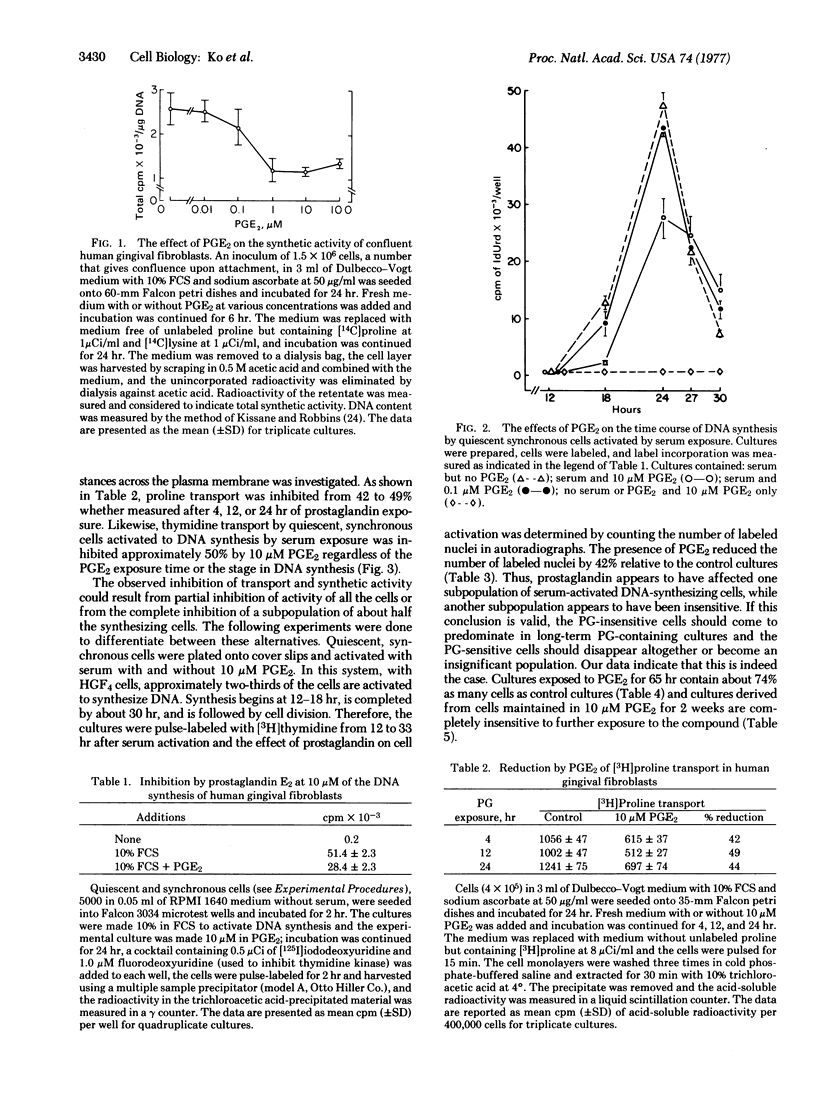
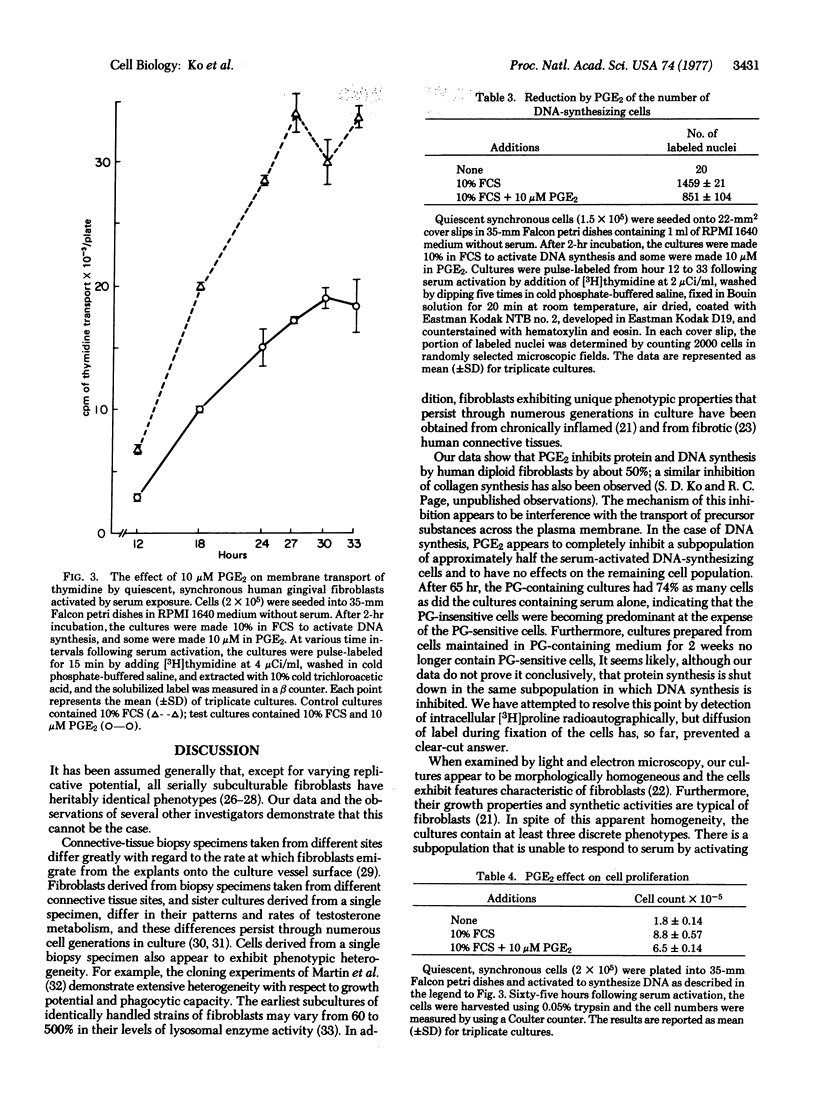
The effects of prostaglandin E2 (PGE2) upon the synthesis of protein and DNA, and membrane transport of proline and thymidine, by human diploid fibroblasts were studied. At a concentration range of 1-10 μM, PGE2 inhibited protein synthesis and membrane transport by 45-50%. Serum-activated DNA synthesis and thymidine transport were also inhibited by approximately 50% in cells made quiescent and synchronous by serum deprivation. To determine whether prostaglandin inhibits some of the cells completely or all of the cells partially, radioautographic and cell-counting experiments were done. In cultures pulse-labeled with [3H]thymidine 12-33 hr after serum activation, prostaglandin exposure reduced the number of labeled nuclei by 42%. Sixty-five hours after serum activation, the total cell numbers present in the PGE2-exposed cultures were reduced by 25%. Furthermore, in the fibroblast cultures derived from cells previously maintained in 10 μM PGE2 for 14 days, PGE2 had no effect on DNA synthesis, indicating that the PGE2-sensitive cells had disappeared from the cultures. Thus, PGE2 appears to inhibit growth and synthesis of a subpopulation of cells while not affecting the remaining insensitive cells. Prostaglandins may play an important role in connective-tissue differentiation and in some pathologic alterations by regulating fibroblast subpopulations.
Keywords: DNA synthesis, mitosis, connective tissue, collagen, gingiva
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenkrantz N., Sondergaard J. Effect of prostaglandins E 1 and F 1 on biosynthesis of collagen. Nat New Biol. 1972 Oct 25;239(95):246–246. doi: 10.1038/newbio239246a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Lichtenstein L. M., Melmon K. L., Henney C. S., Weinstein Y., Shearer G. M. Modulation of inflammation and immunity by cyclic AMP. Science. 1974 Apr 5;184(4132):19–28. doi: 10.1126/science.184.4132.19. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G. S., Beuttal S. C., Lebovitz H. E. Inhibition of cartilage macromolecular synthesis by prostaglandin A. J Pharmacol Exp Ther. 1974 Apr;189(1):213–220. [PubMed] [Google Scholar]
- Ferraris V. A., DeRubertis F. R. Release of prostaglandin by mitogen- and antigen-stimulated leukocytes in culture. J Clin Invest. 1974 Aug;54(2):378–386. doi: 10.1172/JCI107773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goggins J. F., Johnson G. S., Pastan I. The effect of dibutyryl cyclic adenosine monophosphate on synthesis of sulfated acid mucopolysaccharides by transformed fibroblasts. J Biol Chem. 1972 Sep 25;247(18):5759–5764. [PubMed] [Google Scholar]
- Goodson J. M., Dewhirst F. E., Brunetti A. Prostaglandin E2 levels and human periodontal disease. Prostaglandins. 1974 Apr 10;6(1):81–85. doi: 10.1016/s0090-6980(74)80043-2. [DOI] [PubMed] [Google Scholar]
- Hassell T. M., Page R. C., Narayanan A. S., Cooper C. G. Diphenylhydantoin (dilantin) gingival hyperplasia: drug-induced abnormality of connective tissue. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2909–2912. doi: 10.1073/pnas.73.8.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck J. C., Sharma V. K., Hayflick L. Functional failures of cultured human diploid fibroblasts after continued population doublings. Proc Soc Exp Biol Med. 1971 May;137(1):331–333. doi: 10.3181/00379727-137-35571. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Jones C., Puck T. T. Further changes in differentiation state accompanying the conversion of Chinese hamster cells of fibroblastic form by dibutyryl adenosine cyclic 3':5'-monophosphate and hormones. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1648–1652. doi: 10.1073/pnas.68.7.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubscher T. Role of the eosinophil in the allergic reactions. II. Release of prostaglandins from human eosinophilic leukocytes. J Immunol. 1975 Apr;114(4):1389–1393. [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kaufman M., Pinsky L., Straisfeld C., Shanfield B., Zilahi B. Qualitative differences in testosterone metabolism as an indication of cellular heterogeneity in fibroblast monolayers derived from human preputial skin. Exp Cell Res. 1975 Nov;96(1):31–36. doi: 10.1016/s0014-4827(75)80033-4. [DOI] [PubMed] [Google Scholar]
- Kischer C. W. Effects of specific prostaglandins on development of chick embryo skin and down feather organ, in vitro. Dev Biol. 1967 Sep;16(3):203–215. doi: 10.1016/0012-1606(67)90024-3. [DOI] [PubMed] [Google Scholar]
- Klein D. C., Raisz L. G. Prostaglandins: stimulation of bone resorption in tissue culture. Endocrinology. 1970 Jun;86(6):1436–1440. doi: 10.1210/endo-86-6-1436. [DOI] [PubMed] [Google Scholar]
- Lupulescu A. Effect of prostaglandins on protein, RNA, DNA and collagen synthesis in experimental wounds. Prostaglandins. 1975 Oct;10(4):573–579. doi: 10.1016/s0090-6980(75)80003-7. [DOI] [PubMed] [Google Scholar]
- Manner G., Kuleba M. Effect of dibutyryl-cyclic AMP on collagen and non-collagen protein synthesis in cultured human cells. Connect Tissue Res. 1974;2(3):167–175. doi: 10.3109/03008207409152241. [DOI] [PubMed] [Google Scholar]
- Martin G. M., Sprague C. A., Norwood T. H., Pendergrass W. R. Clonal selection, attenuation and differentiation in an in vitro model of hyperplasia. Am J Pathol. 1974 Jan;74(1):137–154. [PMC free article] [PubMed] [Google Scholar]
- Milunsky A., Spielvogel C., Kanfer J. N. Lysosomal enzyme variations in cultured normal skin fibroblasts. Life Sci II. 1972 Nov 22;11(22):1101–1107. doi: 10.1016/0024-3205(72)90219-6. [DOI] [PubMed] [Google Scholar]
- Myatt L., Bray M. A., Gordon D., Morley J. Macrophages on intrauterine contraceptive devices produce prostaglandins. Nature. 1975 Sep 18;257(5523):227–228. doi: 10.1038/257227a0. [DOI] [PubMed] [Google Scholar]
- Narayanan A. S., Page R. C. Biochemical characterization of collagens synthesized by fibroblasts derived from normal and diseased human gingiva. J Biol Chem. 1976 Sep 25;251(18):5464–5471. [PubMed] [Google Scholar]
- Peterkofsky B., Prather W. B. Increased collagen synthesis in Kirsten sarcoma virus-transformed BALB 3T3 cells grown in the presence of dibutyryl cyclic AMP. Cell. 1974 Nov;3(3):291–299. doi: 10.1016/0092-8674(74)90144-5. [DOI] [PubMed] [Google Scholar]
- Pinsky L., Finkelberg R., Straisfeld C., Zilahi B., Kaufman M., Hall G. Testosterone metabolism by serially subcultured fibroblasts from genital and nongenital skin of individual human donors. Biochem Biophys Res Commun. 1972 Jan 31;46(2):364–369. doi: 10.1016/s0006-291x(72)80147-5. [DOI] [PubMed] [Google Scholar]
- Raisz L. G., Koolemans-Beynen A. R. Inhibition of bone collagen synthesis by prostaglandin E2 in organ culture. Prostaglandins. 1974 Dec 10;8(5):377–385. doi: 10.1016/0090-6980(74)90113-0. [DOI] [PubMed] [Google Scholar]
- Robinson D. R., Tashjian A. H., Jr, Levine L. Prostaglandin-stimulated bone resorption by rheumatoid synovia. A possible mechanism for bone destruction in rheumatoid arthritis. J Clin Invest. 1975 Nov;56(5):1181–1188. doi: 10.1172/JCI108195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Jimenez de Asua L. Role of cyclic 3':5'-adenosine monophosphate in the early transport changes induced by serum and insulin in quiescent fibroblasts. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3609–3612. doi: 10.1073/pnas.70.12.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B., Granström E., Green K., Hamberg M., Hammarström S. Prostaglandins. Annu Rev Biochem. 1975;44:669–695. doi: 10.1146/annurev.bi.44.070175.003321. [DOI] [PubMed] [Google Scholar]
- Singal D. P., Goldstein S. Absence of detectable HL-A antigens on cultured fibroblasts in progeria. J Clin Invest. 1973 Sep;52(9):2259–2263. doi: 10.1172/JCI107412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. R., Hayflick L. Variation in the life-span of clones derived from human diploid cell strains. J Cell Biol. 1974 Jul;62(1):48–53. doi: 10.1083/jcb.62.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom T. B., Carpenter C. B., Garovoy M. R., Austen K. F., Merrill J. P., Kaliner M. The modulating influence of cyclic nucleotides upon lymphocyte-mediated cytotoxicity. J Exp Med. 1973 Aug 1;138(2):381–393. doi: 10.1084/jem.138.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velo G. P., Dunn C. J., Giroud J. P., Timsit J., Willoughby D. A. Distribution of prostaglandins in inflammatory exudate. J Pathol. 1973 Nov;111(3):149–158. doi: 10.1002/path.1711110302. [DOI] [PubMed] [Google Scholar]
- Zurier R. B. Prostaglandins, inflammation, and asthma. Arch Intern Med. 1974 Jan;133(1):101–110. [PubMed] [Google Scholar]
- Zurier R. B., Weissmann G., Hoffstein S., Kammerman S., Tai H. H. Mechanisms of lysosomal enzyme release from human leukocytes. II. Effects of cAMP and cGMP, autonomic agonists, and agents which affect microtubule function. J Clin Invest. 1974 Jan;53(1):297–309. doi: 10.1172/JCI107550. [DOI] [PMC free article] [PubMed] [Google Scholar]


