Abstract
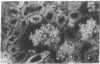
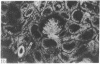
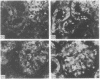
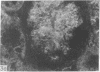
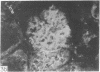
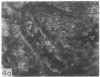
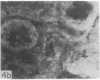
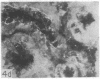
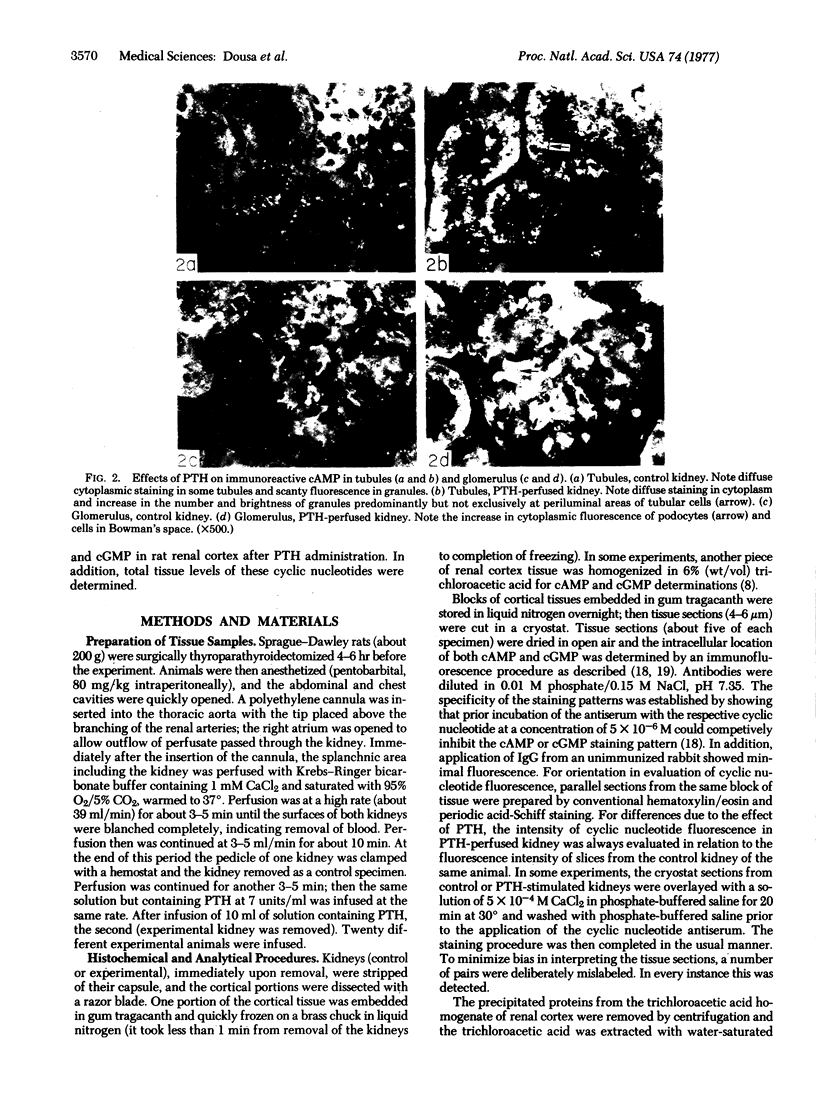
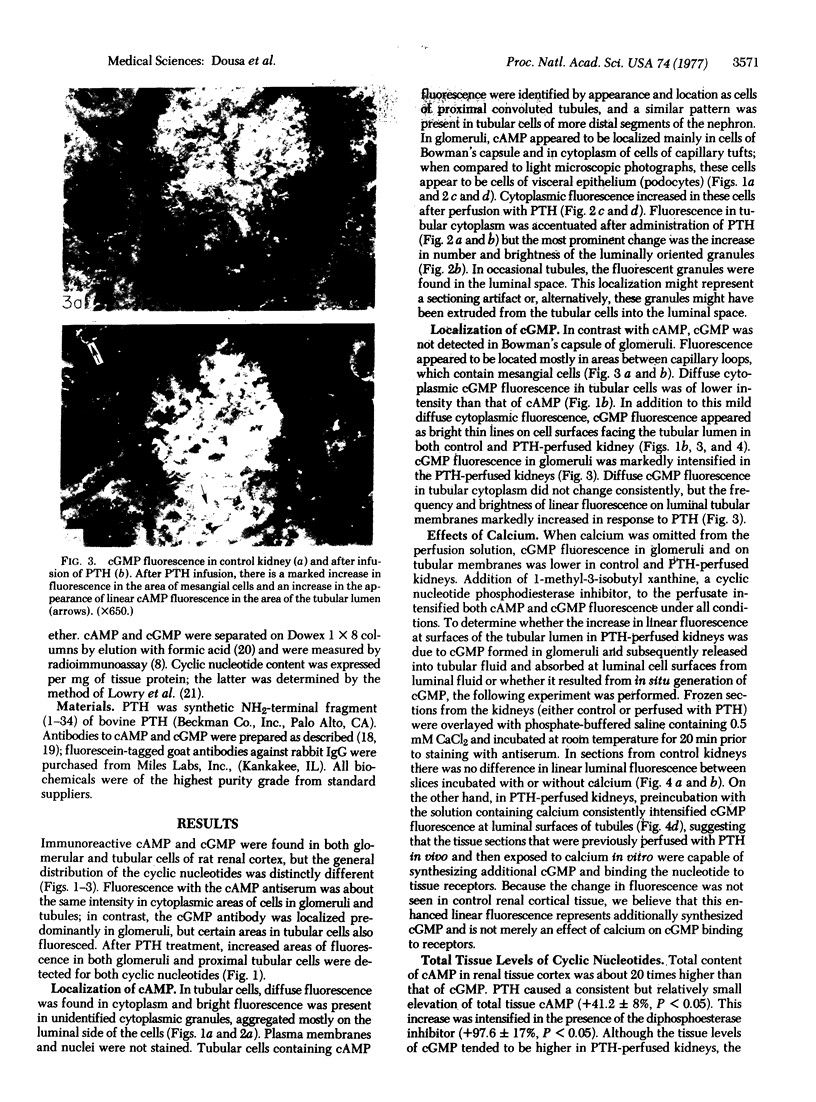
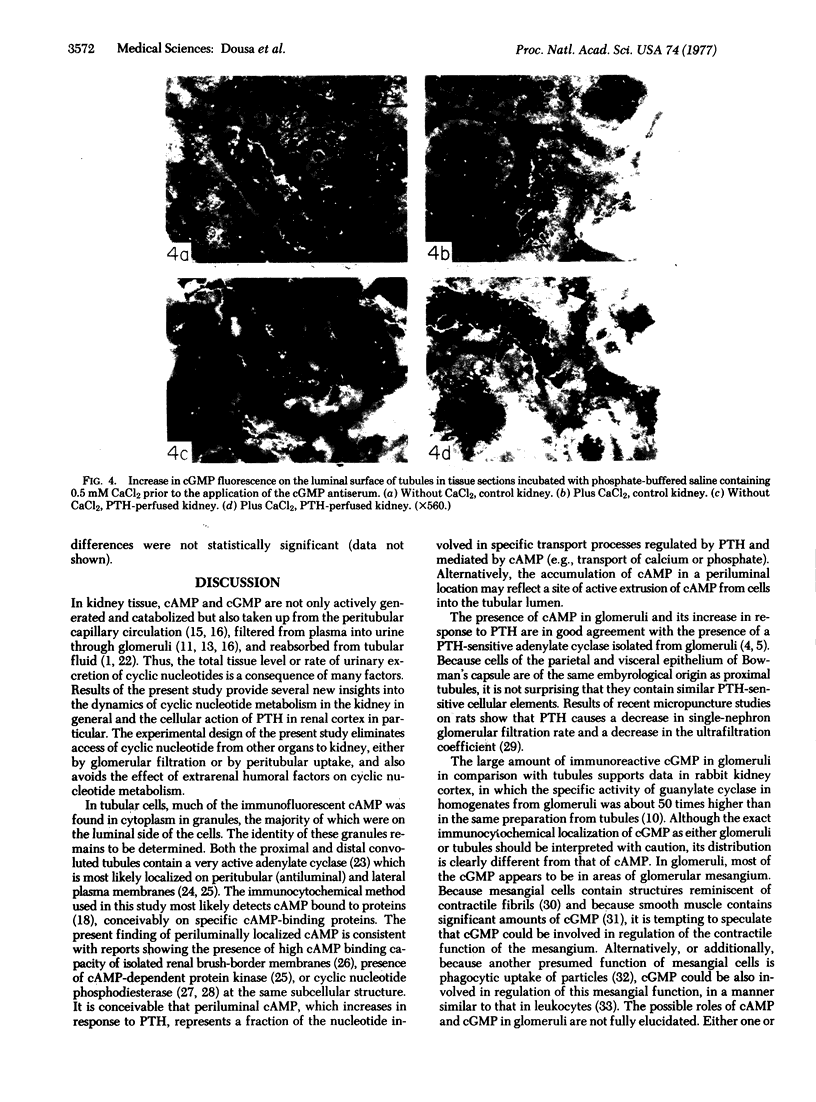
Adenosine 3′:5′-cyclic monophosphate (cAMP) and guanosine 3′:5′-cyclic monophosphate (cGMP) were localized in cells of rat kidney cortex by an immunocytochemical technique before and after perfusion with parathyroid hormone (PTH). In control tissues the cAMP antiserum detected approximately the same intensity of fluorescence in cytoplasmic epithelial cell elements of cortical tubules and glomeruli (cells of Bowman's capsule and podocytes). PTH increased fluorescence in these glomerular cells and increased cAMP fluorescence in cytoplasmic granules in proximal tubular cells. These granules, whose structure has not been identified, were located predominantly on the luminal side of the tubular cells. In control rats, the renal cortical fluorescence detected with the cGMP antiserum was more pronounced in glomeruli (predominantly in the mesangial areas) and lesser amounts of fluorescence were observed in tubules. After PTH treatment, cGMP fluorescence increased in glomeruli and in renal tubular cells. A bright linear pattern of fluorescence was found in the area of the tubular luminal membrane. Perfusion with PTH caused relatively small increases in total tissue cAMP and no consistent increases in total tissue cGMP. Our observations suggest that both cAMP and cGMP are involved in the glomerular and tubular responses to PTH and point out the added dimension that this immunocytochemical technique brings to studies of cyclic nucleotide dynamics in heterogeneous tissues.
Keywords: glomeruli, tubules, calcium
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aurbach G. D., Heath D. A. Parathyroid hormone and calcitonin regulation of renal function. Kidney Int. 1974 Nov;6(5):331–345. doi: 10.1038/ki.1974.118. [DOI] [PubMed] [Google Scholar]
- Beavo J. A., Hardman J. G., Sutherland E. W. Stimulation of adenosine 3',5'-monophosphate hydrolysis by guanosine 3',5'-monophosphate. J Biol Chem. 1971 Jun 25;246(12):3841–3846. [PubMed] [Google Scholar]
- Berridge M. J. The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv Cyclic Nucleotide Res. 1975;6:1–98. [PubMed] [Google Scholar]
- Blonde L., Wehmann R. E., Steiner A. L. Plasma clearance rates and renal clearance of 3H-labeled cyclic AMP and 3H-labeled cyclic GMP in the dog. J Clin Invest. 1974 Jan;53(1):163–172. doi: 10.1172/JCI107534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butlen D., Jard S. Renal handling of 3'-5'-cyclic AMP in the rat. The possible role of luminal 3'-5'-cyclic AMP in the tubular reabsorption of phosphate. Pflugers Arch. 1972;331(2):172–190. doi: 10.1007/BF00587260. [DOI] [PubMed] [Google Scholar]
- Chase L. R., Aurbach G. D. Renal adenyl cyclase: anatomically separate sites for parathyroid hormone and vasopressin. Science. 1968 Feb 2;159(3814):545–547. doi: 10.1126/science.159.3814.545. [DOI] [PubMed] [Google Scholar]
- Coulson R. Metabolism and excretion of exogenous adenosine 3':5'-monophosphate and guanosine 3':5'-monophosphate. Studies in the isolated perfused rat kidney and in the intact rat. J Biol Chem. 1976 Aug 25;251(16):4958–4967. [PubMed] [Google Scholar]
- Criss W. E., Murad F., Kimura H. Properties of guanylate cyclase from rat kidney cortex and transplantable kidney tumors. J Cyclic Nucleotide Res. 1976;2(1):11–19. [PubMed] [Google Scholar]
- Elema J. D., Hoyer J. R., Vernier R. L. The glomerular mesangium: uptake and transport of intravenously injected colloidal carbon in rats. Kidney Int. 1976 May;9(5):395–406. doi: 10.1038/ki.1976.49. [DOI] [PubMed] [Google Scholar]
- Filburn C. R., Sacktor B. Cyclic nucleotide phosphodiesterases of rabbit renal cortex. Characterization of brush border membrane activities. Arch Biochem Biophys. 1976 May;174(1):249–261. doi: 10.1016/0003-9861(76)90344-1. [DOI] [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K., Nicol S. E., Glass D. B., Sanford C. H., Kuehl F. A., Jr, Estensen R. Biologic regulation through opposing influences of cyclic GMP and cyclic AMP: the Yin Yang hypothesis. Adv Cyclic Nucleotide Res. 1975;5:307–330. [PubMed] [Google Scholar]
- Helwig J. J., Bollack C., Mandel P., Goridis C. Renal cortex guanylate cyclase. Preferential enrichment in glomerular membranes. Biochim Biophys Acta. 1975 Feb 19;377(2):463–472. doi: 10.1016/0005-2744(75)90326-5. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Stimulation of phagocytic release of neutral protease from human neutrophils by cholinergic amines and cyclic 3',5'-guanosine monophosphate. J Immunol. 1974 Jan;112(1):210–214. [PubMed] [Google Scholar]
- Insel P., Balakir R., Sacktor B. The binding of cyclic AMP to renal brush border membranes. J Cyclic Nucleotide Res. 1975;1(2):107–122. [PubMed] [Google Scholar]
- Kaminsky N. I., Broadus A. E., Hardman J. G., Jones D. J., Jr, Ball J. H., Sutherland E. W., Liddle G. W. Effects of parathyroid hormone on plasma and urinary adenosine 3',5'-monophosphate in man. J Clin Invest. 1970 Dec;49(12):2387–2395. doi: 10.1172/JCI106458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinne R. K. Polarity of the renal proximal tubular cell: function and enzyme pattern of the isolated plasma membranes. Med Clin North Am. 1975 May;59(3):615–627. doi: 10.1016/s0025-7125(16)32013-2. [DOI] [PubMed] [Google Scholar]
- Kukovetz W. R., Pöch G., Wurm A. Quantitative relations between cyclic AMP and contraction as affected by stimulators of adenylate cyclase and inhibitors of phosphodiesterase. Adv Cyclic Nucleotide Res. 1975;5:395–414. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marx S. J., Aurbach G. D. Renal receptors for calcitonin: coordinate occurrence with calcitonin-activated adenylate cyclase. Endocrinology. 1975 Aug;97(2):448–453. doi: 10.1210/endo-97-2-448. [DOI] [PubMed] [Google Scholar]
- Melson G. L., Chase L. R., Aurbach G. D. Parathyroid hormone-sensitive adenyl cyclase in isolated renal tubules. Endocrinology. 1970 Mar;86(3):511–518. doi: 10.1210/endo-86-3-511. [DOI] [PubMed] [Google Scholar]
- Murad F. Clinical studies and applications of cyclic nucleotides. Adv Cyclic Nucleotide Res. 1973;3:355–383. [PubMed] [Google Scholar]
- Murad F., Manganiello V., Vaughan M. A simple, sensitive protein-binding assay for guanosine 3':5'-monophosphate. Proc Natl Acad Sci U S A. 1971 Apr;68(4):736–739. doi: 10.1073/pnas.68.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S. H., Whitley T. H., Stowe N. W., Steiner A. L. Immunohistochemical localization of 3': 5'-cyclic AMP and 3': 5'-cyclic GMP in rat liver, intestine, and testis. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2022–2026. doi: 10.1073/pnas.72.6.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Pechet M., Fast D. Effect of dibutyryl cyclic adenosine 3',5'-monophosphate, theophylline, and other nucleotides upon calcium and phosphate metabolism. J Clin Invest. 1968 Aug;47(8):1843–1850. doi: 10.1172/JCI105874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz G., Hardman J. G., Schultz K., Baird C. E., Sutherland E. W. The importance of calcium ions for the regulation of guanosine 3':5'-cyclic monophosphage levels. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3889–3893. doi: 10.1073/pnas.70.12.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Ong S. H., Wedner H. J. Cyclic nucleotide immunocytochemistry. Adv Cyclic Nucleotide Res. 1976;7:115–155. [PubMed] [Google Scholar]
- Steiner A. L., Pagliara A. S., Chase L. R., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. II. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mammalian tissues and body fluids. J Biol Chem. 1972 Feb 25;247(4):1114–1120. [PubMed] [Google Scholar]
- White A. A., Zenser T. V. Separation of cyclic 3',5'-nucleoside monophosphates from other nucleotides on aluminum oxide columns. Application to the assay of adenyl cyclase and guanyl cyclase. Anal Biochem. 1971 Jun;41(2):372–396. doi: 10.1016/0003-2697(71)90156-4. [DOI] [PubMed] [Google Scholar]
- Williams R. H., Barish J., Ensinck J. W. Hormone effects upon cyclic nucleotide excretion in man. Proc Soc Exp Biol Med. 1972 Feb;139(2):447–454. doi: 10.3181/00379727-139-36162. [DOI] [PubMed] [Google Scholar]