Abstract
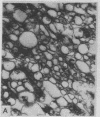
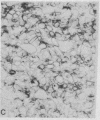
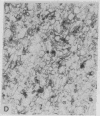
Intestinal Ca2+ transport was studied in membrane vesicles isolated from microvillus, Golgi, and lateral-basal membrane preparations. Ca2+ uptake by these vesicles was measured by determination of 45Ca2+ associated with these membranes after collection by micropore filtration. Golgi membranes showed the highest initial rate and equilibration level of Ca2+ uptake. Approximately 90% of this Ca2+ uptake was into an osmotically responsive space, suggesting that what was measured was predominantly Ca2+ translocation. Vitamin D-deficient rats showed a markedly diminished rate of uptake and level of equilibration. These data indicate that a Ca2+-translocating process was associated with Golgi membranes to a greater extent than with surface membranes and that this process was markedly decreased in vitamin D-deficient rats. The results suggest that the Golgi apparatus participates in intestinal Ca2+ absorption.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams T. H., Wong R. G., Norman A. W. Studies on the mechanism of action of calciferol. II. Effects of the polyene antibiotic, filipin, on vitamin D-mediated calcium transport. J Biol Chem. 1970 Sep 10;245(17):4432–4442. [PubMed] [Google Scholar]
- Birge S. J., Gilbert H. R. Indentification of an intestinal sodium and calcium-dependent phosphatase stimulated by parathyroid hormone. J Clin Invest. 1974 Sep;54(3):710–717. doi: 10.1172/JCI107809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borle A. B. Calcium metabolism at the cellular level. Fed Proc. 1973 Sep;32(9):1944–1950. [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
- Case R. M., Clausen T. The relationship between calcium exchange and enzyme secretion in the isolated rat pancreas. J Physiol. 1973 Nov;235(1):75–102. doi: 10.1113/jphysiol.1973.sp010379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell R., Walker W. A., Isselbacher K. J. Small intestinal absorption of horseradish peroxidase. A cytochemical study. Lab Invest. 1971 Jul;25(1):42–48. [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca H. F., Schnoes H. K. Metabolism and mechanism of action of vitamin D. Annu Rev Biochem. 1976;45:631–666. doi: 10.1146/annurev.bi.45.070176.003215. [DOI] [PubMed] [Google Scholar]
- Friedman H. I., Cardell R. R., Jr Effects of puromycin on the structure of rat intestinal epithelial cells during fat absorption. J Cell Biol. 1972 Jan;52(1):15–40. doi: 10.1083/jcb.52.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- Kowarski S., Schachter D. Vitamin D and adenosine triphosphatase dependent on divalent cations in rat intestinal mucosa. J Clin Invest. 1973 Nov;52(11):2765–2773. doi: 10.1172/JCI107472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martin D. L., Melancon M. J., Jr, DeLuca H. F. Vitamin D stimulated, calcium-dependent adenosine triphosphatase from brush borders of rat small intestine. Biochem Biophys Res Commun. 1969 Jun 27;35(6):819–823. doi: 10.1016/0006-291x(69)90697-4. [DOI] [PubMed] [Google Scholar]
- Melancon M. J., Jr, DeLuca H. F. Vitamin D stimulation of calcium-dependent adenosine triphosphatase in chick intestinal brush borders. Biochemistry. 1970 Apr 14;9(8):1658–1664. doi: 10.1021/bi00810a002. [DOI] [PubMed] [Google Scholar]
- Messer M., Dahlqvist A. A one-step ultramicro method for the assay of intestinal disaccharidases. Anal Biochem. 1966 Mar;14(3):376–392. doi: 10.1016/0003-2697(66)90280-6. [DOI] [PubMed] [Google Scholar]
- Mircheff A. K., Wright E. M. Analytical isolation of plasma membranes of intestinal epithelial cells: identification of Na, K-ATPase rich membranes and the distribution of enzyme activities. J Membr Biol. 1976 Sep 17;28(4):309–333. doi: 10.1007/BF01869703. [DOI] [PubMed] [Google Scholar]
- Omdahl J. L., DeLuca H. F. Regulation of vitamin D metabolism and function. Physiol Rev. 1973 Apr;53(2):327–372. doi: 10.1152/physrev.1973.53.2.327. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Weiser M. M. Galactosyltransferase activities in human sera: detection of a cancer-associated isoenzyme. Biochem Biophys Res Commun. 1975 Jul 22;65(2):545–551. doi: 10.1016/s0006-291x(75)80181-1. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Weiser M. M. Role of cell membrane galactosyltransferase in concanavalin A agglutination of erythrocytes. Biochem J. 1975 Jan;146(1):213–221. doi: 10.1042/bj1460213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley J. P., Gotterer G. S. Distribution of (Na+-K+)-stimulated ATPase activity in rat intestinal mucosa. Biochim Biophys Acta. 1969 Apr;173(3):456–468. doi: 10.1016/0005-2736(69)90010-8. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Bygrave F. L. Methodology for in vitro studies of Ca-2+ transport. Anal Biochem. 1975 Jul;67(1):44–54. doi: 10.1016/0003-2697(75)90270-5. [DOI] [PubMed] [Google Scholar]
- Rodewald R. Selective antibody transport in the proximal small intestine of the neonatal rat. J Cell Biol. 1970 Jun;45(3):635–640. doi: 10.1083/jcb.45.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- Sampson H. W., Matthews J. L., Martin J. H., Kunin A. S. An electron microscopic localization of calcium in the small intestine of normal, rachitic, and vitamin-D-treated rats. Calcif Tissue Res. 1970;5(4):305–316. doi: 10.1007/BF02017560. [DOI] [PubMed] [Google Scholar]
- Schachter D., Kowarski S., Finkelstein J. D., Ma R. I. Tissue concentration differences during active transport of calcium by intestine. Am J Physiol. 1966 Nov;211(5):1131–1136. doi: 10.1152/ajplegacy.1966.211.5.1131. [DOI] [PubMed] [Google Scholar]
- Spencer R., Charman M., Wilson P., Lawson E. Vitamin d-stimulated intestinal calcium absorption may not involve calcium-binding protein directly. Nature. 1976 Sep 9;263(5573):161–163. doi: 10.1038/263161a0. [DOI] [PubMed] [Google Scholar]
- Taylor A. N., Wasserman R. H. Immunofluorescent localization of vitamin D-dependent calcium-binding protein. J Histochem Cytochem. 1970 Feb;18(2):107–115. doi: 10.1177/18.2.107. [DOI] [PubMed] [Google Scholar]
- Warner R. R., Coleman J. R. Electron probe analysis of calcium transport by small intestine. J Cell Biol. 1975 Jan;64(1):54–74. doi: 10.1083/jcb.64.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman R. H., Corradino R. A., Taylor A. N. Vitamin D-dependent calcium-binding protein. Purification and some properties. J Biol Chem. 1968 Jul 25;243(14):3978–3986. [PubMed] [Google Scholar]
- Wasserman R. H., Taylor A. N. Vitamin d3-induced calcium-binding protein in chick intestinal mucosa. Science. 1966 May 6;152(3723):791–793. doi: 10.1126/science.152.3723.791. [DOI] [PubMed] [Google Scholar]
- Weisberg H., Rhodin J., Glass G. B. Intestinal vitamin B12 absorption in the dog. 3. Demonstration of the intracellular pathway of absorption by light and electron microscope autoradiography. Lab Invest. 1968 Nov;19(5):516–525. [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Whaley W. G., Dauwalder M., Kephart J. E. Golgi apparatus: influence on cell surfaces. Science. 1972 Feb 11;175(4022):596–599. doi: 10.1126/science.175.4022.596. [DOI] [PubMed] [Google Scholar]
- Whur P., Herscovics A., Leblond C. P. Radioautographic visualization of the incorporation of galactose-3H and mannose-3H by rat thyroids in vitro in relation to the stages of thyroglobulin synthesis. J Cell Biol. 1969 Nov;43(2):289–311. doi: 10.1083/jcb.43.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R. G., Norman A. W. Studies on the mechanism of action of calciferol. VIII. The effects of dietary vitamin D and the polyene antibiotic, filipin, in vitro, on the intestinal cellular uptake of calcium. J Biol Chem. 1975 Apr 10;250(7):2411–2419. [PubMed] [Google Scholar]
- Zagury D., Uhr J. W., Jamieson J. D., Palade G. E. Immunoglobulin synthesis and secretion. II. Radioautographic studies of sites of addition of carbohydrate moieties and intracellular transport. J Cell Biol. 1970 Jul;46(1):52–63. doi: 10.1083/jcb.46.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]