Abstract
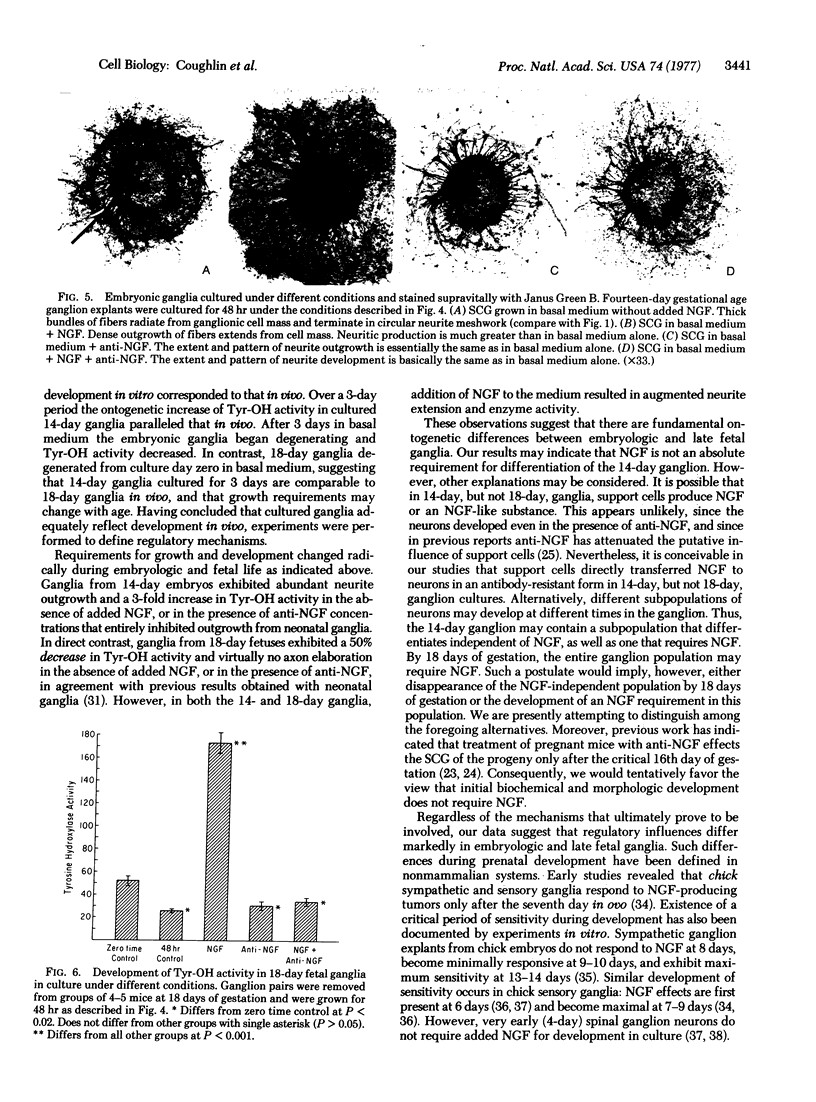
The morphologic and biochemical development of the embryonic mouse superior cervical ganglion was characterized in vivo and in tissue culture. From 13 days of gestation, when the superior cervical ganglion was first visible, to birth at 19 days, tyrosine hydroxylase [tyrosine 3-monooxygenase; L-tyrosine, tetrahydropteridine:oxygen oxidoreductase (3-hydroxylating); EC 1.14.16.2] activity increased 100-fold in vivo. Explants of ganglia from 14-day embryos exhibited abundant neurite outgrowth in basal medium without added nerve growth factor (NGF), and increases in tyrosine hydroxylase activity paralleled that observed in vivo. Ganglia from 14-day embryos elaborated neurites and exhibited 3-fold increases in enzyme activity in vitro in the presence of antiserum to NGF (anti-NGF) or NGF + anti-NGF. In direct contrast, ganglia from 18-day fetuses failed to grow without added NGF or in medium containing anti-NGF or NGF + anti-NGF: virtually no axon outgrowth occurred and tyrosine hydroxylase activity decreased by half. These observations suggest that developmental regulatory mechanisms change radically during embryologic and fetal life of mammalian superior cervical ganglion.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augulis V., Sigg E. B. Supravital staining and fixation of brain and spinal cord by intravascular perfusion. Stain Technol. 1971 Jul;46(4):183–190. doi: 10.3109/10520297109067851. [DOI] [PubMed] [Google Scholar]
- Black I. B., Geen S. C. Trans-synaptic regulation of adrenergic neuron development: inhibition by ganglionic blockade. Brain Res. 1973 Dec 7;63:291–302. doi: 10.1016/0006-8993(73)90096-6. [DOI] [PubMed] [Google Scholar]
- Black I. B., Hendry I. A., Iversen L. L. Effects of surgical decentralization and nerve growth factor on the maturation of adrenergic neurons in a mouse sympathetic ganglion. J Neurochem. 1972 May;19(5):1367–1377. doi: 10.1111/j.1471-4159.1972.tb01461.x. [DOI] [PubMed] [Google Scholar]
- Black I. B., Hendry I. A., Iversen L. L. The role of post-synaptic neurones in the biochemical maturation of presynaptic cholinergic nerve terminals in a mouse sympathetic ganglion. J Physiol. 1972 Feb;221(1):149–159. doi: 10.1113/jphysiol.1972.sp009745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black I. B., Hendry I. A., Iversen L. L. Trans-synaptic regulation of growth and development of adrenergic neurones in a mouse sympathetic ganglion. Brain Res. 1971 Nov;34(2):229–240. doi: 10.1016/0006-8993(71)90278-2. [DOI] [PubMed] [Google Scholar]
- Black I. B., Hendry I., Iversen L. L. Differences in the regulation of tyrosine hydroxylase and dopa decarboxylase in sympathetic ganglia and adrenals. Nat New Biol. 1971 May 5;231(18):27–29. [PubMed] [Google Scholar]
- Black I. B. Increased tyrosine hydroxylase activity in frontal cortex and cerebellum after reserpine. Brain Res. 1975 Sep 12;95(1):170–176. doi: 10.1016/0006-8993(75)90219-x. [DOI] [PubMed] [Google Scholar]
- Black I. B., Joh T. H., Reis D. J. Accumulation of tyrosine hydroxylase molecules during growth and development of the superior cervical ganglion. Brain Res. 1974 Jul 19;75(1):133–144. doi: 10.1016/0006-8993(74)90775-6. [DOI] [PubMed] [Google Scholar]
- Black I. B., Mytilineou C. Trans-synaptic regulation of the development of end organ innervation by sympathetic neurons. Brain Res. 1976 Jan 23;101(3):503–521. doi: 10.1016/0006-8993(76)90474-1. [DOI] [PubMed] [Google Scholar]
- Bray D. Surface movements during the growth of single explanted neurons. Proc Natl Acad Sci U S A. 1970 Apr;65(4):905–910. doi: 10.1073/pnas.65.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley J. H., Campbell G. R., Burnstock G. An analysis of the interactions between sympathetic nerve fibers and smooth muscle cells in tissue culture. Dev Biol. 1973 Aug;33(2):344–361. doi: 10.1016/0012-1606(73)90142-5. [DOI] [PubMed] [Google Scholar]
- Cohen A. M. Factors directing the expression of sympathetic nerve traits in cells of neural crest origin. J Exp Zool. 1972 Feb;179(2):167–182. doi: 10.1002/jez.1401790204. [DOI] [PubMed] [Google Scholar]
- Cohen S., Levi-Montalcini R., Hamburger V. A NERVE GROWTH-STIMULATING FACTOR ISOLATED FROM SARCOM AS 37 AND 180. Proc Natl Acad Sci U S A. 1954 Oct;40(10):1014–1018. doi: 10.1073/pnas.40.10.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. PURIFICATION OF A NERVE-GROWTH PROMOTING PROTEIN FROM THE MOUSE SALIVARY GLAND AND ITS NEURO-CYTOTOXIC ANTISERUM. Proc Natl Acad Sci U S A. 1960 Mar;46(3):302–311. doi: 10.1073/pnas.46.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin M. D. Early development of parasympathetic nerves in the mouse submandibular gland. Dev Biol. 1975 Mar;43(1):123–139. doi: 10.1016/0012-1606(75)90136-0. [DOI] [PubMed] [Google Scholar]
- Coughlin M. D. Target organ stimulation of parasympathetic nerve growth in the developing mouse submandibular gland. Dev Biol. 1975 Mar;43(1):140–158. doi: 10.1016/0012-1606(75)90137-2. [DOI] [PubMed] [Google Scholar]
- Crain S. M., Peterson E. R. Development of neural connections in culture. Ann N Y Acad Sci. 1974 Mar 22;228(0):6–34. doi: 10.1111/j.1749-6632.1974.tb20499.x. [DOI] [PubMed] [Google Scholar]
- Dibner M. D., Black I. B. The effect of taget organ removal on the development of sympathetic neurons. Brain Res. 1976 Feb 13;103(1):93–102. doi: 10.1016/0006-8993(76)90689-2. [DOI] [PubMed] [Google Scholar]
- Klingman G. I. In utero immunosympathectomy of mice. Int J Neuropharmacol. 1966 Mar;5(2):163–170. doi: 10.1016/0028-3908(66)90019-0. [DOI] [PubMed] [Google Scholar]
- Klingman G. I., Klingman J. D. Catecholamines in peripheral tissues of mice and cell counts of sympathetic ganglia after the prenatal and postnatal administration of the nerve growth factor antiserum. Int J Neuropharmacol. 1967 Nov;6(6):501–508. doi: 10.1016/0028-3908(67)90050-0. [DOI] [PubMed] [Google Scholar]
- Ko C. P., Burton H., Johnson M. I., Bunge R. P. Synaptic transmission between rat superior cervical ganglion neurons in dissociated cell cultures. Brain Res. 1976 Dec 3;117(3):461–485. doi: 10.1016/0006-8993(76)90753-8. [DOI] [PubMed] [Google Scholar]
- LEVI-MONTALCINI R., HAMBURGER V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool. 1951 Mar;116(2):321–361. doi: 10.1002/jez.1401160206. [DOI] [PubMed] [Google Scholar]
- LEVITT M., SPECTOR S., SJOERDSMA A., UDENFRIEND S. ELUCIDATION OF THE RATE-LIMITING STEP IN NOREPINEPHRINE BIOSYNTHESIS IN THE PERFUSED GUINEA-PIG HEART. J Pharmacol Exp Ther. 1965 Apr;148:1–8. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Letourneau P. C. Possible roles for cell-to-substratum adhesion in neuronal morphogenesis. Dev Biol. 1975 May;44(1):77–91. doi: 10.1016/0012-1606(75)90378-4. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Nerve growth factor. Physiol Rev. 1968 Jul;48(3):534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Ludueña M. A. Nerve cell differentiation in vitro. Dev Biol. 1973 Aug;33(2):268–284. doi: 10.1016/0012-1606(73)90137-1. [DOI] [PubMed] [Google Scholar]
- Mackay A. V. The long-term regulation of tyrosine hydroxylase activity in cultured sympathetic ganglia: role of ganglionic noradrenaline content. Br J Pharmacol. 1974 Aug;51(4):509–520. doi: 10.1111/j.1476-5381.1974.tb09669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains R. E., Patterson P. H. Primary cultures of dissociated sympathetic neurons. I. Establishment of long-term growth in culture and studies of differentiated properties. J Cell Biol. 1973 Nov;59(2 Pt 1):329–345. doi: 10.1083/jcb.59.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B., Bamburg J. R. Studies on the action of nerve growth factor. I. Characterization of a simplified in vitro culture system for dorsal root and sympathetic ganglia. Dev Biol. 1976 Mar;49(1):11–19. doi: 10.1016/0012-1606(76)90254-2. [DOI] [PubMed] [Google Scholar]
- Norr S. C. In vitro analysis of sympathetic neuron differentiation from chick neural crest cells. Dev Biol. 1973 Sep;34(1):16–38. doi: 10.1016/0012-1606(73)90336-9. [DOI] [PubMed] [Google Scholar]
- Partlow L. M., Larrabee M. G. Effects of a nerve-growth factor, embryo age and metabolic inhibitors on growth of fibres and on synthesis of ribonucleic acid and protein in embryonic sympathetic ganglia. J Neurochem. 1971 Nov;18(11):2101–2118. doi: 10.1111/j.1471-4159.1971.tb05069.x. [DOI] [PubMed] [Google Scholar]
- Patterson P. H., Chun L. L. The influence of non-neuronal cells on catecholamine and acetylcholine synthesis and accumulation in cultures of dissociated sympathetic neurons. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3607–3610. doi: 10.1073/pnas.71.9.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon S., Nomura J., Shooter E. M. The isolation of the mouse nerve growth factor protein in a high molecular weight form. Biochemistry. 1967 Jul;6(7):2202–2209. doi: 10.1021/bi00859a043. [DOI] [PubMed] [Google Scholar]
- Varon S., Raiborn C., Burnham P. A. Implication of a nerve growth factor-like antigen in the support derived by ganglionic neurons from their homologous glia in dissociated cultures. Neurobiology. 1974;4(5):317–327. [PubMed] [Google Scholar]
- WINICK M., GREENBERG R. E. CHEMICAL CONTROL OF SENSORY GANGLIA DURING A CRITICAL PERIOD OF DEVELOPMENT. Nature. 1965 Jan 9;205:180–181. doi: 10.1038/205180a0. [DOI] [PubMed] [Google Scholar]