Abstract
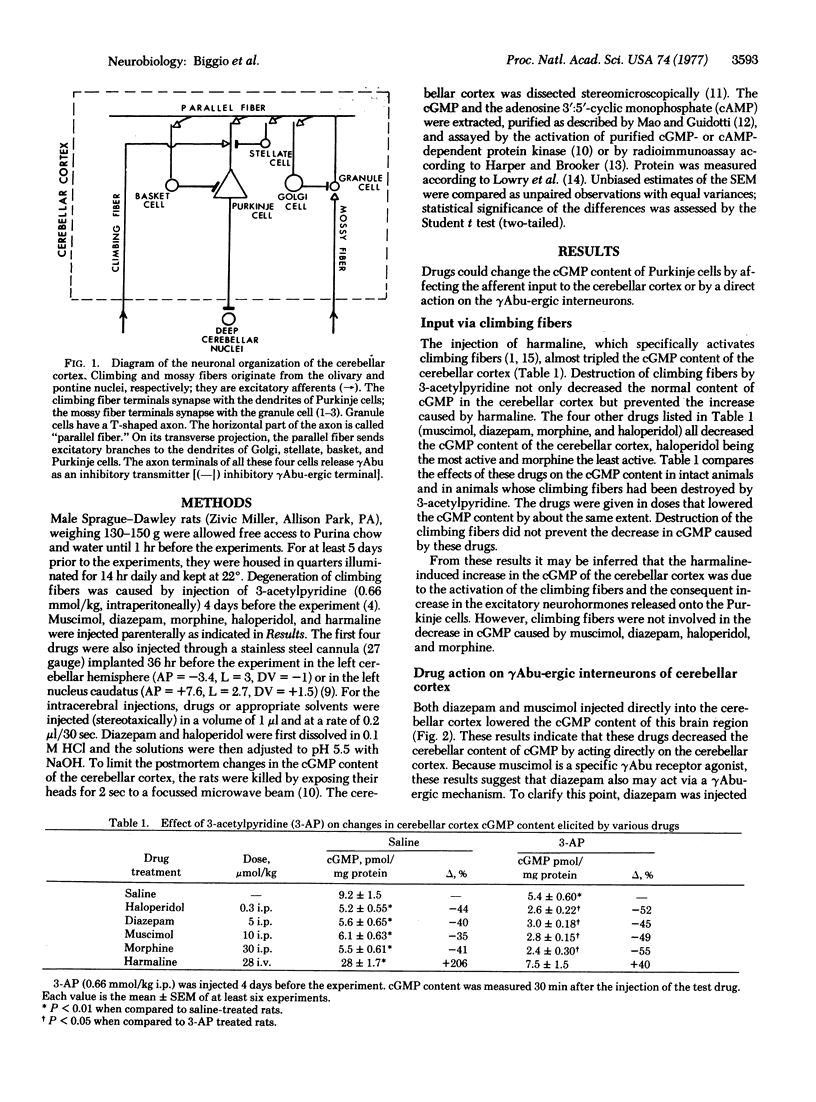
The cerebellum consists of two parts: the cerebellar nuclei whose connections to the various parts of the central nervous system coordinate muscle movements, and the cerebellar cortex which exerts an inhibitory influence on the cerebellar nuclei through the release of γ-aminobutyric acid (γAbu) from Purkinje cells. The activity of Purkinje cells is regulated by two excitatory inputs to the cerebellar cortex—the climbing and mossy fibers—and by a neuronal network within the cortex which inhibits the activity of Purkinje cells through the release of γAbu from interneurons. The net activity of Purkinje cells is related to their content of guanosine 3′:5′-cyclic monophosphate (cGMP) which increases or decreases according to changes in the activity of climbing and mossy fibers as well as to changes in the activation of γAbu receptors. When these receptors are activated, the cGMP of Purkinje cells decreases; when they are inhibited, the cGMP increases.
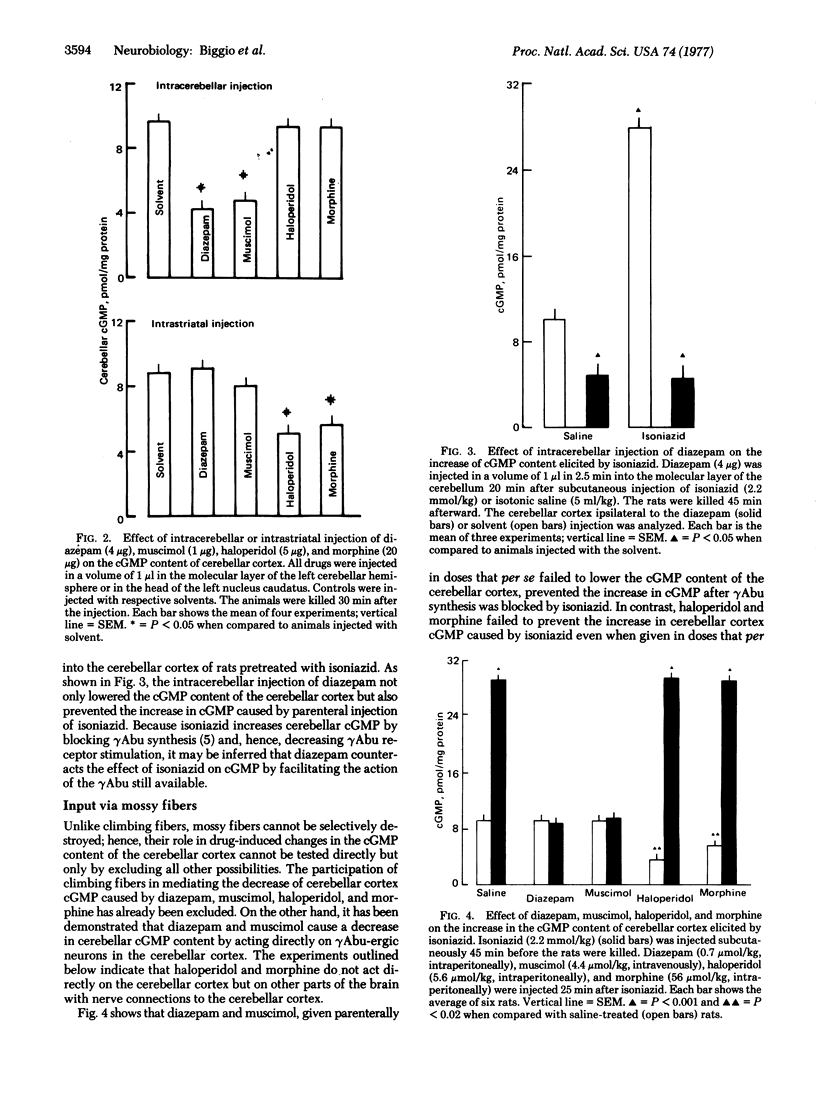
The cGMP content of the cerebellar cortex is altered by drugs that change either the excitatory input of climbing or mossy fibers or the inhibitory input mediated by the activation of γAbu receptors. Mechanisms by which various drugs alter the cerebellar content of cGMP were investigated. By using various experimental designs, it was shown that diazepam and muscimol lowered the cGMP content by activating γAbu receptors. In contrast, morphine and haloperidol lowered the cerebellar cortex cGMP by decreasing the excitation of mossy fibers whereas harmaline increased the cGMP by increasing the excitation of the climbing fibers.
Keywords: γ-aminobutyric acid, haloperidol, harmaline, morphine, Purkinje cells
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. I., Tsukahara N. Cerebrocerebellar communication systems. Physiol Rev. 1974 Oct;54(4):957–1006. doi: 10.1152/physrev.1974.54.4.957. [DOI] [PubMed] [Google Scholar]
- Biggio G., Costa E., Guidotti A. Pharmacologically induced changes in the 3':5'-cyclic guanosine monophosphate content of rat cerebellar cortex: difference between apomorphine, haloperidol and harmaline. J Pharmacol Exp Ther. 1977 Jan;200(1):207–215. [PubMed] [Google Scholar]
- Biggio G., Guidotti A. Climbing fiver activation and 3', 5'-cyclic guanosine monophosphate (cGMP) content in cortex and deep nuclei of cerebellum. Brain Res. 1976 May 7;107(2):365–373. doi: 10.1016/0006-8993(76)90233-x. [DOI] [PubMed] [Google Scholar]
- Costa E., Guidotti A., Mao C. C., Suria A. New concepts on the mechanism of action of benzodiazepines. Life Sci. 1975 Jul 15;17(2):167–185. doi: 10.1016/0024-3205(75)90501-9. [DOI] [PubMed] [Google Scholar]
- Enna S. J., Snyder S. H. Gamma-aminobutyric acid (GABA) receptor binding in mammalian retina. Brain Res. 1976 Oct 8;115(1):174–179. doi: 10.1016/0006-8993(76)90834-9. [DOI] [PubMed] [Google Scholar]
- Guidotti A., Cheney D. L., Trabucchi M., Doteuchi M., Wang C. Focussed microwave radiation: a technique to minimize post mortem changes of cyclic nucleotides, dopa and choline and to preserve brain morphology. Neuropharmacology. 1974 Dec;13(12):1115–1122. doi: 10.1016/0028-3908(74)90061-6. [DOI] [PubMed] [Google Scholar]
- Koslow S. H., Racagni G., Costa E. Mass fragmentographic measurement of norepinephrine dopamine, serotonin and acetylcholine in seven discrete nuclei of the rat tel-diencephalon. Neuropharmacology. 1974 Dec;13(12):1123–1130. doi: 10.1016/0028-3908(74)90062-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mao C. C., Guidotti A., Landis S. Cyclic GMP: reduction of cerebellar concentrations in "nervous" mutant mice. Brain Res. 1975 Jun 13;90(2):335–339. doi: 10.1016/0006-8993(75)90316-9. [DOI] [PubMed] [Google Scholar]
- Mao C. C., Guidotti A. Simultaneous isolation of adenosine 3',5'-cyclic monophosphate (cAMP) and guanosine 3',5'-cyclic monophosphate (cGMP) in small tissue samples. Anal Biochem. 1974 May;59(1):63–68. doi: 10.1016/0003-2697(74)90009-8. [DOI] [PubMed] [Google Scholar]
- Mao C. C., Marco E., Revuelta A., Bertilsson L., Costa E. The turnover rate of gamma-aminobutyric acid in the nuclei of telencephalon: implications in the pharmacology of antipsychotics and of a minor tranquilizer. Biol Psychiatry. 1977 Jun;12(3):359–371. [PubMed] [Google Scholar]
- Pemberton J. M., Fisher P. R. 2,4-D plasmids and persistence. Nature. 1977 Aug 25;268(5622):732–733. doi: 10.1038/268732a0. [DOI] [PubMed] [Google Scholar]
- de Montigny C., Lamarre Y. Rhythmic activity induced by harmaline in the olivo-cerebello-bulbar system of the cat. Brain Res. 1973 Apr 13;53(1):81–95. doi: 10.1016/0006-8993(73)90768-3. [DOI] [PubMed] [Google Scholar]


