Abstract
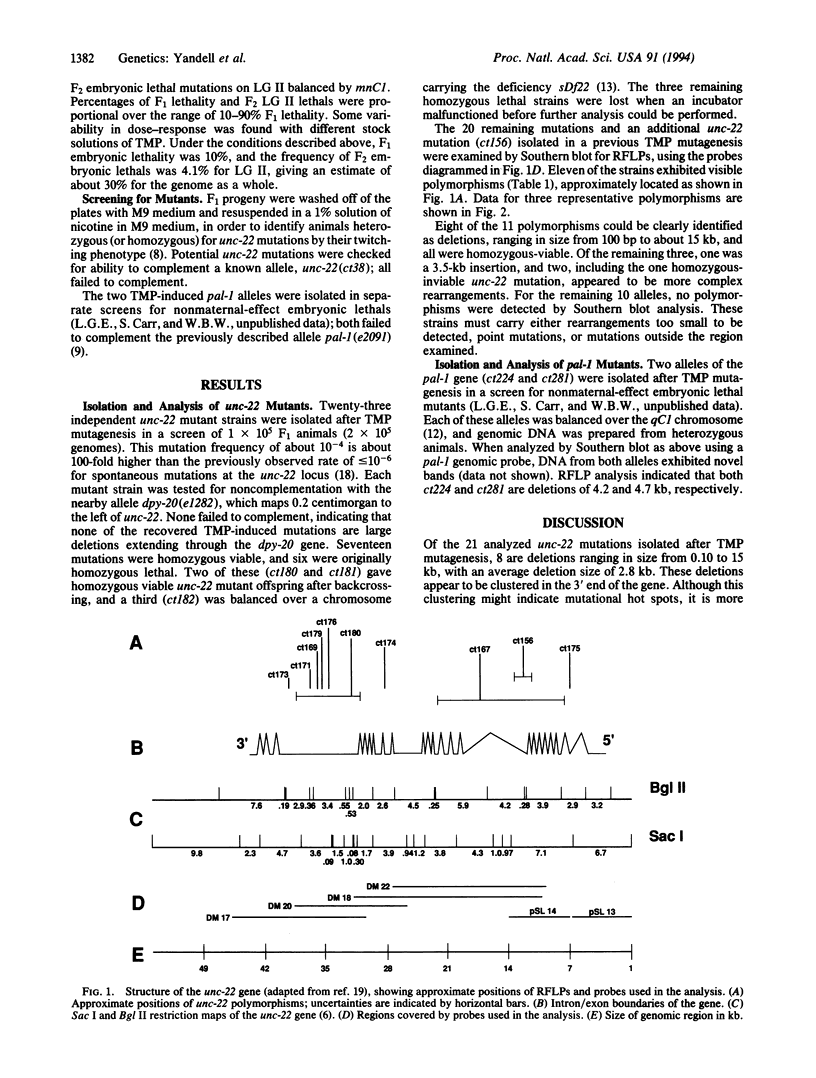
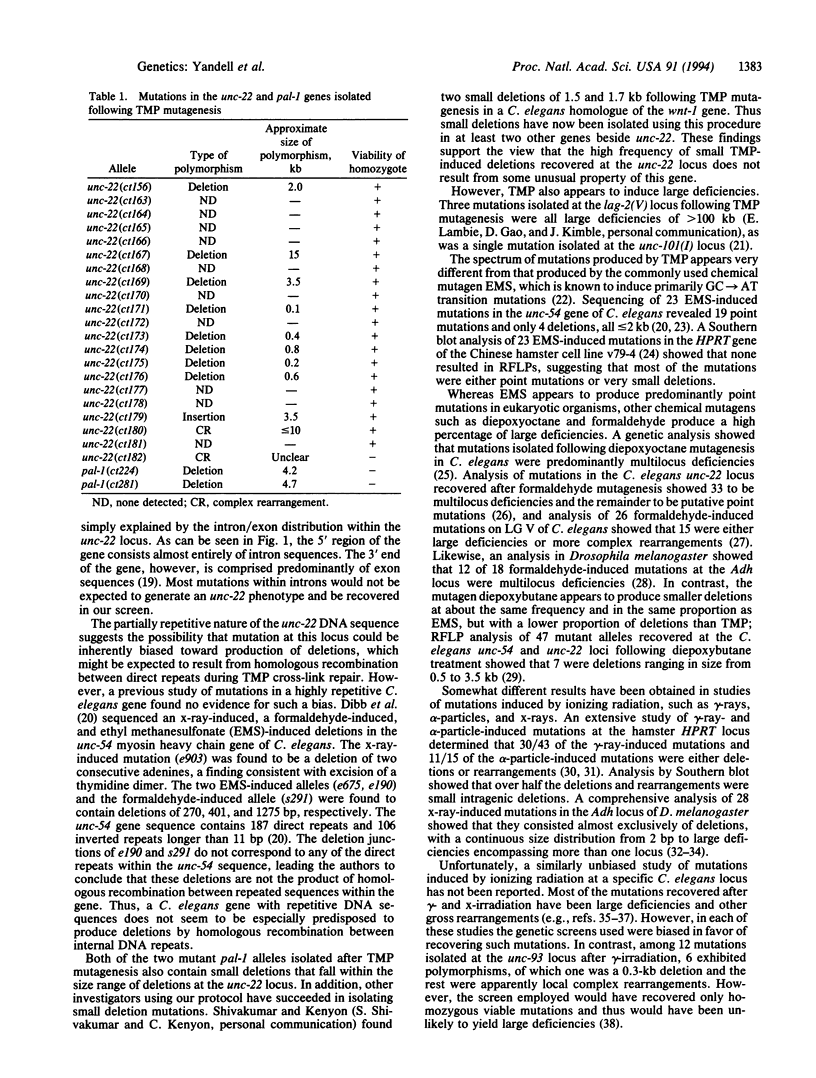
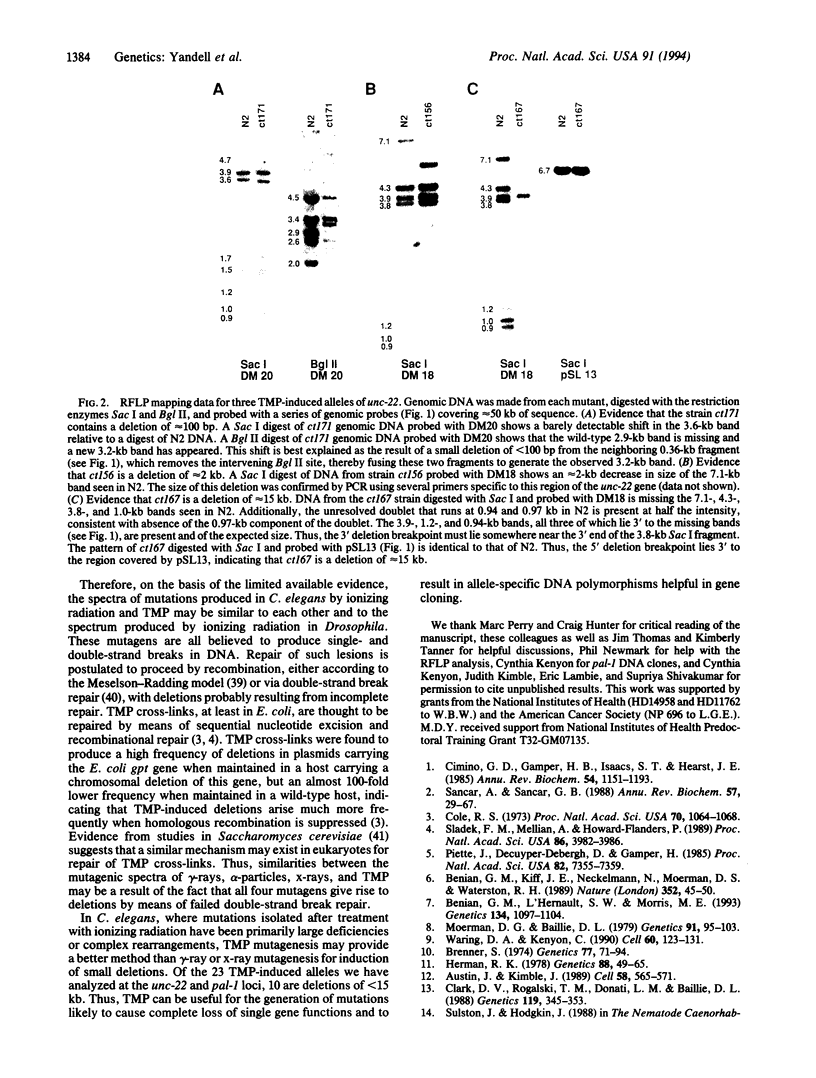
To examine the mutagenic spectrum of 4,5',8-trimethylpsoralen (TMP) in Caenorhabditis elegans, we isolated mutations in the unc-22 and pal-1 genes following TMP mutagenesis and analyzed them for restriction fragment length polymorphisms by Southern blot. Eleven of 21 unc-22 mutations exhibited restriction fragment length polymorphisms, 8 of which were deletions of between 0.10 and 15 kb in length. Both of two pal-1 mutations were also small deletions within this size range. Comparison of our results with previous studies on mutagenesis by gamma-rays and x-rays suggests that the mutagenic spectrum of TMP may be similar. TMP should be useful in generating mutations that cause complete loss of function of single genes and that are likely to result in allele-specific DNA polymorphisms.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaron C. S. X-ray-induced mutations affecting the level of the enzyme alcohol dehydrogenase in Drosophila melanogaster: frequency and genetic analysis of null-enzyme mutants. Mutat Res. 1979 Nov;63(1):127–137. doi: 10.1016/0027-5107(79)90109-x. [DOI] [PubMed] [Google Scholar]
- Anderson P., Brenner S. A selection for myosin heavy chain mutants in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4470–4474. doi: 10.1073/pnas.81.14.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M., Aaron C. S., Tsubota S. The genetics of a small autosomal region of Drosophila melanogaster, including the structural gene for alcohol dehydrogenase. V. Characterization of X-ray-induced Adh null mutations. Genetics. 1982 Nov;102(3):421–435. doi: 10.1093/genetics/102.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin J., Kimble J. Transcript analysis of glp-1 and lin-12, homologous genes required for cell interactions during development of C. elegans. Cell. 1989 Aug 11;58(3):565–571. doi: 10.1016/0092-8674(89)90437-6. [DOI] [PubMed] [Google Scholar]
- Bejsovec A., Anderson P. Functions of the myosin ATP and actin binding sites are required for C. elegans thick filament assembly. Cell. 1990 Jan 12;60(1):133–140. doi: 10.1016/0092-8674(90)90723-r. [DOI] [PubMed] [Google Scholar]
- Benian G. M., Kiff J. E., Neckelmann N., Moerman D. G., Waterston R. H. Sequence of an unusually large protein implicated in regulation of myosin activity in C. elegans. Nature. 1989 Nov 2;342(6245):45–50. doi: 10.1038/342045a0. [DOI] [PubMed] [Google Scholar]
- Benian G. M., L'Hernault S. W., Morris M. E. Additional sequence complexity in the muscle gene, unc-22, and its encoded protein, twitchin, of Caenorhabditis elegans. Genetics. 1993 Aug;134(4):1097–1104. doi: 10.1093/genetics/134.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimino G. D., Gamper H. B., Isaacs S. T., Hearst J. E. Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem. 1985;54:1151–1193. doi: 10.1146/annurev.bi.54.070185.005443. [DOI] [PubMed] [Google Scholar]
- Clark D. V., Rogalski T. M., Donati L. M., Baillie D. L. The unc-22(IV) region of Caenorhabditis elegans: genetic analysis of lethal mutations. Genetics. 1988 Jun;119(2):345–353. doi: 10.1093/genetics/119.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S. Repair of DNA containing interstrand crosslinks in Escherichia coli: sequential excision and recombination. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1064–1068. doi: 10.1073/pnas.70.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibb N. J., Brown D. M., Karn J., Moerman D. G., Bolten S. L., Waterston R. H. Sequence analysis of mutations that affect the synthesis, assembly and enzymatic activity of the unc-54 myosin heavy chain of Caenorhabditis elegans. J Mol Biol. 1985 Jun 25;183(4):543–551. doi: 10.1016/0022-2836(85)90170-6. [DOI] [PubMed] [Google Scholar]
- Fire A., Albertson D., Harrison S. W., Moerman D. G. Production of antisense RNA leads to effective and specific inhibition of gene expression in C. elegans muscle. Development. 1991 Oct;113(2):503–514. doi: 10.1242/dev.113.2.503. [DOI] [PubMed] [Google Scholar]
- Greenwald I. S., Horvitz H. R. unc-93(e1500): A behavioral mutant of Caenorhabditis elegans that defines a gene with a wild-type null phenotype. Genetics. 1980 Sep;96(1):147–164. doi: 10.1093/genetics/96.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R. K. Crossover suppressors and balanced recessive lethals in Caenorhabditis elegans. Genetics. 1978 Jan;88(1):49–65. doi: 10.1093/genetics/88.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen R. C., Baillie D. L. Formaldehyde mutagenesis of the eT1 balanced region in Caenorhabditis elegans: dose-response curve and the analysis of mutational events. Mutat Res. 1988 Sep;201(1):137–147. doi: 10.1016/0027-5107(88)90120-0. [DOI] [PubMed] [Google Scholar]
- Levin J. Z., Horvitz H. R. The Caenorhabditis elegans unc-93 gene encodes a putative transmembrane protein that regulates muscle contraction. J Cell Biol. 1992 Apr;117(1):143–155. doi: 10.1083/jcb.117.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud J., Fossett N. G., Arbour-Reily P., McDaniel M., Tucker A., Chang S. H., Lee W. R. DNA sequence analysis of X-ray induced Adh null mutations in Drosophila melanogaster. Environ Mol Mutagen. 1991;18(3):157–160. doi: 10.1002/em.2850180303. [DOI] [PubMed] [Google Scholar]
- Meneely P. M., Herman R. K. Lethals, steriles and deficiencies in a region of the X chromosome of Caenorhabditis elegans. Genetics. 1979 May;92(1):99–115. doi: 10.1093/genetics/92.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman D. G., Baillie D. L. Formaldehyde mutagenesis in the nematode Caenorhabditis elegans. Mutat Res. 1981 Feb;80(2):273–279. doi: 10.1016/0027-5107(81)90100-7. [DOI] [PubMed] [Google Scholar]
- Moerman D. G., Baillie D. L. Genetic Organization in CAENORHABDITIS ELEGANS: Fine-Structure Analysis of the unc-22 Gene. Genetics. 1979 Jan;91(1):95–103. doi: 10.1093/genetics/91.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman D. G., Waterston R. H. Spontaneous unstable unc-22 IV mutations in C. elegans var. Bergerac. Genetics. 1984 Dec;108(4):859–877. doi: 10.1093/genetics/108.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell J., Mandel H. C., Krauss M., Sofer W. Genetic and cytogenetic analysis of the Adh region in Drosophila melanogaster. Genetics. 1977 Jul;86(3):553–566. doi: 10.1093/genetics/86.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Decuyper-Debergh D., Gamper H. Mutagenesis of the lac promoter region in M13 mp10 phage DNA by 4'-hydroxymethyl-4,5',8-trimethylpsoralen. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7355–7359. doi: 10.1073/pnas.82.21.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth R. E., Cuddeford C., Baillie D. L. Mutagenesis in Caenorhabditis elegans. II. A spectrum of mutational events induced with 1500 r of gamma-radiation. Genetics. 1985 Mar;109(3):493–511. doi: 10.1093/genetics/109.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffran W. A., Smith E. D., Chan S. K. Induction of multiple plasmid recombination in Saccharomyces cerevisiae by psoralen reaction and double strand breaks. Nucleic Acids Res. 1991 Oct 25;19(20):5681–5687. doi: 10.1093/nar/19.20.5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Sladek F. M., Melian A., Howard-Flanders P. Incision by UvrABC excinuclease is a step in the path to mutagenesis by psoralen crosslinks in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3982–3986. doi: 10.1073/pnas.86.11.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sun H., Treco D., Schultes N. P., Szostak J. W. Double-strand breaks at an initiation site for meiotic gene conversion. Nature. 1989 Mar 2;338(6210):87–90. doi: 10.1038/338087a0. [DOI] [PubMed] [Google Scholar]
- Thacker J., Fleck E. W., Morris T., Rossiter B. J., Morgan T. L. Localization of deletion breakpoints in radiation-induced mutants of the hprt gene in hamster cells. Mutat Res. 1990 Oct;232(2):163–170. doi: 10.1016/0027-5107(90)90121-j. [DOI] [PubMed] [Google Scholar]
- Thacker J., Ganesh A. N. Molecular analysis of spontaneous and ethyl methanesulphonate-induced mutations of the hprt gene in hamster cells. Mutat Res. 1989 Jan;210(1):103–112. doi: 10.1016/0027-5107(89)90049-3. [DOI] [PubMed] [Google Scholar]
- Thacker J. The nature of mutants induced by ionising radiation in cultured hamster cells. III. Molecular characterization of HPRT-deficient mutants induced by gamma-rays or alpha-particles showing that the majority have deletions of all or part of the hprt gene. Mutat Res. 1986 May;160(3):267–275. doi: 10.1016/0027-5107(86)90137-5. [DOI] [PubMed] [Google Scholar]
- Trent C., Purnell B., Gavinski S., Hageman J., Chamblin C., Wood W. B. Sex-specific transcriptional regulation of the C. elegans sex-determining gene her-1. Mech Dev. 1991 Mar;34(1):43–55. doi: 10.1016/0925-4773(91)90090-s. [DOI] [PubMed] [Google Scholar]
- Waring D. A., Kenyon C. Selective silencing of cell communication influences anteroposterior pattern formation in C. elegans. Cell. 1990 Jan 12;60(1):123–131. doi: 10.1016/0092-8674(90)90722-q. [DOI] [PubMed] [Google Scholar]



