Abstract
Background
The use of minimally invasive surgery (MIS) techniques for pancreatic and liver operations remains ill defined. We sought to compare inpatient outcomes among patients undergoing open versus MIS pancreas and liver operations using a nationally representative cohort.
Methods
We queried the Nationwide Inpatient Sample database for all major pancreatic and hepatic resections performed between 2000 and 2011. Appropriate International Classification of Diseases, 9th Revision (ICD-9) coding modifiers for laparoscopy and robotic assist were used to categorize procedures as MIS. Demographics, comorbidities, and inpatient outcomes were compared between the open and MIS groups.
Results
A total of 65,033 resections were identified (pancreas, n = 36,195 [55.7%]; liver, n = 28,035 [43.1%]; combined pancreas and liver, n = 803 [1.2%]). The overwhelming majority of operations were performed open (n = 62,192, 95.6%), whereas 4.4% (n = 2,841) were MIS. The overall use of MIS increased from 2.3% in 2000 to 7.5% in 2011. Compared with patients undergoing an open operation, MIS patients were older and had a greater incidence of multiple comorbid conditions. After operation, the incidence of complications for MIS (pancreas, 35.4%; liver, 29.5%) was lower than for open (pancreas, 41.6%; liver, 33%) procedures (all P < .05) resulting in a shorter median length of stay (8 vs 7 days; P = .001) as well as a lower in-hospital mortality (5.1% vs 2.8%; P = .001).
Conclusion
During the last decade, the number of MIS pancreatic and hepatic operations has increased, with nearly 1 in 13 HPB cases now being performed via an MIS approach. Despite MIS patients tending to have more preoperative medical comorbidities, postoperative morbidity, mortality, and duration of stay compared favorably with open surgery.
During the last decade, minimally invasive surgery (MIS) has become used increasingly for a wide array of operative procedures. The MIS approach holds the promise of better cosmesis, fewer wound complications, a shorter hospital stay, and a quicker return to baseline functional status.1–6 Although all of these benefits have not been completely borne out in studies examining MIS versus open approaches for various surgical procedures,7,8 patients remain interested in the MIS approach.9 Laparoscopic cholecystectomy has been standard-of-care for nearly two decades, whereas the MIS approach for other complex abdominal operations has increased over time.10 The MIS approach has gained particular acceptance for colorectal,11–13 urologic,14 bariatric,15 and gynecological16 procedures. The MIS approach has also long been applied to certain aspects of hepatopancreaticobiliary (HPB) surgery such as distal pancreatectomy17 and minor hepatic resection.7,18 More recently, the MIS approach has gained increased acceptance for other more complicated HPB operative procedures such as pancreatoduodenectomy19,20 and major hepatic resections.21,22
Previous studies have reported on the feasibility and safety of laparoscopic pancreatic and hepatic procedures.23–26 Recently, several centers have published data on the robotic approach to pancreatic and hepatic lesions.27–29 Most studies have noted that the MIS approach to pancreatic and hepatic surgery is safe, feasible, and can lead to comparable results as the open approach.30,31 Data on the MIS approach for HPB surgery remain, however, relatively scarce. In fact, most studies reporting on outcome comparing open versus the MIS approach for HPB surgery have been derived from the experience of single institutions.26,32,33 Data on the MIS approach for HPB surgery have been limited in that virtually all reports are derived from specialized, tertiary hepatobiliary centers.23–29 Previous studies that have included only academic medical centers may not reflect population-wide outcomes for HPB MIS surgery. Several previous studies have noted differences in HPB outcomes when comparing data from academic centers versus population-level data.34,35
To better understand the relative use of MIS in HPB surgery, it is important and necessary to evaluate nationally representative data from the entire United States. Therefore, the objective of the current study was to define the overall use and temporal trends in the use of the MIS versus open approach for HPB surgery using data from the National Inpatient Sample (NIS) database. In addition, we examined the factors associated with receipt of MIS HPB surgery, as well as characterized in-hospital outcomes after MIS versus open HPB surgery.
METHODS
Data source
We queried the NIS database that is maintained by the Agency for Healthcare Research and Quality. The NIS database is the largest publicly available all-payer inpatient care database in the United States. The database contains discharge data from 1,045 hospitals in 46 states representing an approximate 20% sample of US hospitals.36
Patient selection and data collection
All patients who underwent a major hepatic or pancreatic resection between 2000 and 2011 were identified using the appropriate International Classification of Diseases, 9th Revision (ICD-9) procedure codes: partial hepatectomy (50.22), hepatic lobectomy (50.3), pancreatectomy (52.0), partial pancreatectomy (52.5), proximal pancreatectomy (52.51), distal pancreatectomy (52.52), radical subtotal pancreatectomy (52.53), other partial pancreatectomy (52.59), total pancreatectomy (52.6), and radical pancreaticoduodenectomy (52.7). Appropriate ICD-9 coding modifiers for laparoscopy and robotic-assist were used to categorize procedures as MIS (laparoscopy: 54.21, robotic: 17.4, 17.41, 17.42, 17.43, 17.49). To minimize the number of patients in which the robotic modifier was unrelated to the liver or pancreas procedure, all patients who had additional procedure codes for urologic (602–606) or gynecologic (683–689) operations were excluded.
Standard patient characteristics were collected, including age, sex, and race. The primary payer, hospital teaching status, and hospital location were collected when available. Patient comorbidities and in-hospital perioperative complications (myocardial infarction, pneumonia, cerebrovascular accident, venous thromboembolism, acute renal failure, urinary tract infection, liver failure, wound complication and infection, ileus, fistula, shock, hemorrhage, and reoperation) were ascertained using the corresponding ICD-9 diagnostic codes.
Statistical analysis
Standard measures of frequencies and central tendency were calculated to summarize characteristics of patients, procedures, and in-hospital outcomes. Demographics, comorbidities, and multivariable-adjusted outcomes for complications and death were compared among patients undergoing open versus MIS procedures. We used multivariable logistic regression to adjust for variables, including sex, age, and comorbid conditions, that were significant on univariable analysis (P < .05). The χ2 analysis was used for comparison of categorical variables and t test or Wilcoxon rank-sum test was used for continuous variables (significance level <.05). SAS version 9.3 (SAS Institute, Cary, NC) was used for all statistical analyses.
RESULTS
Patient and hospital characteristics
A total of 65,033 resections were identified (pancreas, n = 36,195, 55.7%; liver, n = 28,035, 43.1%; combined pancreas and liver, n = 803, 1.2%). There were slightly more females (n = 33,159, 51.2%) than males (n = 31,604, 48.8%) in the cohort (Table I). The median age was 60 years (interquartile range [IQR] 49–70) and the majority of patients were white (n = 38,463, 73.7%). Most cases were performed as an elective operation (n = 44,465; 77.4%), at a teaching hospital (n = 45,883; 80.7%), and in an urban setting (n = 55,232; 97.1%). Most operations were performed as an open procedure (n = 62,192; 95.6%), whereas the remaining 2,841 cases (4.4%) were MIS. The median length of stay (LOS) among all patients was 8 days (IQR 6, 14). The most common perioperative condition among patients was hypertension (n = 25,020; 38.6%) followed by malignancy (n = 15,690; 24.2%) and diabetes (n = 10,940; 16.9%).
Table I.
Patient and hospital characteristics by operative approach
| Open (n = 62,108) | MIS (n = 2,841) | P value | |
|---|---|---|---|
| Age, yr (IQR) | 60 (48,70) | 64 (54,72) | .0001 |
| Male sex | 30,367 (48.8) | 1,354 (48.8) | .99 |
| Ethnicity | |||
| White | 36,720 (73.5) | 1,743 (78.4) | |
| Black | 4,949 (9.9) | 169 (7.6) | .0001 |
| Hispanic | 4,497 (9.0) | 156 (7.0) | |
| Other | 1,745 (3.5) | 53 (2.4) | |
| Comorbidities | |||
| Hypertension | 23,760 (38.3) | 1,260 (45.5) | <.0001 |
| Diabetes | 10,390 (16.7) | 550 (19.8) | <.0001 |
| Chronic lung disease | 3,909 (6.3) | 224 (8.1) | .0002 |
| Malignancy | 14,885 (24.0) | 805 (29.0) | <.0001 |
| Obesity | 2,997 (4.8) | 184 (6.6) | <.0001 |
| Chronic kidney disease | 1,730 (2.8) | 65 (2.3) | .17 |
| Congestive heart failure | 1,909 (3.1) | 85 (3.1) | .99 |
| Primary payer | |||
| Medicare | 23,604 (38.1) | 1,228 (44.3) | |
| Medicaid | 4,923 (7.9) | 152 (5.5) | |
| Private/HMO Insurance | 28,961 (46.7) | 1,266 (45.7) | .0001 |
| Self-pay | 2,186 (3.5) | 55 (2.0) | |
| Other | 2,008 (3.2) | 62 (2.2) | |
| Hospital location | |||
| Urban | 53,093 (97.1) | 2,139 (98.8) | .0001 |
| Rural | 1,616 (3.0) | 26 (1.2) | |
| Hospital teaching status | |||
| Teaching | 43,935 (80.3) | 1,898 (87.7) | .0001 |
| Nonteaching | 10,774 (19.7) | 267 (12.3) |
HMO, Health maintenance organization; IQR, interquartile range; MIS, minimally invasive surgery.
Case breakdown and operative approach
At the time of the operation, pancreas resection (n = 36,195; 55.7%) was performed more often than liver resection (n = 28,035; 43.1%; Table II). The most common pancreatic operation was a pancreatoduodenectomy, accounting for 51.2% (n = 17,613) of all open pancreas cases and 53.2% (n = 973) of all MIS pancreas cases. Other common pancreatic procedures included distal pancreatectomy (open: 33.0% [n = 11,371] vs MIS: 35.3% [n = 646]). The most common surgery involving the liver was a partial hepatectomy, accounting for 66.8% (n = 18,060) of all open liver cases and 72.0% (n = 711) of all MIS liver cases; hepatic lobectomy was performed less often (open: 30.1% [n = 8,341] vs MIS: 23.2% [n = 229]).
Table II.
Procedure type stratified by operative approach
| Open (n = 62,192) | All MIS (n = 2,841) |
Laparoscopic assisted (n = 2,578) |
Robotic assisted (n = 263) |
P value | |
|---|---|---|---|---|---|
| Pancreas | 34,367 (55.3) | 1,828 (64.3) | 1,638 (63.5) | 190 (72.2) | .001 |
| Partial pancreatectomy | .54 | ||||
| Proximal | 538 (0.9) | 27 (1.0) | 25 (1.0) | 2 (0.8) | .77 |
| Distal | 11,371 (18.3) | 646 (22.7) | 530 (20.6) | 116 (44.1) | .05 |
| Subtotal | 337 (0.5) | 18 (0.6) | 15 (0.6) | 3 (1.1) | .99 |
| Other | 2,467 (4.0) | 105 (3.7) | 94 (3.7) | 11 (4.2) | .02 |
| Total pancreatectomy | 2,041 (3.3) | 59 (2.1) | 55 (2.0) | 4 (1.5) | .001 |
| Whipple | 17,613 (28.3) | 973 (34.2) | 919 (35.6) | 54 (20.5) | .10 |
| Liver | 27,047 (43.5) | 988 (34.8) | 915 (35.5) | 74 (27.8) | .001 |
| Lobectomy | 8,341 (13.4) | 229 (8.1) | 215 (8.3) | 14 (5.3) | .001 |
| Partial hepatectomy | 18,060 (29.0) | 711 (25.0) | 656 (25.4) | 55 (20.9) | .001 |
| Marsupialization | 646 (1.0) | 48 (1.7) | 44 (1.8) | 4 (1.5) | .001 |
| Combined pancreas/liver | 778 (1.2) | 25 (0.9) | 25 (1.0) | 0 |
All percentages (in parentheses) reflect proportion of cases within the given column (total open or MIS cases as indicated). MIS, Minimally invasive surgery.
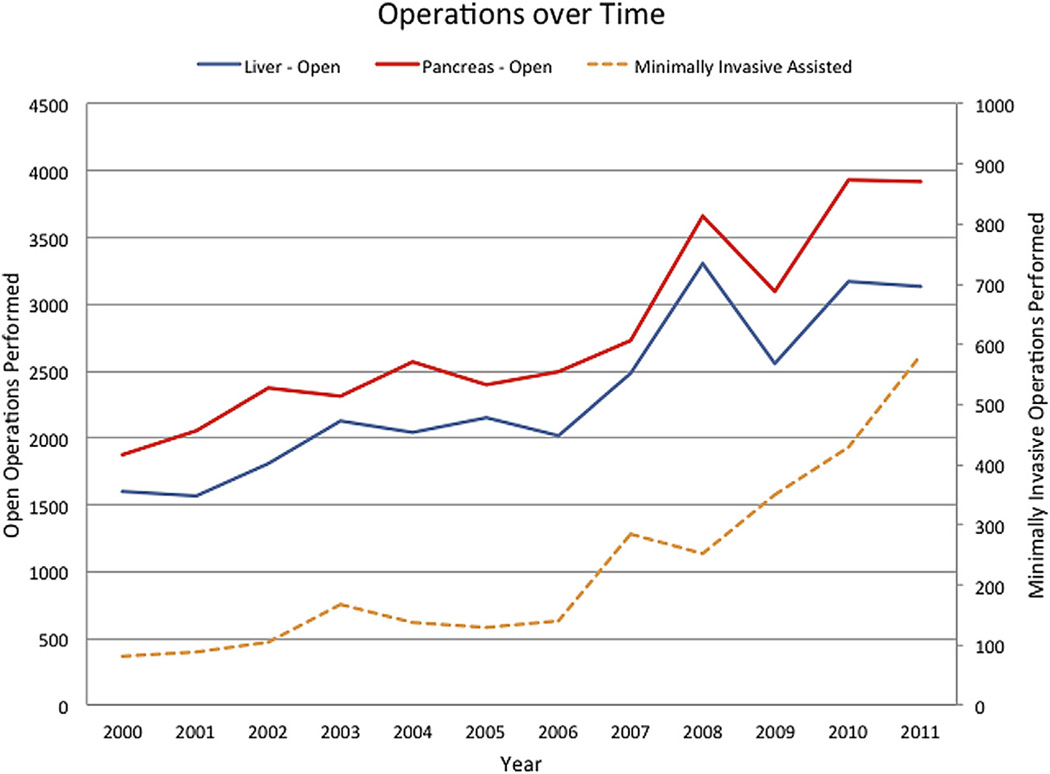
The overwhelming majority of operations were performed open (n = 62,192, 95.7%), whereas 4.4% (n = 2,841) of hepato-pancreatic cases were MIS. Among MIS cases, most were laparoscopic (n = 2,578, 90.7%) whereas a small subset was performed with a robotic approach (n = 263, 9.3%). The overall number of MIS cases increased from 81 (2.3%) liver and pancreas cases in 2000 to 581 (7.5%) in 2011 (Fig 1). Among laparoscopic pancreatic operations, pancreatoduodenectomy (n = 919, 56.1%) was most commonly performed, whereas a distal pancreatectomy was the most common robotic pancreatic resection (n = 116; 61.1%). Among liver cases, partial hepatectomy was the most common operation for both laparoscopic (n = 656; 71.7%) and robotic (n = 55; 75.3%) cases. Among MIS cases, robotic operations accounted for 10.4% of pancreas cases and 7.4% of liver cases. Robotic-assisted operations, first recorded in 2008 (n = 9; 0.1%of all cases), increased to 122 cases (1.6%of all cases) in 2011. Of note, by 2011 more than 1 in 5 (21.0%) of all MIS cases were performed via a robotic approach, with the majority of robotic cases (75%) being performed on the pancreas. Both the number of MIS pancreaticoduodenectomies (2000–2005: n = 288, 28.8% vs 2006–2011:n = 711, 71.2%) and major hepatectomies (2000–2005: n = 71, 29.5% vs 2006–2011: n = 170, 70.5%) increased over the time periods examined (both P < .001).
Fig 1.
Total number of operations over time stratified by operative approach.
Factors associated with operative approach
When comparing cohorts, patients undergoing an MIS operation were often older (open: 60 years [IQR: 48, 70] vs MIS: 64 years [IQR: 54, 72]; P = .0001) and obese (body mass index >30) (open: n = 2,997, 4.8% vs MIS: n = 184, 6.6%) (both P < .05); in addition, patients undergoing an MIS approach were more likely to have comorbidities, including hypertension (open: n = 23,760, 38.3% vs MIS: n = 1,260, 45.5%; odds ratio [OR] 1.35, 95% 1.25–1.45; P = .001) and diabetes (open: n = 10,390, 16.7% vs MIS: n = 550, 19.8%; OR 1.23, 95% 1.12–1.14; P = .001) (Table I). Patients undergoing an MIS operation were also more likely to have Medicare coverage (open: 38.1% vs MIS: 44.3%; OR 1.69, 95% 1.42–2.00; P = .001). MIS operations were more commonly performed at a teaching hospital (open: 80.3% vs MIS: 87.7%; OR 1.74, 95% 1.53–1.98; P = .001) and in an urban setting (open: 97.1% vs MIS 98.8%; OR 2.50, 95% 1.69–3.70; P = .001). After adjustment on multivariate analyses, each of these factors remained independently associated with receipt of MIS surgery (Table III).
Table III.
Univariate and multivariate analysis of factors associated with MIS versus open approach
| Univariate analysis | Adjusted analysis | |||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | P value | OR | 95% CI | P value |
| Age >60 yrs | 0.68 | 0.62–0.73 | .001 | 0.75 | 0.69–0.82 | .001 |
| Female sex | 0.99 | 1.07–0.91 | .98 | — | — | — |
| Race | ||||||
| White | Ref | — | — | — | — | — |
| Black | 0.72 | 0.61–0.84 | <.001 | 0.79 | 0.67–0.94 | .006 |
| Hispanic | 0.73 | 0.62–0.86 | .002 | 0.80 | 0.67–0.95 | .01 |
| Chronic lung disease | 1.31 | 1.14–1.51 | .002 | 1.23 | 1.07–1.42 | .004 |
| Diabetes | 1.23 | 1.12–1.14 | .001 | 1.07 | 0.97–1.19 | .15 |
| Hypertension | 1.35 | 1.25–1.45 | .001 | 1.20 | 1.10–1.30 | .001 |
| Malignancy | 1.30 | 1.19–1.41 | .001 | 1.27 | 1.17–1.39 | .001 |
| Obesity | 1.40 | 1.20–1.64 | .001 | 1.18 | 1.01–1.39 | .04 |
| Teaching hospital | 1.74 | 1.53–1.98 | .001 | 1.71 | 1.49–1.97 | .001 |
| Urban hospital | 2.50 | 1.69–3.70 | .001 | 2.74 | 1.76–4.27 | .001 |
| Elective case | 2.08 | 1.85–2.34 | .001 | 1.97 | 1.75–2.22 | .001 |
| LOS >8 days | 0.73 | 0.69–0.81 | .001 | 0.82 | 0.76–0.90 | .001 |
| Reoperation | 0.51 | 0.38–0.68 | .001 | 0.55 | 0.41–0.73 | .001 |
| Death | — | — | — | 0.67 | 0.52–0.85 | .001 |
| Complications | 0.84 | 0.78–0.91 | .001 | 0.83 | 0.74–0.90 | .001 |
CI, Confidence interval; LOS, length of stay; MIS, minimally invasive surgery; OR, odds ratio.
Open versus MIS outcomes
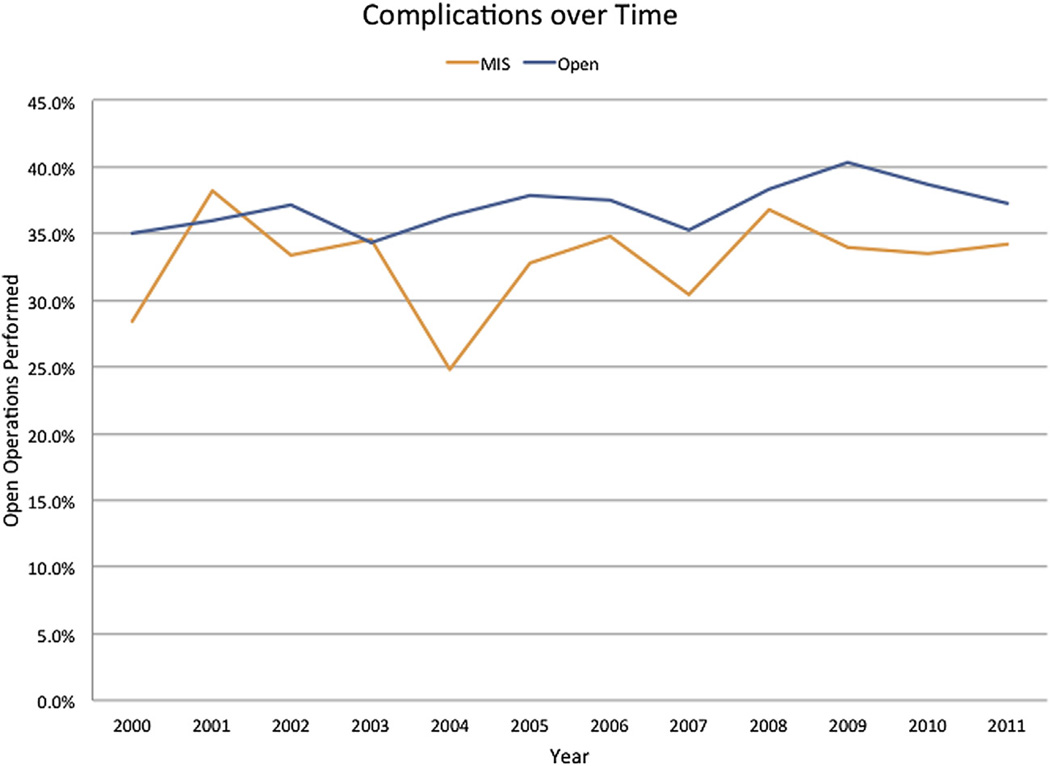
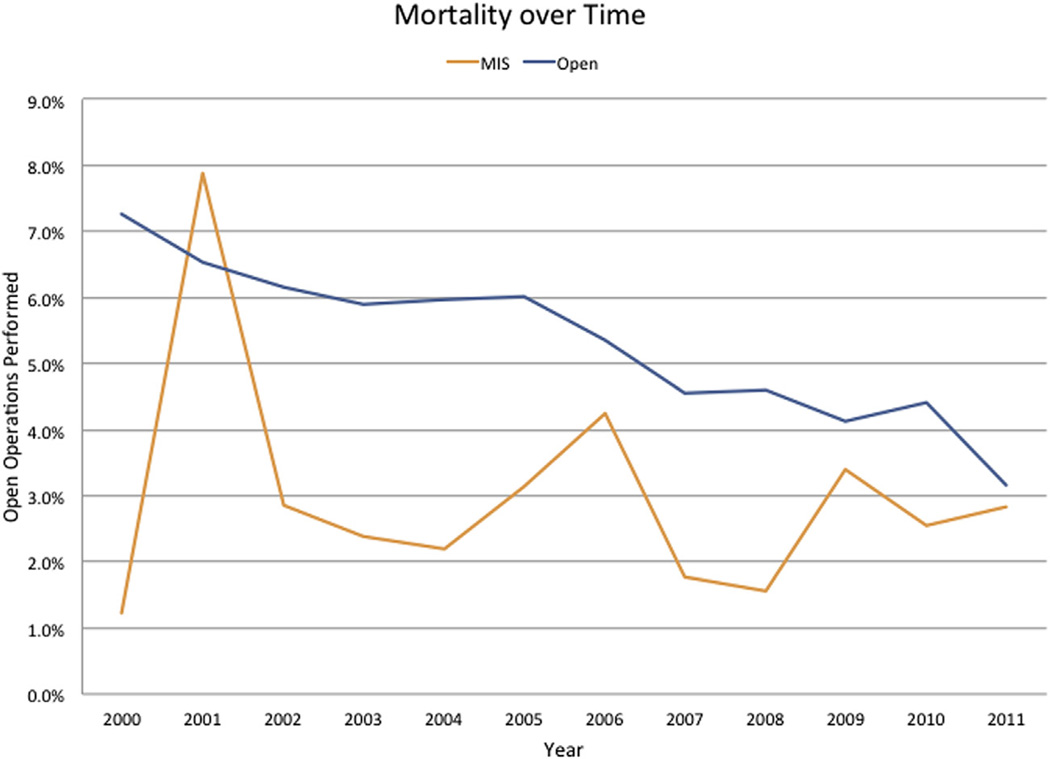
The incidence of complications was lower after an MIS procedure compared with an open procedure (OR 0.84, 95% 0.78–0.91; P = .001) (Fig 2). Specifically, on multivariate analysis, complications were less common with an MIS approach for pancreas (open: 41.6% vs MIS: 35.4%; OR 0.81, 95% 0.72–0.89; P = .001), yet were about the same for liver (open: 33.0% vs MIS: liver: 29.5%; OR 0.91, 95% 0.79–1.05; P = .22) procedures. The MIS associated decrease in complications was mostly related to fewer gastrointestinal (open: 15.6% vs MIS: 14.0%; OR 0.88, 95% 0.79–0.99; P = .03) and renal infectious (open: 6.1% vs MIS: 4.3%; OR 0.79, 95% 0.66–0.96; P = .02). Of note, infectious complications were comparable in both groups (open: 5.9% vs MIS: 5.2%; OR 0.85, 95% 0.71–1.02; P = .09) (Table IV). Reoperation was less common among patients undergoing an MIS pancreas (open: 3.8% vs MIS: 1.8%; OR 0.60, 95% 0.42–0.85; P < .001) and tended to be less for liver (open: 2.8% vs MIS: 1.4%; OR 0.59, 95% 0.33–1.05; P = .07) procedure. Compared with patients who underwent an open operation, patients who had an MIS approach had a slightly shorter median duration of stay (open: 8 vs MIS: 7 days; P < .001), as well as lower in-hospital mortality (open: 5.1% vs MIS: 2.8%; P = .001) (Fig 3). LOS and in-hospital mortality also was less among patients who underwent a “major” MIS HPB procedure (open vs MIS pancreaticoduodenectomy: median LOS, 10 (7, 17) vs 9 (6, 14) days (P = .001) and mortality 5.1% vs 2.9%; OR 0.64, 95% 0.47–0.86; P = .004; open vs MIS major hepatic resection: median LOS, 7 (5, 10) vs 6 (4, 8) days (P = .001) and mortality 5.4% vs 2.9%; OR 0.67, 95% 0.45–1.0; P = .01). On multivariate analysis after controlling for competing risk factors, all patients undergoing an MIS operation were more likely to have a lower incidence of in-hospital complications (OR 0.83, 95% confidence interval [95% CI] 0.74–0.90), reoperation (OR 0.55, 95% CI 0.41–0.73), and in-hospital mortality (OR 0.67, 95% CI 0.52–0.85), as well as a shorter LOS (OR 0.82, 95% CI 0.76–0.90) (all P < .001) (Table III).
Fig 2.
In-hospital complications over time stratified by operative approach.
Table IV.
Perioperative outcomes by procedure type and operative approach
| All open | Open pancreas | Open liver | All MIS | MIS pancreas | MIS liver |
P value |
|
|---|---|---|---|---|---|---|---|
| Any complications | 23,411 (37.7) | 13,887 (41.6) | 9,209 (33.0) | 927 (33.4) | 634 (35.4) | 281 (29.5) | .001 |
| Myocardial infarction | 2,188 (3.5) | 1,349 (4.0) | 818 (2.9) | 105 (3.8) | 66 (3.7) | 39 (4.1) | .46 |
| Pneumonia | 1,393 (2.2) | 872 (2.6) | 495 (1.8) | 44 (1.6) | 36 (2.0) | 8 (0.8) | .02 |
| Cerebrovascular accident | 413 (0.7) | 218 (0.7) | 185 (0.7) | 22 (0.8) | 17 (1.0) | 5 (0.5) | .42 |
| Thromboembolic event | 1,795 (2.9) | 1,121 (3.4) | 643 (2.3) | 75 (2.7) | 57 (3.2) | 18 (1.9) | .57 |
| Acute renal failure | 3,785 (6.1) | 2,125 (6.4) | 1,601 (5.7) | 120 (4.3) | 76 (4.2) | 42 (4.4) | .001 |
| Liver failure | 1,116 (1.8) | 280 (0.8) | 817 (2.9) | 35 (1.3) | 12 (0.7) | 21 (2.2) | .04 |
| Urinary tract infection | 4,148 (6.7) | 2,574 (7.7) | 1,513 (5.4) | 154 (5.6) | 106 (5.9) | 48 (5.0) | .02 |
| Wound complication | 803 (1.3) | 491 (1.5) | 296 (1.1) | 27 (1.0) | 22 (1.2) | 5 (0.5) | .14 |
| Gastrointestinal complication | 9,663 (15.6) | 5,799 (17.4) | 3,757 (13.4) | 387 (14.0) | 272 (15.2) | 112 (11.7) | .02 |
| Shock | 435 (0.7) | 271 (0.8) | 156 (0.6) | 21 (0.8) | 15 (0.8) | 5 (0.5) | .72 |
| Infection | 3,673 (5.9) | 2,586 (7.7) | 1,013 (3.6) | 143 (5.2) | 110 (6.1) | 29 (3.0) | .098 |
| Hemorrhage | 2,807 (4.5) | 1,659 (5.0) | 1,115 (4.0) | 112 (4.0) | 71 (4.0) | 37 (3.9) | .23 |
| Reoperation | 2,078 (3.3) | 1,274 (3.8) | 772 (2.8) | 48 (1.7) | 33 (1.8) | 13 (1.4) | <.001 |
| Other | 2,651 (4.3) | 1,535 (4.6) | 1,079 (3.9) | 109 (3.9) | 69 (3.9) | 39 (4.1) | .391 |
| LOS >8 days | 30,429 (49.0) | 21,238 (63.6) | 8,777 (31.4) | 1,154 (41.6) | 914 (51.1) | 221 (23.2) | .001 |
| Death | 3,161 (5.1) | 1,603 (4.8) | 1,405 (5.0) | 77 (2.8) | 49 (2.7) | 25 (2.6) | .001 |
LOS, Length of stay; MIS, minimally invasive surgery.
Fig 3.
In-hospital mortality over time stratified by operative approach.
DISCUSSION
In 1910, an internist from Stockholm named Han Christian Jacobaeus reported on the abdominal visualization of a small series of patients with ascites and coined the term “laparoscopy.”37 During the past 100 years, there have been tremendous technological innovations and advancements in the field of MIS.38 MIS is now used in virtually all realms of abdominal surgery. In fact, MIS is often the preferred operative approach for many gynecological procedures (eg, tubal ligation, etc),16 as well as general abdominal surgical procedures (eg, antireflux operations, bariatric surgery, etc).15 During the last decade, the MIS approach also has gained increasing acceptance and utilization for colorectal and thoracic procedures.11–13,39
In addition, MIS has now expanded beyond pure laparoscopy to include robotic approaches. Although previous studies have documented the trends and outcomes of laparoscopic and robotic approaches for colorectal and thoracic surgical procedures,12,40,41 national data on HPB MIS has remained poorly defined. Most previous data on the topic of HPB MIS have come from highly specialized, tertiary centers of excellence.23–25,27–29 The current study is important because we defined the overall use and growth trend of HPB MIS in a nationally representative cohort. By using a dataset of nationally representative hospitals, we were more able to characterize the general adoption of MIS techniques for HPB surgery across a broader cohort of institutions. Interestingly, we noted that the overall use of the MIS approach for HPB remained relatively low. In fact, during the 10-year time period examined, the overwhelming majority (95.7%) of operations were performed open, with only 4.4% of hepato-pancreatic cases being MIS. Perhaps not surprisingly, we did note an increased trend in the overall use of the MIS approach for HPB operations. Specifically, by 2011---the last year examined---the proportion of HPB MIS cases had increased to 7.5% compared with only 2.3% in 2000. Of note, although the incidence of complications, as well as LOS, for MIS was lower than for open procedures the differences were not dramatic.
Several reports have noted the expanded use of MIS in other subspecialty operations. For example, Bardakcioglu et al12 reported a marked increase in the use of laparoscopic colectomy after 2004 with a particular inflection of more MIS colon cases after 2008. In several separate studies, the use of video-assisted thoracic surgery similarly has been shown to have increased in the late 2000s such that between 15 and 30% of all lobectomies in the United States are now performed using a MIS approach.42,43 In the current study, we noted a similar increase in the use of the MIS approach for HPB cases. Specifically, the overall number of MIS cases increased from 81 (2.3%) liver and pancreas cases in 2000 to 581 (7.5%) in 2011 (Fig 1). The proportion of all distal pancreatectomies performed via a MIS approach increased from 2.5% in 2000 to 11.8% in 2011; in comparison, although there were no MIS pancreaticoduodenectomy cases recorded in 2000, the proportion had increased to 8.8% by 2011. A similar trend was noted for minor and major hepatic resections (Fig 1).
It is interesting to note that, although there was an increase over time, overall use of the MIS approach for HPB surgery remained low at less than 10%. Interestingly, the use of robotic surgery in HPB was particularly low, with only 1.6% of all cases using this technology in 2011. The reason for the low use of MIS for liver and pancreas operations compared with other abdominal procedures is undoubtedly multifactorial. In part, the lower use of MIS is probably related to the fact that HPB cases tend to be technically complex, with steep learning curves for even open liver and pancreas resections.44,45 In addition, there is the further learning curve associated with techniques inherent to laparoscopic and robotic operations.46,47 Kluger et al48 recently described the importance of development of proper skills in laparoscopic techniques to achieve acceptable perioperative outcomes. Although it is likely that the learning curve for these technically demanding operations is a major contributor to the current low use of MIS HPB surgery, as current trainees gain greater exposure to these techniques the adoption of MIS into complex abdominal operations will likely increase.49
In the current study, we also noted that the majority of MIS operations were performed in teaching hospitals that were located in an urban setting. These findings are consistent with data suggesting that surgeons at nonteaching hospitals often perceive barriers to MIS procedures including limited access to operating room equipment, inadequate mentoring, as well as a lack of experience.50 The finding that pancreaticoduodenectomy (MIS: 53.2%) was the most common pancreas MIS procedure may be related to the fact that most MIS surgery occurred in large, urban centers. Similar to the introduction of MIS approaches for other surgical procedures,51,52 HPB MIS is likely to be centralized at large, teaching hospitals early in the adoption of these techniques.
In addition to hospital-level factors, several patient characteristics were found to be associated with receipt of HPB MIS. Unlike one previous report looking at the disparities in the use of MIS for colorectal disease,53 Medicaid insurance status did not adversely impact the chance of a patient being offered an MIS approach for their HPB procedure. Patients of minority race were, however, less likely to undergo HPB MIS, suggesting a possible socioeconomic barrier to access. Interestingly, other patient specific factors such as age and perioperative comorbidities did not decrease a patient’s chance of undergoing HPB MIS. In fact, patients undergoing MIS were older and had a greater incidence of several comorbidities, which may be related, in part, to more MIS procedures being performed at teaching hospitals. Recent advances in HPB surgery have improved perioperative morbidity and mortality.54 As such, more patients with a greater comorbidity profile are being offered operative resection. Furthermore, as surgeons gain comfort and skills with MIS techniques over time, the restriction of these procedures to the “ideal” patient may be lessening as suggested by our data.
Regarding postoperative outcomes, we found that metrics such as overall complications, mortality, and LOS were roughly comparable after MIS versus open HPB surgery. Specifically, although complications were approximately 25% less common with an MIS approach for pancreas, morbidity was about the same for liver procedures. In addition, the risk of infectious complications was noted to be similar in both the MIS and open HPB groups. Nguyen et al23 had similarly reported comparable morbidity among patients undergoing either open minor or major hepatic resections, whereas Pericleous et al55 had noted the same among pancreatic surgery patients. LOS was shorter in the MIS group, with the difference being about 1 day on average. These data are consistent with previous prospective data based on patients undergoing colorectal surgery, which noted decreased hospital stay and improved quality of life with an MIS approach.56 Taken together, the data strongly suggest that an MIS approach for HPB surgery can be associated with comparable morbidity and mortality, as well as a slightly decreased LOS for well selected patients.
There are a number of limitations that need to be considered when interpreting the data. Accuracy of coding for comorbid conditions is variable57,58 and often selected by the data entry personnel, favoring diagnoses with higher reimbursement rates.59 The greater emphasis on coding complications for reimbursement purpose may explain, in part, the relatively stable incidence of perioperative complications recorded over time.60 Any coding bias was, however, likely random with regard to the MIS versus open groups. As with all retrospective studies, the relative use of the MIS versus the open approach was undoubtedly affected by differential selection of patients who were felt to be better suited for a certain approach. Although we controlled for as many confounding factors as possible with multivariable logistic regression, we could not account for inherent selection bias, patient preference and anatomic differences.
In conclusion, the number of MIS pancreatic and hepatic operations has increased during the last decade with nearly 1 in 13 HPB cases now being performed via an MIS approach. Despite MIS patients tending to have more preoperative medical comorbidities, postoperative morbidity and mortality, as well as LOS compared favorably with open surgery. As with other operative disciplines, HPB MIS will undoubtedly continue to expand over time. Further studies comparing MIS with open surgery will be needed to better elucidate proper patient selection, as well as characterize outcomes in a prospective setting.
Footnotes
Presented at the 9th Annual Academic Surgical Congress in San Diego, CA, February 4–6, 2014.
REFERENCES
- 1.Juo YY, Hyder O, Haider AH, Camp M, Lidor A, Ahuja N. Is minimally invasive colon resection better than traditional approaches? First comprehensive national examination with propensity score matching. JAMA Surg. 2014;149:177–184. doi: 10.1001/jamasurg.2013.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thakur V, Schlachta CM, Jayaraman S. Minilaparoscopic versus conventional laparoscopic cholecystectomy a systematic review and meta-analysis. Ann Surg. 2011;253:244–258. doi: 10.1097/SLA.0b013e318207bf52. [DOI] [PubMed] [Google Scholar]
- 3.Levy BF, Tilney HS, Dowson HM, Rockall TA. A systematic review of postoperative analgesia following laparoscopic colorectal surgery. Colorectal Dis. 2010;12:5–15. doi: 10.1111/j.1463-1318.2009.01799.x. [DOI] [PubMed] [Google Scholar]
- 4.Krane MK, Fichera A. Laparoscopic rectal cancer surgery: where do we stand? World J Gastroenterol. 2012;18:6747–6755. doi: 10.3748/wjg.v18.i46.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kendrick ML. Laparoscopic and robotic resection for pancreatic cancer. Cancer J. 2012;18:571–576. doi: 10.1097/PPO.0b013e31827b8f86. [DOI] [PubMed] [Google Scholar]
- 6.Qiu J, Chen S, Pankaj P, Wu H. Laparoscopic hepatectomy for hepatic colorectal metastases--- a retrospective comparative cohort analysis and literature review. PloS One. 2013;8:e60153. doi: 10.1371/journal.pone.0060153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao AM, Ahmed I. Laparoscopic versus open liver resection for benign and malignant hepatic lesions in adults. Cochrane Database Syst Rev. 2013:CD010162. doi: 10.1002/14651858.CD010162.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Lawrie TA, Medeiros LR, Rosa DD, da Rosa MI, Edelweiss MI, Stein AT, et al. Laparoscopy versus laparotomy for FIGO stage I ovarian cancer. Cochrane Database Syst Rev. 2013:CD005344. doi: 10.1002/14651858.CD005344.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Rao A, Kynaston J, MacDonald ER, Ahmed I. Patient preferences for surgical techniques: should we invest in new approaches? Surg Endosc. 2010;24:3016–3025. doi: 10.1007/s00464-010-1078-y. [DOI] [PubMed] [Google Scholar]
- 10.Tsui C, Klein R, Garabrant M. Minimally invasive surgery: national trends in adoption and future directions for hospital strategy. Surg Endosc. 2013;27:2253–2257. doi: 10.1007/s00464-013-2973-9. [DOI] [PubMed] [Google Scholar]
- 11.Theophilus M, Platell C, Spilsbury K. Long term survival following laparoscopic and open colectomy for colon cancer: a meta-analysis of randomised controlled trials. Colorectal Dis. 2014;16:O75–O81. doi: 10.1111/codi.12483. [DOI] [PubMed] [Google Scholar]
- 12.Bardakcioglu O, Khan A, Aldridge C, Chen J. Growth of laparoscopic colectomy in the United States: analysis of regional and socioeconomic factors over time. Ann Surg. 2013;258:270–274. doi: 10.1097/SLA.0b013e31828faa66. [DOI] [PubMed] [Google Scholar]
- 13.Antoniou SA, Antoniou GA, Koch OO, Pointner R, Granderath FA. Robot-assisted laparoscopic surgery of the colon and rectum. Surg Endosc. 2012;26:1–11. doi: 10.1007/s00464-011-1867-y. [DOI] [PubMed] [Google Scholar]
- 14.Hakimi AA, Ghavamian R. Feasibility of minimally invasive lymphadenectomy in bladder and prostate cancer surgery. Urol Clin North Am. 2011;38:407–418. doi: 10.1016/j.ucl.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Batchelder AJ, Williams R, Sutton C, Khanna A. The evolution of minimally invasive bariatric surgery. J Surg Res. 2013;183:559–566. doi: 10.1016/j.jss.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 16.Schlaerth AC, Abu-Rustum NR. Role of minimally invasive surgery in gynecologic cancers. Oncologist. 2006;11:895–901. doi: 10.1634/theoncologist.11-8-895. [DOI] [PubMed] [Google Scholar]
- 17.Jin T, Altaf K, Xiong JJ, Huang W, Javed MA, Mai G, et al. A systematic review and meta-analysis of studies comparing laparoscopic and open distal pancreatectomy. HPB (Oxford) 2012;14:711–724. doi: 10.1111/j.1477-2574.2012.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao A, Rao G, Ahmed I. Laparoscopic vs. open liver resection for malignant liver disease. A systematic review. Surgeon. 2012;10:194–201. doi: 10.1016/j.surge.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Gumbs AA, Rodriguez Rivera AM, Milone L, Hoffman JP. Laparoscopic pancreatoduodenectomy: a review of 285 published cases. Ann Surg Oncol. 2011;18:1335–1341. doi: 10.1245/s10434-010-1503-4. [DOI] [PubMed] [Google Scholar]
- 20.Ammori BJ, Ayiomamitis GD. Laparoscopic pancreaticoduodenectomy and distal pancreatectomy: a UK experience and a systematic review of the literature. Surg Endosc. 2011;25:2084–2099. doi: 10.1007/s00464-010-1538-4. [DOI] [PubMed] [Google Scholar]
- 21.Cardinal JS, Reddy SK, Tsung A, Marsh JW, Geller DA. Laparoscopic major hepatectomy: pure laparoscopic approach versus hand-assisted technique. J Hepato-biliary pancreatic Sci. 2013;20:114–119. doi: 10.1007/s00534-012-0553-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin NC, Nitta H, Wakabayashi G. Laparoscopic major hepatectomy: a systematic literature review and comparison of 3 techniques. Ann Surg. 2013;257:205–213. doi: 10.1097/SLA.0b013e31827da7fe. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg. 2009;250:831–841. doi: 10.1097/SLA.0b013e3181b0c4df. [DOI] [PubMed] [Google Scholar]
- 24.Morino M, Morra I, Rosso E, Miglietta C, Garrone C. Laparoscopic vs open hepatic resection: a comparative study. Surg Endosc. 2003;17:1914–1918. doi: 10.1007/s00464-003-9070-4. [DOI] [PubMed] [Google Scholar]
- 25.Kaneko H, Takagi S, Otsuka Y, Tsuchiya M, Tamura A, Katagiri T, et al. Laparoscopic liver resection of hepatocellular carcinoma. Am J Surg. 2005;189:190–194. doi: 10.1016/j.amjsurg.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen KT, Marsh JW, Tsung A, Steel JJ, Gamblin TC, Geller DA. Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg. 2011;146:348–356. doi: 10.1001/archsurg.2010.248. [DOI] [PubMed] [Google Scholar]
- 27.Tsung A, Geller DA, Sukato DC, Sabbaghian S, Tohme S, Steel J, et al. Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg. 2014;259:549–555. doi: 10.1097/SLA.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 28.Cirocchi R, Partelli S, Coratti A, Desiderio J, Parisi A, Falconi M. Current status of robotic distal pancreatectomy: a systematic review. Surg Oncol. 2013;22:201–207. doi: 10.1016/j.suronc.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Cirocchi R, Partelli S, Trastulli S, Coratti A, Parisi A, Falconi M. A systematic review on robotic pancreaticoduodenectomy. Surg Oncol. 2013;22:238–246. doi: 10.1016/j.suronc.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura M, Nakashima H. Laparoscopic distal pancreatectomy and pancreatoduodenectomy: is it worthwhile? A meta-analysis of laparoscopic pancreatectomy. J Hepatobiliary Pancreat Sci. 2013;20:421–428. doi: 10.1007/s00534-012-0578-7. [DOI] [PubMed] [Google Scholar]
- 31.Yin Z, Fan X, Ye H, Yin D, Wang J. Short- and long-term outcomes after laparoscopic and open hepatectomy for hepatocellular carcinoma: a global systematic review and meta-analysis. Ann Surg Oncol. 2013;20:1203–1215. doi: 10.1245/s10434-012-2705-8. [DOI] [PubMed] [Google Scholar]
- 32.Mesleh MG, Stauffer JA, Bowers SP, Asbun HJ. Cost analysis of open and laparoscopic pancreaticoduodenectomy: a single institution comparison. Surg Endosc. 2013;27:4518–4523. doi: 10.1007/s00464-013-3101-6. [DOI] [PubMed] [Google Scholar]
- 33.Cannon RM, Scoggins CR, Callender GG, McMasters KM, Martin RC., 2nd Laparoscopic versus open resection of hepatic colorectal metastases. Surgery. 2012;152:567–573. doi: 10.1016/j.surg.2012.07.013. discussion 573-4. [DOI] [PubMed] [Google Scholar]
- 34.Asiyanbola B, Chang D, Gleisner AL, Nathan H, Choti MA, Schulick RD, et al. Operative mortality after hepatic resection: are literature-based rates broadly applicable? J Gastrointest Surg. 2008;12:842–851. doi: 10.1007/s11605-008-0494-y. [DOI] [PubMed] [Google Scholar]
- 35.Syin D, Woreta T, Chang DC, Cameron JL, Pronovost PJ, Makary MA. Publication bias in surgery: implications for informed consent. J Surg Res. 2007;143:88–93. doi: 10.1016/j.jss.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 36.HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP) Rockville, MD: Agency for Healthcare Research and Quality; 2007–2009. Available from: http://www.hcup-us.ahrq.gov/nisoverview.jsp. [PubMed] [Google Scholar]
- 37.Harrell AG, Heniford BT. Minimally invasive abdominal surgery: lux et veritas past, present, and future. Am J Surg. 2005;190:239–243. doi: 10.1016/j.amjsurg.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 38.Lee WJ, Chan CP, Wang BY. Recent advances in laparoscopic surgery. Asian J Endosc Surg. 2013;6:1–8. doi: 10.1111/ases.12001. [DOI] [PubMed] [Google Scholar]
- 39.Flores RM, Park BJ, Dycoco J, Aronova A, Hirth Y, Rizk NP, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg. 2009;138:11–18. doi: 10.1016/j.jtcvs.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 40.Kent M, Wang T, Whyte R, Curran T, Flores R, Gangadharan S. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg. 2014;97:236–242. doi: 10.1016/j.athoracsur.2013.07.117. [DOI] [PubMed] [Google Scholar]
- 41.Halabi WJ, Kang CY, Jafari MD, Nguyen VQ, Carmichael JC, Mills S, et al. Robotic-assisted colorectal surgery in the United States: a nationwide analysis of trends and outcomes. World J Surg. 2013;37:2782–2790. doi: 10.1007/s00268-013-2024-7. [DOI] [PubMed] [Google Scholar]
- 42.Paul S, Altorki NK, Sheng S, Lee PC, Harpole DH, Onaitis MW, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg. 2010;139:366–378. doi: 10.1016/j.jtcvs.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 43.Paul S, Sedrakyan A, Chiu YL, Nasar A, Port JL, Lee PC, et al. Outcomes after lobectomy using thoracoscopy vs thoracotomy: a comparative effectiveness analysis utilizing the Nationwide Inpatient Sample database. Eur J Cardiothorac Surg. 2013;43:813–817. doi: 10.1093/ejcts/ezs428. [DOI] [PubMed] [Google Scholar]
- 44.Tseng JF, Pisters PW, Lee JE, Wang H, Gomez HF, Sun CC, et al. The learning curve in pancreatic surgery. Surgery. 2007;141:694–701. doi: 10.1016/j.surg.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Nathan H, Cameron JL, Choti MA, Schulick RD, Pawlik TM. The volume-outcomes effect in hepato-pancreato-biliary surgery: hospital versus surgeon contributions and specificity of the relationship. J Am Coll Surg. 2009;208:528–538. doi: 10.1016/j.jamcollsurg.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Schreuder HW, Wolswijk R, Zweemer RP, Schijven MP, Verheijen RH. Training and learning robotic surgery, time for a more structured approach: a systematic review. BJOG. 2012;119:137–149. doi: 10.1111/j.1471-0528.2011.03139.x. [DOI] [PubMed] [Google Scholar]
- 47.Miskovic D, Ni M, Wyles SM, Tekkis P, Hanna GB. Learning curve and case selection in laparoscopic colorectal surgery: systematic review and international multicenter analysis of 4852 cases. Dis Colon Rectum. 2012;55:1300–1310. doi: 10.1097/DCR.0b013e31826ab4dd. [DOI] [PubMed] [Google Scholar]
- 48.Kluger MD, Vigano L, Barroso R, Cherqui D. The learning curve in laparoscopic major liver resection. J Hepatobiliary Pancreat Sci. 2013;20:131–136. doi: 10.1007/s00534-012-0571-1. [DOI] [PubMed] [Google Scholar]
- 49.McCoy AC, Gasevic E, Szlabick RE, Sahmoun AE, Sticca RP. Are open abdominal procedures a thing of the past? An analysis of graduating general surgery residents’ case logs from 2000 to 2011. J Surg Educ. 2013;70:683–689. doi: 10.1016/j.jsurg.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Birch DW, Misra M, Farrokhyar F. The feasibility of introducing advanced minimally invasive surgery into surgical practice. Can J Surg. 2007;50:256–260. [PMC free article] [PubMed] [Google Scholar]
- 51.Flowers JL, Bailey RW, Scovill WA, Zucker KA. The Baltimore experience with laparoscopic management of acute cholecystitis. Am J Surg. 1991;161:388–392. doi: 10.1016/0002-9610(91)90604-c. [DOI] [PubMed] [Google Scholar]
- 52.Schauer PR, Ikramuddin S, Gourash W, Ramanathan R, Luketich J. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 2000;232:515–529. doi: 10.1097/00000658-200010000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robinson CN, Balentine CJ, Sansgiry S, Berger DH. Disparities in the use of minimally invasive surgery for colorectal disease. J Gastrointest Surg. 2012;16:897–903. doi: 10.1007/s11605-012-1844-3. discussion 903-4. [DOI] [PubMed] [Google Scholar]
- 54.Barbas AS, Turley RS, Mallipeddi MK, Lidsky ME, Reddy SK, White RR, et al. Examining reoperation and readmission after hepatic surgery. J Am Coll Surg. 2013;216:915–923. doi: 10.1016/j.jamcollsurg.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 55.Pericleous S, Middleton N, McKay SC, Bowers KA, Hutchins RR. Systematic review and meta-analysis of case-matched studies comparing open and laparoscopic distal pancreatectomy: is it a safe procedure? Pancreas. 2012;41:993–1000. doi: 10.1097/MPA.0b013e31824f3669. [DOI] [PubMed] [Google Scholar]
- 56.Kuhry E, Schwenk WF, Gaupset R, Romild U, Bonjer HJ. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev. 2008:CD003432. doi: 10.1002/14651858.CD003432.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romano PS, Mark DH. Bias in the coding of hospital discharge data and its implications for quality assessment. Med Care. 1994;32:81–90. doi: 10.1097/00005650-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 58.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: an overview. Med Care. 2002;40(8 Suppl):IV-26–IV-35. doi: 10.1097/00005650-200208001-00004. [DOI] [PubMed] [Google Scholar]
- 59.Lee LA, Morell RC. Rare complications and national databases. Anesth Analg. 2009;109:1357–1359. doi: 10.1213/ANE.0b013e3181b763d6. [DOI] [PubMed] [Google Scholar]
- 60.Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Yamashita K, et al. Perioperative management of hepatic resection toward zero mortality and morbidity: analysis of 793 consecutive cases in a single institution. J Am Coll Surg. 2010;211:443–449. doi: 10.1016/j.jamcollsurg.2010.06.005. [DOI] [PubMed] [Google Scholar]