Abstract
The rhesus glycoproteins, Rh B glycoprotein (RHBG) and Rh C glycoprotein (RHCG), are recently identified ammonia transporters. Rhcg expression is necessary for normal male fertility, but its specific cellular expression is unknown, and Rhbg has not been reported to be expressed in the male reproductive tract. This study sought to determine the specific cellular expression of Rhcg, to determine whether Rhbg is expressed in the male reproductive tract, and, if so, to determine which cells express Rhbg using real-time RT-PCR, immunoblot analysis, and immunohistochemistry. Both Rhbg and Rhcg were expressed throughout the male reproductive tract. In the testis, high levels of Rhbg were expressed in Leydig cells, and Rhcg was expressed in spermatids during the later stages of their maturation (steps 13–16) in stages I–VIII of the seminiferous epithelium cycle. In the epididymis, basolateral Rhbg was present in narrow cells in the initial segment, in principal cells in the upper corpus, and in clear cells throughout the epididymis. Apical Rhcg immunolabel was present in principal cells in the caput and upper corpus epididymidis and in clear cells in the middle and lower corpus and cauda epididymidis. In the vas deferens, apical Rhcg immunolabel and basolateral Rhbg immunolabel were present in some principal cells and colocalized with H+-ATPase immunolabel. We conclude that both Rhbg and Rhcg are highly expressed in specific cells in the male reproductive tract where they can contribute to multiple components of male fertility.
Introduction
The ammonia transporter family, which includes Mep proteins in yeast, AMT proteins in many bacteria and plants, and rhesus (Rh) glycoproteins in mammalian species, is a recently identified extended family of integral membrane proteins that mediate critical roles in transmembrane ammonia transport (Winkler 2005, Weiner 2006, Knepper 2008, Weiner & Verlander 2010). Mammals express three ammonia transporter family members, Rh A glycoprotein (RHAG), RHBG, and RHCG (Liu et al. 2000, 2001, Marini et al. 2000, Weiner & Verlander 2010). Rh glycoproteins are integral membrane proteins with 12 transmembrane segments. They are present in plasma membranes in a homotrimeric form and enable specific transport of ammonia, but no other known solutes, across plasma membranes.
Rh glycoproteins are expressed specifically in tissues in which ammonia transport is necessary for normal biological functioning. They are highly expressed in the kidneys, where ammonia formation and transport are central to renal regulation of acid–base homeostasis (Eladari et al. 2002, Quentin et al. 2003, Verlander et al. 2003, Weiner & Verlander 2011). In liver, Rh glycoproteins are expressed in perivenous hepatocytes, which mediate high-affinity ammonia metabolism (Weiner et al. 2003). Sweat contains ammonia in concentrations ~100-fold greater than in plasma (Brusilow & Gordes 1964, 1968, Liu et al. 2001), and Rh glycoproteins are highly expressed in sweat glands (Liu et al. 2001).
Another tissue that expresses Rh glycoproteins is the male reproductive tract. RHCG is expressed in both the testis and epididymis (Liu et al. 2000), and its expression, at least as determined by global knockout studies, is necessary for normal male fertility (Biver et al. 2008). However, both the testis and epididymis contain multiple cell types with distinct cellular functions, and the specific cells in these organs that express Rhcg have not been identified. In the studies reporting the initial cloning of mammalian RHBG, northern blots did not identify RHBG mRNA expression in male reproductive organs (Liu et al. 2001). However, we have shown that RHBG is expressed in several tissues in which it was not identified in the initial cloning report (Handlogten et al. 2005, Han et al. 2009). Thus, whether RHBG is expressed in the male reproductive tract is unclear.
In view of the importance of RHCG in male fertility, but lack of knowledge regarding its specific cellular expression, and the uncertainty regarding whether RHBG is expressed in the male reproductive organs, the purpose of the current study was to perform a detailed examination of RHCG and RHBG mRNA and protein expression in the testis and epididymis. Our results show distinct cell-specific RHCG and RHBG expression in the testis and cell-specific expression combined with axial heterogeneity in the epididymis and vas deferens. These observations indicate that RHCG and RHBG are likely to be involved in multiple components of male fertility.
Ammonia exists in two molecular forms, and NH3, in aqueous solutions. In this manuscript, we use the term ‘ammonia’ to refer to the combination of these two molecular forms. When referring specifically to one of these molecular forms, we use the terms ‘NH3’ or ‘ .’
Materials and methods
Animals
Adult wild-type C57Bl/6 mice were used in these studies. All animal studies were approved by the University of Florida College of Medicine and the North Florida/South Georgia Veterans Health System IACUC.
Antibodies
Affinity-purified antibodies to RHCG and RHBG generated in our laboratory have been characterized previously (Verlander et al. 2003, Weiner et al. 2003, Mak et al. 2006). Antibodies to the a4 subunit of H+-ATPase were kindly provided by Dr Fiona Karet (University of Cambridge, UK) and rabbit polyclonal antibody for glutamine synthetase (cat #AB73593) was purchased from Abcam (Cambridge, MA, USA).
mRNA extraction
Adult C57Bl/6 male mice were anesthetized with inhalant isoflurane. After the mice were killed, the kidney, testis, caput, corpus and cauda epididymidis, and vas deferens were removed rapidly, placed into RNAlater (Qiagen), and stored in a −70 °C freezer until used. Total RNA was extracted from kidney, testis, and epididymis using RNeasy Mini Kit (Qiagen) and stored in a −70 °C freezer until used.
Real-time RT-PCR
Real-time RT-PCR amplification of Rhbg and Rhcg was performed as described in detail previously (Weiner et al. 2003, Handlogten et al. 2005). Briefly, total RNA was reverse transcribed using SuperScript First Strand Synthesis System for RT-PCR (Invitrogen) and random hexamer primers. For real-time PCR amplification of Rhbg, we used forward primer, 5′-GCCTGCAGAGTGTGTTTCCA-3′; reverse primer, 5′-GAGCTGATACACGGCCTGAGA-3′; and fluorescent probe, 6Fam-TGGCACTCCGCTGACCCTTGG-Tamra. For Rhcg, the forward primer was 5′-GGATACCCCTTCTTGGACTCTTC-3′, the reverse primer was 5′-TGCCTTGGAACATGGGAAAT-3′, and the fluorescent probe was 6Fam-AGCCTCCGCCTGCTCC-CCAAC-Tamra. Real-time RT-PCR was performed on an ABI Prism GeneAmp 7500 Sequence Detection System. We used a two-step cycle protocol including an initial 95 °C denaturation step for 15 s and then 60 °C for 1 min and then 40–50 cycles of alternating temperatures. 18S RNA was amplified in all experiments as an internal control using commercially available primers and probes (Applied Biosystems). All experiments included samples not treated with RT and samples to which RNA was not added as internal controls. All results were confirmed by repeating the experiments on specimens from at least three different mice.
Protein preparation
Animals were anesthetized with inhalant isoflurane, and the testes and epididymides were rapidly removed. The epididymides were dissected cleaned of connective tissues and divided into caput, corpus, and cauda regions. The tissues were frozen in liquid nitrogen and stored at −70 °C until used. The tissues were homogenized using microtube pestles (USA Scientific, Ocala, FL, USA), and proteins were extracted using T-PER Tissue Protein Extraction Reagent (Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer’s recommended procedures. An aliquot was obtained for protein determination using a BCA assay, and the remainder was stored frozen at −70 °C until used.
Immunoblotting procedure
Ten micrograms of protein were electrophoresed on 10% PAGE ReadyGel (Bio-Rad). Gels were then transferred electrophoretically to nitrocellulose membranes, blocked with 5 g/dl nonfat dry milk, and incubated at 4 °C overnight with primary antibody diluted in Blotto buffer (50 mM Tris, 150 mM NaCl, 5 mM Na2EDTA, and 0.05% Tween 20, pH 7.6) with 5 g/dl nonfat dry milk. Loading and transfer equivalence were assessed with Ponceau S staining. After washing, membranes were exposed to secondary antibody goat anti-rabbit IgG (Millipore, Billerica, MA, USA) conjugated to HRP at a dilution of 1:5000. Sites of antibody–antigen reaction were visualized using ECL (SuperSignal West Pico Substrate, Pierce) and a Kodak Image Station 440CF digital imaging system. Band density was quantified using Kodak 1D, version 5.0, Software (Kodak Scientific Imaging, New Haven, CT, USA). Absence of saturation was confirmed by examining pixel intensity distribution in all immunoblots.
Immunohistochemistry
Following isoflurane anesthesia, testis and epididymis were preserved by in vivo cardiac perfusion with PBS (pH 7.4) followed by periodate–lysine–2% paraformaldehyde and then immersed for 24–48 h at 4 °C in this fixative. For light microscopy, 2- or 3 μm-thick sections were cut from paraffin-embedded testis or epididymis.
Immunolocalization was accomplished using standard immunoperoxidase procedures. The sections were deparaffinized in xylene and ethanols, rehydrated, and then rinsed in PBS. Endogenous peroxidase activity was blocked by incubating sections in Peroxidase Blocking Reagent (DakoCytomation, Carpinteria, CA, USA) for 45 min. Sections were blocked for 15 min with Serum-Free Protein Block (DakoCytomation) and then incubated overnight with primary antibody. Sections were washed with PBS and incubated for 30 min with polymer-linked, peroxidase-conjugated goat anti-rabbit IgG (MACH2, Biocare Medical, Concord, CA, USA), again washed with PBS, and then exposed to diaminobenzidine (DAB) for 5 min. Sections were washed in distilled water, then dehydrated with xylene, mounted, and observed by light microscopy. Comparisons of labeling were made only between sections of the same thickness from the same immunohistochemistry experiment. Sections were examined and photographed with a Nikon E600 microscope equipped with differential interference contrast (DIC) optics and interfaced with a DXM1200F digital camera and ACT-1 Software (Nikon). Color correction was performed using Adobe Photoshop CS5 Software (Adobe Systems, Inc.). All immunohistochemistry was performed on tissue sections from at least four different mice.
Double-immunolabeling procedure
Double immunolabeling was accomplished using immunoperoxidase procedures described in detail previously (Kim et al. 2009). To distinguish the cell types, tissue sections were labeled with H+-ATPase using Vector SG (Vector Laboratories, Burlingame, CA, USA) as the chromogen to produce a blue label, as described earlier. The above procedure was repeated with the substitution of a second primary antibody (Rhbg and Rhcg) and the substitution of DAB for Vector SG. This label was easily distinguishable from the blue label produced by the Vector SG for detection of H+-ATPase. Sections were then washed with glass-distilled water, dehydrated and mounted, and observed by light microscopy. All double-immunolabel immunohistochemistry was performed on tissue sections from at least four different mice.
Statistical analysis
Results are presented as means±S.E.M. Statistical analyses were performed using Student’s t-test, and P<0.05 was taken as statistically significant.
Results
Rhbg and Rhcg mRNA expression
We first determined whether Rhbg and Rhcg mRNA were expressed in the testis and epididymis. To do so, and to enable detection of mRNA with greater sensitivity than the northern blotting techniques used in initial studies of the male reproductive tract, we used real-time RT-PCR with gene-specific primers and TaqMan probe, as we have described previously (Verlander et al. 2003, Weiner et al. 2003, Handlogten et al. 2005, Han et al. 2009). We identified expression of both Rhbg and Rhcg mRNA in both testis and epididymis (Fig. 1A and B). Neither Rhbg nor Rhcg mRNA was detected in the absence of reverse transcriptase, confirming that mRNA, not genomic DNA, was amplified (not shown).
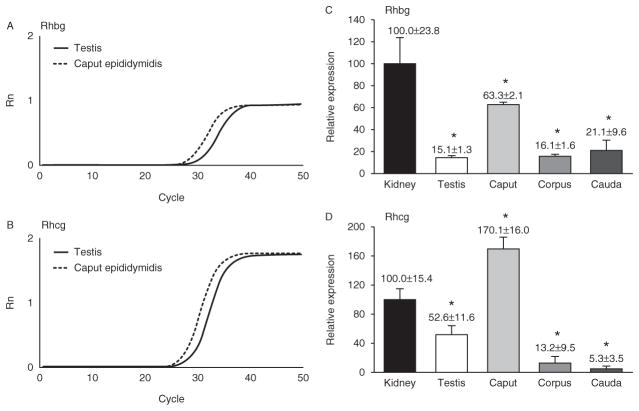
Figure 1.
Real-time RT-PCR relative expression of Rhcg and Rhbg mRNA in the mouse. mRNA was reverse transcribed and amplified using gene-specific primers and TaqMan probe for Rhcg and Rhbg. (A) Amplification curves for Rhbg in the testis and the caput epididymidis. No amplification was identified in the absence of RTor when sample mRNAwas not included. (B) Amplification curves for Rhcg in the testis and the caput epididymidis. No amplification was identified in the absence of RT or when sample mRNA was not included. (C) Relative expression levels of Rhbg mRNA in the testis and different regions of the epididymis. Rhbg is present in all male reproductive tract regions tested, with axial heterogeneity in its expression levels in different portions of the epididymis. Rhbg mRNA is expressed relative to renal cortical mRNA determined simultaneously. (D) Relative expression levels of Rhcg mRNA in the testis and different regions of the epididymis. Rhcg is present in all male reproductive tract regions tested, with axial heterogeneity in its expression levels in different portions of the epididymis. Rhcg mRNA is expressed relative to renal cortical mRNA determined simultaneously. Values are means±S.E.M. *P<0.05 vs kidney. n=3 for each sample. Kidney cortex mRNA was used as a positive control.
Because there is known axial heterogeneity of cell types and function in the epididymis, we examined differences in Rhbg and Rhcg mRNA expression in different regions of the epididymis. Kidney was used as an internal control to assess relative abundance because RHBG and RHCG expression in the kidney is high (Weiner & Verlander 2010, 2011, 2013) and because expression is essential for normal renal ammonia metabolism (Biver et al. 2008, Lee et al. 2009, 2010, 2013, Bishop et al. 2010, 2013). Rhbg mRNA in the testis was relatively modest, ~20% of the level in the kidney. In the epididymis, Rhbg mRNA expression was maximal in the caput, at ~60% of renal expression. There was a lower level of expression in the corpus and cauda epididymidis (Fig. 1C). Rhcg mRNA expression in the testis was ~50% of levels in the kidney. In the epididymis, Rhcg mRNA expression was maximal in the caput, where expression level was ~160% of that observed in the kidney (Fig. 1D). Similar to the pattern for Rhbg, expression in the corpus and cauda epididymidis was significantly less than that in the caput epididymidis, ~10% of the expression in the caput. Thus, both Rhbg and Rhcg mRNA are expressed in both the testis and epididymis, their highest levels of expression are in the caput, and there is axial variation in both Rhbg and Rhcg mRNA expression in the epididymis.
RHBG and RHCG protein expression
We then examined RHBG and RHCG protein expression in the testis and epididymis. Because Rh glycoprotein mRNA expression was the greatest in the caput epididymidis, we specifically examined expression in this region. Immunoblot analysis demonstrated expression of RHBG and RHCG proteins in both the testis and epididymis (Fig. 2). For both RHBG and RHCG, detected molecular weights exhibited slight variations in different portions of the male reproductive tract, consistent with tissue-specific differences in glycosylation as reported previously (Weiner et al. 2003, Handlogten et al. 2005).
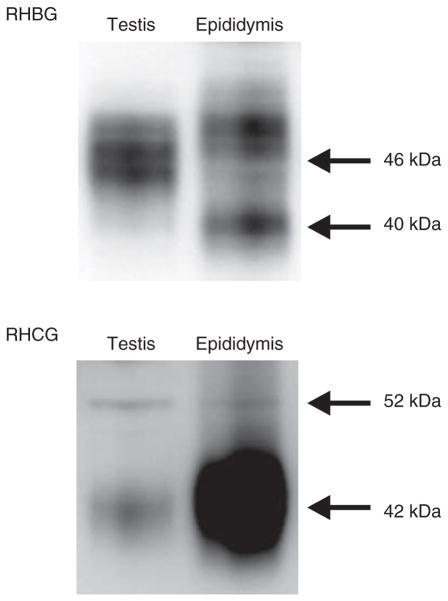
Figure 2.
Immunoblot analysis of RHCG and RHBG in the male reproductive tract. (Upper panel) Immunoblot analysis of RHBG protein expression. RHBG is present in both testis and epididymis.
The predominant expression of RHBG is at ~46 kDa in the testis and 40 and 49 kDa in the epididymis, consistent with tissue-specific variations in glycosylation. (Lower panel) Immunoblot analysis of RHCG protein expression. The predominant expression of RHCG is at 42 and 52 kDa in the testis and at 42 kDa in the epididymis. These variations are consistent with tissue-specific variations in RHCG glycosylation.
Cellular localization of RHBG and RHCG in testis
We next determined which cells in the testis expressed RHBG and RHCG. RHBG immunolabel was detected only in interstitial cells and not in the seminiferous tubules (Fig. 3A and B). High-power micrographs suggested that the interstitial cells with RHBG immunolabel had a location and morphology suggestive of Leydig cells.
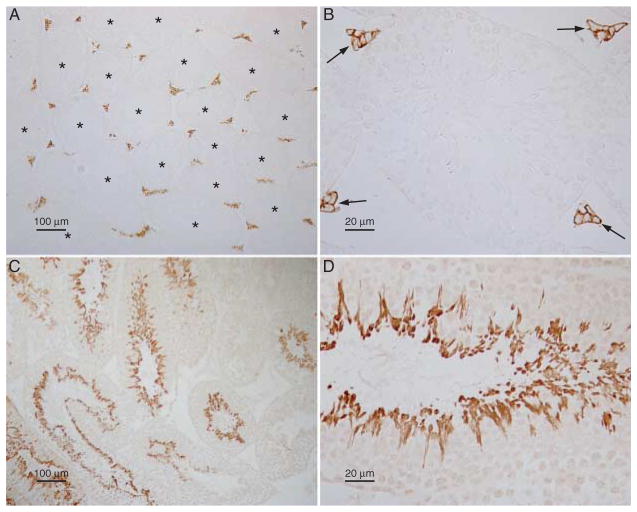
Figure 3.
RHBG and RHCG localization in the testis. (A and B) Low- and high-power micrographs respectively of RHBG immunolabeling in the testis. No RHBG immunolabel is present in seminiferous tubules (asterisks). However, strong plasma membrane RHBG immunolabel is present in interstitial cells with morphology and localization consistent with identification as Leydig cells (arrows).
No immunolabel was observed in experiments in which primary antibody was omitted (data not shown). (C and D) Representative low- and high-power micrographs respectively of RHCG immunolabel in the testis. RHCG exhibits significant variation in immunolabel intensity in different seminiferous tubules, consistent with stage-dependent variations in RHCG expression. High-power micrographs (D) spermatids, but not Leydig cells, display RHCG immunolabel. Controls in which primary antibody was omitted had no detectable immunolabel. Results are representative of findings from at least four mice.
To confirm that RHBG was expressed in Leydig cells, we performed double-immunolabel experiments using antibodies to the Leydig cell-specific protein glutamine synthetase (Holash et al. 1993, van Straaten et al. 2006). We identified that RHBG immunolabel was found only in glutamine synthetase-positive cells in the testis and that all cells with cytoplasmic glutamine synthetase expression expressed Rhbg (Fig. 4). Thus, RHBG is expressed specifically in Leydig cells in the testis.
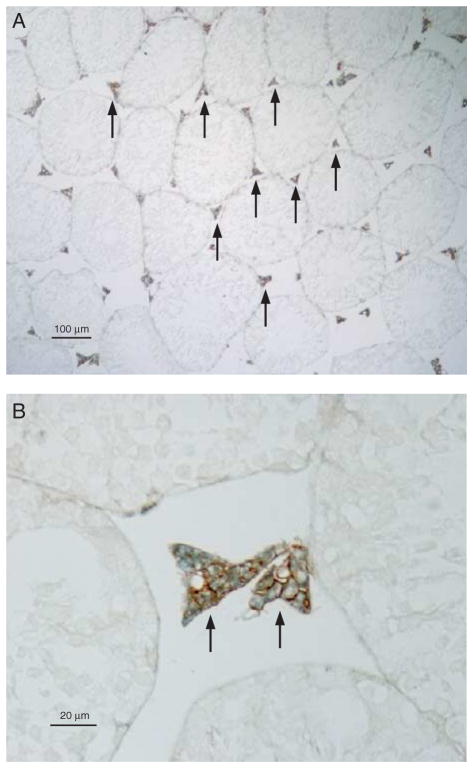
Figure 4.
Double-immunolabel for RHBG (brown) and glutamine synthetase (blue) in the testis. Glutamine synthetase immunolabel was used as a Leydig cell-specific marker. (A) Low-power micrograph and (B) High-power micrograph. All cells with RHBG immunolabel (brown) also exhibited cytoplasmic glutamine synthetase immunolabel (blue), and all cells with glutamine synthetase immunolabel expressed RHBG immunolabel (arrows). These findings indicate RHBG immunolabel expression in Leydig cells in the testis. Results are representative of findings from at least four mice.
Because testis expresses RHCG mRNA and protein, we also examined the cellular pattern of RHCG immunolabel expression. Strong RHCG immunolabel was present in seminiferous tubules, with no detectable expression in interstitial cells (Fig. 3C). High-power micrographs showed RHCG immunolabel exclusively in the head and midpiece of spermatids (Fig. 3D). In addition, spermatids in some tubules were strongly labeled, while there was no labeling in other tubules, suggesting stage-specific variations in RHCG expression. The stages of the seminiferous epithelial cycle where RHCG was expressed were determined using staging criteria described by Meistrich & Hess (2013). RHCG expression was absent in tubules from stage IX through XII, indicating that RHCG was not seen at or before step 12 of spermatid maturation, as all earlier steps of spermatid maturation are present in these stages but RHCG immunostaining was absent. The most apical layer of spermatids stained in tubules between stages I and VIII. This indicates that RHCG expression is confined to steps 13–16 of spermatid maturation, which are the most apical cells during stages I–VIII. Thus, both RHBG and RHCG are expressed in the testis, but their cellular localization differs. RHCG has a highly restricted pattern of expression in seminiferous epithelium and is present in only the more mature spermatids but not in other spermatogenic cells.
Cellular localization of RHBG in the efferent ductules
We next determined the expression of these ammonia transporter family members in the efferent ductules. No significant expression of either RHBG or RHCG immunolabel was detectable (Fig. 5A and B). Intact antigenic preservation was demonstrated by showing normal localization and expression of H+-ATPase immunolabel (Fig. 5C and D).
Figure 5.
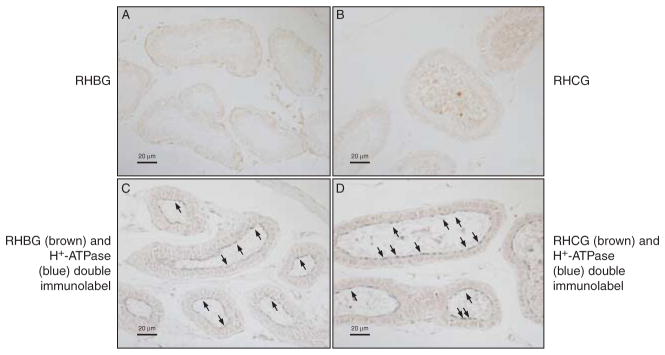
RHBG and RHCG immunolabel expression in the efferent ductules. (A) Efferent ductules stained for RHBG immunolabel expression. No significant immunolabel is detectable. (B) Efferent ductules stained for RHCG immunolabel. No significant immunolabel is detectable. (C) Double-immunolabel of RHBG (brown) with H+-ATPase (blue) to confirm retention of antigenic sites. Apical H+-ATPase immunolabel is present (arrows), but no RHBG immunolabel is detectable. (D) Double-immunolabel of RHCG (brown) with H+-ATPase (blue) to confirm retention of antigenic sites. Apical H+-ATPase immunolabel is present (arrows), but no RHCG immunolabel is detectable. Results are representative of findings from at least four mice.
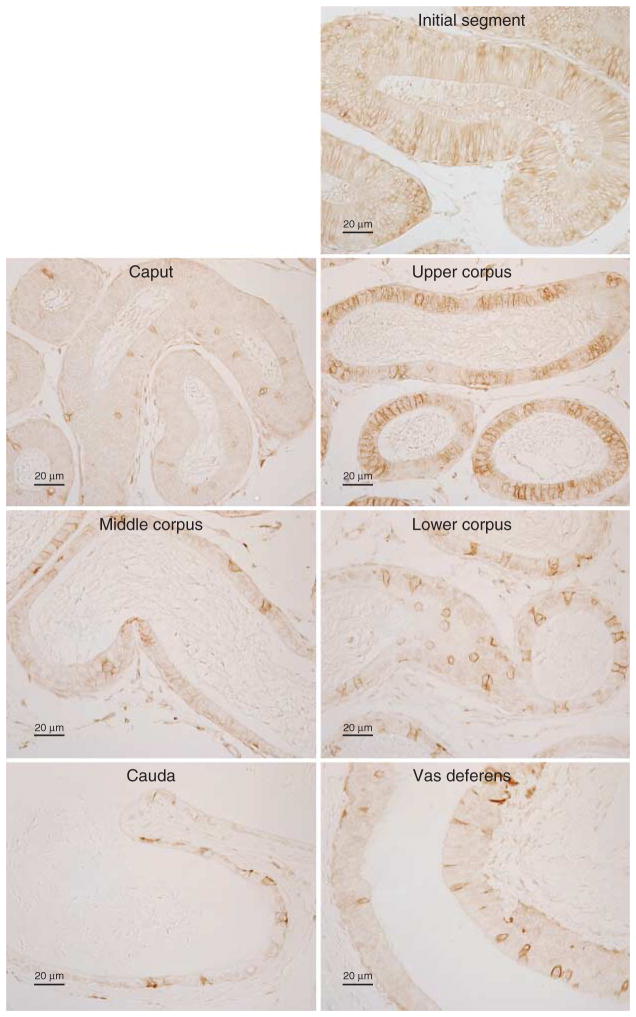
Cellular localization of RHBG in the epididymis
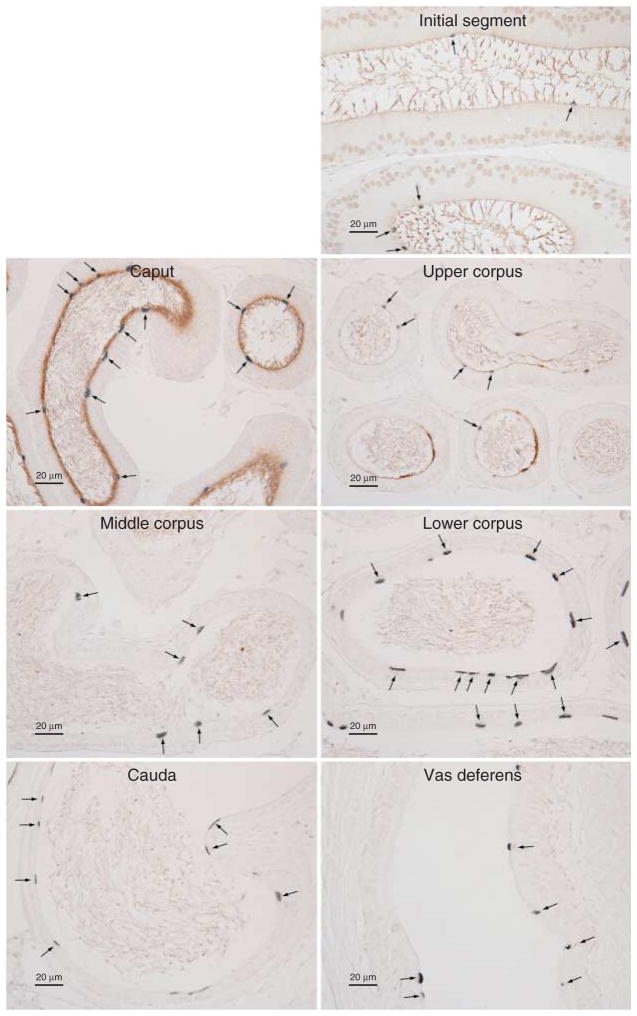
Because our mRNA and protein analysis studies of tissue homogenates demonstrated for the first time, to our knowledge, that RHBG is expressed in the epididymis, we determined its specific localization using immunohistochemistry. Because of axial variations in the epididymis, we paid specific attention to RHBG expression in the different anatomic regions. Figure 6 shows representative findings. Basolateral RHBG immunolabel was expressed in all regions of the epididymis. In the initial segment and caput epididymidis, only a subpopulation of cells showed basolateral RHBG immunolabeling; the proportion of cells expressing RHBG appeared to be greater in the initial segment than in the caput epididymidis. In the upper corpus, all cells expressed basolateral RHBG, with varying expression in different cells. In the middle and lower corpus epididymidis, the cauda epididymidis, and the vas deferens, only some cells exhibited basolateral RHBG immunolabel. No immunolabel was observed when primary antibody was omitted from the immunohistochemistry procedure as a control (data not shown).
Figure 6.
RHBG immunolabel in the epididymis. In the caput epididymidis, a small population of cells expressed moderate-intensity basolateral RHBG immunolabel; the majority of cells, however, did not express detectable immunolabel. In the upper corpus epididymidis, all epithelial cells exhibited basolateral RHBG immunoreactivity, albeit at varying levels of immunolabel intensity. In the middle and lower corpus and cauda epididymidis, and vas deferens, basolateral RHBG immunolabel was present in a minority of epithelial cells. Results are representative of findings from at least four mice.
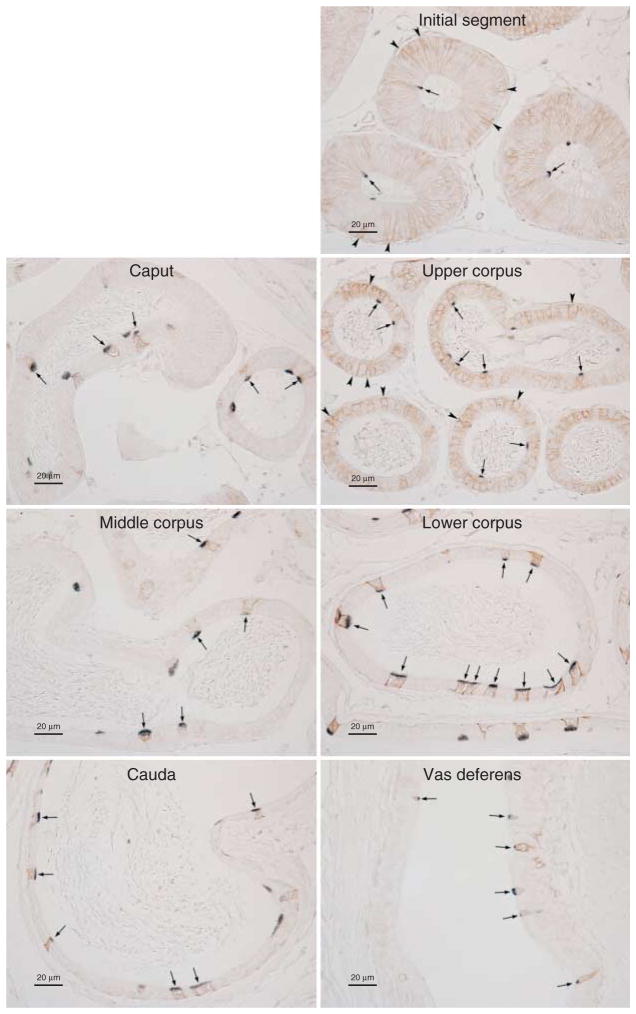
Double immunolabeling of H+-ATPase and RHBG in the epididymis
The heterogeneous expression of RHBG in the epididymis suggested cell-specific differences in its expression. To test this, we performed double-immunolabel immunohistochemistry using antibodies to RHBG and to H+-ATPase. Figure 7 shows representative results. Throughout the epididymis and vas deferens, RHBG immunolabel was found in H+-ATPase-positive cells. Thus, RHBG is present in narrow cells and some principal cells in the initial segment, principal cells, and clear cells in the upper corpus epididymidis and in clear cells throughout the epididymis. Table 1 summarizes RHBG immunolabel expression in the epididymis.
Figure 7.
Colocalization of RHBG (brown) with H+-ATPase (blue) in the epididymis. In order to determine specific epididymal cell types expressing RHBG, we colocalized RHBG with H+-ATPase. In the initial segment, basolateral RHBG immunoreactivity (brown) is present in H+-ATPase-positive narrow cells (arrows) and H+-ATPase-negative principal cells (arrowheads). In the caput epididymidis, basolateral RHBG immunoreactivity is present exclusively in H+-ATPase-positive clear cells (arrows). In the upper corpus, both H+-ATPase-positive clear cells (arrows) and a subset of H+-ATPase-negative principal cells (arrowheads) expressed basolateral RHBG immunolabel. In the middle and lower corpus and cauda epididymidis, H+-ATPase-positive clear cells (arrows), but not H+-ATPase-negative principal cells, expressed basolateral RHBG immunolabel. In the vas deferens, basolateral RHBG was present only in H+-ATPase-positive cells (arrows). In all regions, intraluminal sperm did not label for either RHBG or H+-ATPase. Results are representative of findings from at least four mice.
Table 1.
Summary of expression of RHCG, RHBG, and H+-ATPase in the efferent ductules, epididymis, and vas deferens.
| Efferent duct
|
Caput
|
Corpus
|
Cauda
|
Vas deferens
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial segment
|
Head
|
Upper
|
Middle
|
Lower
|
|||||||||||
| Ciliated cells | Non-ciliated cells | PCs | Narrow cells | PCs | Clear cells | PCs | Clear cells | PCs | Clear cells | PCs | Clear cells | PCs | Clear cells | Epithelial cells | |
| RHCG | − | − | − | − | + | − | + | − | − | + | − | + | − | + | + |
| RHBG | − | − | + | + | − | + | + | + | − | + | − | + | − | + | + |
| H+-ATPase | − | + | − | + | − | + | − | + | − | + | − | + | − | + | + |
PCs, principal cells.
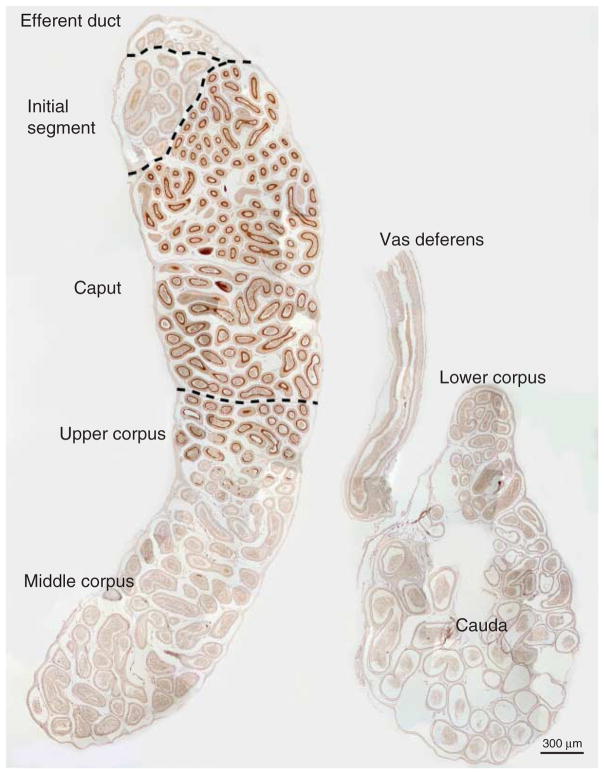
RHCG immunolocalization in the epididymis
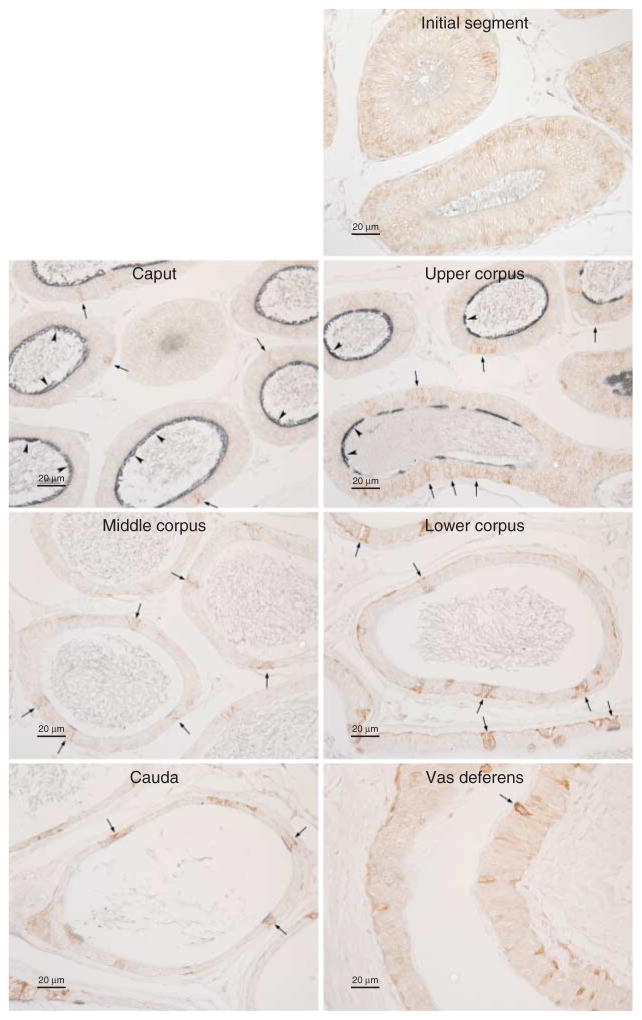
We next determined the cell-specific expression of RHCG in the epididymis. Figure 8 shows a low-power micrograph of RHCG immunolabel in the epididymis. RHCG immunoreactivity was greatest in the caput epididymidis, intermediate in the upper corpus epididymidis, and lowest in the middle and lower corpus epididymidis and vas deferens.
Figure 8.
Low-power micrograph of RHCG immunolabel in the epididymis. In the initial segment, RHCG immunolabel was not identified. High-intensity immunolabeling is present in the caput epididymidis, intermediate-intensity in the upper corpus epididymidis, and only low-intensity immunolabelling in the middle corpus, lower corpus and the cauda epididymidis. No immunolabeling was detected when primary antibody was omitted (not shown). Results are representative of findings from at least four mice.
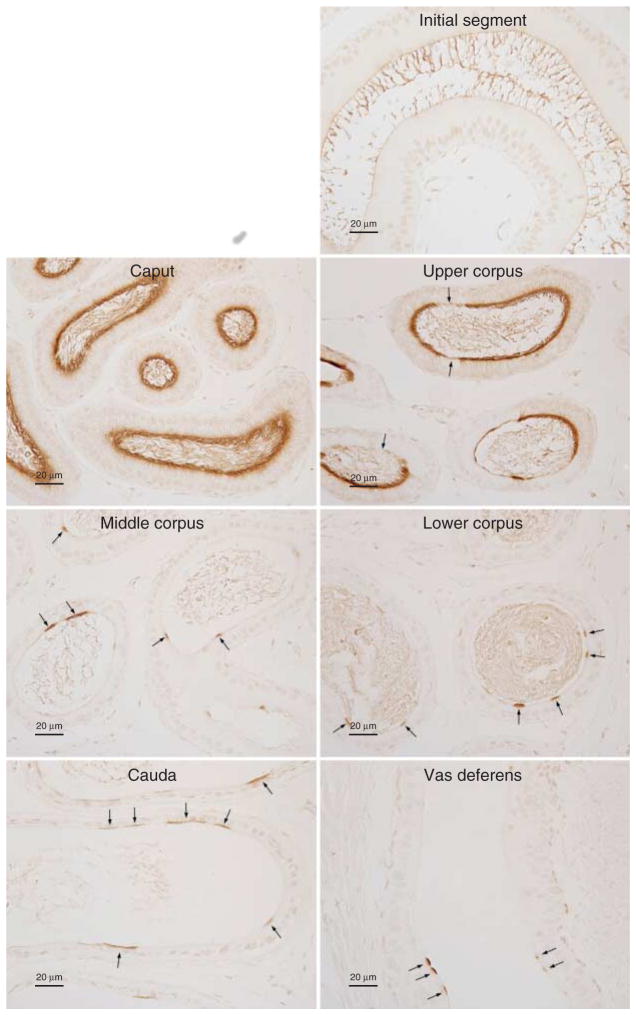
Higher-power micrographs identified significant cellular heterogeneity in RHCG expression in the epididymis. In the caput and upper corpus, most, but not all, of the cells had significant RHCG immunolabel (Fig. 9). In contrast, only a minority of cells in the middle and lower corpus had detectable RHCG immunolabel. In both the cauda epididymidis and the vas deferens, only a small population of cells expressed RHCG. In contrast to RHBG, which exhibited exclusively basolateral immunolabel, RHCG immunolabel was exclusively apical.
Figure 9.
High-power micrographs of RHCG immunolabel in the epididymis. In the initial segment, RHCG immunolabel was not identified. In the caput epididymidis, the majority of cells exhibit strong apical RHCG immunolabel. In the upper corpus, apical RHCG was expressed by the majority of cells. Again, significant cellular heterogeneity in RHCG immunolabel was present, with some cells exhibiting either very low or undetectable RHCG immunolabel (arrows). In the middle and lower corpus and cauda epididymidis, and the vas deferens, the majority of cells did not immunolabel for RHCG. However, a subpopulation of cells exhibit moderately strong apical RHCG immunolabel (arrows). No immunolabel was detected when primary antibody was omitted (data not shown). Results are representative of findings from at least four mice.
Double immunolabeling of H+-ATPase and RHCG in epididymis
Because only a subpopulation of epididymal cells expressed RHCG immunolabel, we used double immunolabeling to determine the specific cell types that expressed RHCG. To do so, we colocalized RHCG with the vacuolar H+-ATPase. Figure 10 shows representative findings. In the initial segment, significant RHCG immunolabel was not detected, either in H+-ATPase-positive or H+-ATPase-negative cells. In the caput and upper corpus epididymidis, strong apical RHCG immunolabel was present only in H+-ATPase-negative cells. In the middle and lower corpus and cauda epididymidis and vas deferens, apical RHCG immunolabel colocalized with H+-ATPase.
Figure 10.
Double immunolabeling of H+-ATPase and RHCG in the epididymis. In the initial segment, RHCG immunolabel was not present in H+-ATPase-positive (narrow) cells (arrows). In the caput and upper corpus, intense apical RHCG immunolabel was limited to H+-ATPase-negative (principal) cells. H+-ATPase-positive (clear) cells (arrows) do not exhibit RHCG immunolabel. In the middle corpus, apical RHCG immunolabel was limited to H+-ATPase-positive clear cells (arrows) and no detectable RHCG immunolabel was found in H+-ATPase-negative principal cells. H+-ATPase was expressed on the apical membrane of clear cells. In the lower corpus and cauda epididymidis and in the vas deferens colocalization of H+-ATPase and RHCG was present (arrows), similar to middle corpus. Results are representative of findings from at least four mice.
Double immunolabel of RHBG with RHCG
To further confirm the cellular expression of RHCG in these more distal epididymal regions, we colocalized it with RHBG (Fig. 11). Clear cells, identified by basolateral RHBG (see results from Fig. 7), expressed apical RHCG immunolabel; principal cells had neither basolateral RHBG nor apical RHCG immunoreactivity. Table 1 summarizes these findings. Thus, apical RHCG immunolabel exhibits both axial and cell-specific heterogeneity in the epididymis.
Figure 11.
Double-immunolabel of RHCG and RHBG in the epididymis. In the initial segment, basolateral RHBG immunoreactivity (brown) is present in narrow cells and principal cells. In the caput epididymidis, apical RHCG immunoreactivity (blue) is present in principal cells (arrowheads) and basolateral RHBG immunoreactivity is present in clear cells (arrows). In the upper corpus, apical RHCG immunoreactivity is present in principal cells (arrowheads) and basolateral RHBG immunoreactivity is present in both principal cells and clear cells (arrows). In the middle corpus, apical RHCG immunolabel and basolateral RHBG immunolabel are limited to clear cells (arrows). In the lower corpus and cauda epididymidis and in the vas deferens, apical RHCG and basolateral RHBG are present (arrows), as in the middle corpus. Results are representative of findings from at least four mice.
Discussion
The current studies identify for the first time, to our knowledge, the cell-specific expression of RHBG and RHCG in the male reproductive tract and provide several new and important findings. First, we demonstrate for the first time, to our knowledge, that RHBG mRNA and protein are expressed throughout the male reproductive tract. In the testis, high levels of Rhbg are expressed in Leydig cells. In the epididymis, basolateral RHBG immunolabel is present in H+-ATPase-positive cells throughout the epididymis and also in H+-ATPase-negative cells in the upper corpus epididymidis. Thus, RHBG is present in male reproductive organs and it is expressed in specific cells known to have important roles in male fertility. RHCG expression exhibits both axial and cellular heterogeneity in the epididymis. This expression pattern of RHBG and RHCG in the male reproductive tract suggests important roles for these ammonia transporter family members in male fertility.
The first major finding reported here is that RHBG is expressed in both the testis and epididymis and that its expression demonstrates highly specific cellular expression in the male reproductive tract. We show this by demonstrating RHBG mRNA and protein expression in both testis and epididymis homogenates, by demonstrating heterogeneous RHBG protein expression in these organs using immunohistochemistry, and by identifying the specific cell types that express RHBG using double-immunolabel immunohistochemistry.
In the testis, we show for the first time, to our knowledge, that Leydig cells express RHBG, as demonstrated by the histological distribution of RHBG immunoreactivity consistent with Leydig cell distribution and confirmed by double-immunolabeling of RHBG with the Leydig cell-specific marker, glutamine synthetase. We speculate that RHBG-mediated ammonia transport may regulate Leydig cell steroidogenesis. Leydig cells are known to produce both testosterone and estrogen (Raeside et al. 2006, Carreau et al. 2007, 2011, Svechnikov et al. 2010), and humans with chronic liver disease often have elevated plasma ammonia levels in conjunction with altered sex steroid hormone levels (Chopra et al. 1973, Galvao-Teles et al. 1973). Another potential role of Rhbg in Leydig cells may be to facilitate ammonia metabolism. Leydig cells express high levels of glutamine synthetase, an enzyme that mediates the reaction of ammonia with glutamate to form glutamine, thereby decreasing ammonia concentration (Holash et al. 1993, van Straaten et al. 2006). The combined expression in the same cell of RHBG, an integral membrane ammonia transporter, with glutamine synthetase could enable high-affinity ammonia metabolism; this expression pattern is also present in the perivenous hepatocytes in the liver (Weiner et al. 2003), cells that mediate a critical role in hepatic ammonia metabolism (Haussinger 1983, Haussinger et al. 1985).
The testis also expresses RHCG, although in different cell populations than those that express RHBG. In particular, RHCG is expressed only during the later stages (steps 13–16) of spermatid maturation, leading to stage-specific differences in its expression during the seminiferous epithelial cycle. This expression of RHCG is consistent with the expression of Rhcg mRNA in the seminiferous epithelium that was reported in studies using in situ hybridization (Liu et al. 2000) but further indicates that expression is confined to one cell type during one specific maturational period. These results suggest that that RHCG-mediated transport of NH3 could play a role in spermatid maturation during the later stages of spermatid development. Alternatively, as the yeast ammonia transporter family member, Mep2, can regulate cellular function through mechanisms independent of ammonia transport (Lorenz & Heitman 1998, Biswas & Morschhauser 2005), it is possible that Rhcg may also regulate spermatid maturation and/or function through mechanisms not involving ammonia transport.
In the epididymis, both RHBG and RHCG are widely expressed, typically in different epithelial membrane domains in the same cells. The finding of polarized expression of both ammonia transporter family members, RHBG and RHCG, suggests that ammonia is transported by specific epididymal epithelial cell types. Several lines of evidence support this hypothesis. First, intraluminal ammonia concentrations are high in the epididymis. Direct measurements have shown ammonia concentrations of 800-2800 μmol/l in epididymal fluid in a wide variety of species (Johnson et al. 1972, Jones 1978). In contrast, normal plasma ammonia levels are 20–30 μmol/l, indicating that the epididymal fluid ammonia concentration is 25- to 100-fold greater than plasma and suggesting epididymal ammonia secretion. Secondly, Rhcg deletion lowers epididymal fluid pH (Biver et al. 2008), a finding easily explained through inhibition of RHCG-mediated NH3 transport. Lastly, the coexistence of basolateral RHBG, apical H+-ATPase, and apical RHCG in clear cells in the middle and lower corpus and cauda epididymidis is similar to that observed in the renal collecting duct (Weiner & Hamm 2007, Weiner & Verlander 2010, 2011), a known site of transepithelial ammonia secretion (Weiner & Verlander 2010, 2011) that requires RHBG and RHCG expression (Biver et al. 2008, Bishop et al. 2010, Lee et al. 2009, 2010). Thus, ammonia secretion by the epididymis involving RHBG and RHCG is likely to play a critical role in both epididymal ammonia secretion and determination of epididymal fluid pH.
The current study is the first, to our knowledge, to identify RHBG expression in the male reproductive tract. A previous study using northern analysis did not detect Rhbg mRNA expression (Liu et al. 2001), whereas the current study, using RT-PCR, immunoblot analysis, and immunohistochemistry, demonstrates RHBG mRNA and protein expression in testis, epididymis, and vas deferens. The increased sensitivity of real-time RT-PCR compared with northern blot analysis probably explains the different results of these studies. Similar identification of RHBG expression using real-time RT-PCR, immunoblot analysis and immunohistochemistry in tissues not reported to contain Rhbg mRNA by northern analysis has been made for several other tissues, including lung, stomach, small intestine, and colon (Handlogten et al. 2005, Han et al. 2009).
Our identification of RHCG expression in epididymis provides important new information. We show specific colocalization of RHCG with H+-ATPase, a pattern identical to that in the kidney, where RHCG and H+-ATPase are co-expressed in intercalated cells. Intercalated cells have many similarities to epididymal clear cells (Paunescu et al. 2004, Pastor-Soler et al. 2005, Da Silva et al. 2010) and are responsible for renal collecting duct ammonia secretion. Thus, it is likely that RHCG mediates an important role in epididymis ammonia secretion and that this process involves parallel transport by clear cells of both NH3 and H+. This finding significantly expands our understanding of the function of these cells by suggesting critical roles not only in epididymal fluid acidification but also in epididymal fluid ammonia secretion. In the initial report identifying RHCG, Rhcg mRNA expression was demonstrated in both the mouse and human testis; protein expression was not reported (Liu et al. 2000). A subsequent report showed apical RHCG immunolabel in the epididymis but did not determine the cell-specific expression pattern (Biver et al. 2008). Thus, the current study is consistent with these previous studies and provides important new information regarding RHCG expression in the male reproductive tract.
In summary, the current study provides the first characterization, to our knowledge, of RHCG and RHBG cellular distribution in the male reproductive organs, especially testis and epididymis, by real-time RT-PCR, immunoblot analysis, and immunolocalization. RHBG is present in Leydig cells in the testis and in the basolateral membrane of H+-secreting cells in the epididymis. RHCG demonstrates both axial and cell-specific heterogeneity in the epididymis and is also expressed in sperm. Ammonia transport by RHCG and RHBG is likely to mediate critical roles in multiple components of male fertility.
Acknowledgments
The authors thank the staff of the University of Florida College of Medicine Electron Microscopy Core Facility for expert preparation of tissues for immunohistochemistry.
Funding
These studies were supported by funds from the National Institutes for Health (NIH) (NIDDK R01-045788), Merit Review Grant program of the Department of Veterans Affairs (1I01BX000818), and the National Research Foundation of Korea (2011-0016068).
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Bishop JM, Verlander JW, Lee HW, Nelson RD, Weiner AJ, Handlogten ME, Weiner ID. Role of the rhesus glycoprotein, Rh B glycoprotein, in renal ammonia excretion. American Journal of Physiology. Renal Physiology. 2010;299:F1065–F1077. doi: 10.1152/ajprenal.00277.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop JM, Lee HW, Handlogten ME, Han KH, Verlander JW, Weiner ID. Intercalated cell-specific Rh B glycoprotein deletion diminishes renal ammonia excretion response to hypokalemia. American Journal of Physiology. Renal Physiology. 2013;304:F422–F431. doi: 10.1152/ajprenal.00301.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas K, Morschhauser J. The Mep2p ammonium permease controls nitrogen starvation-induced filamentous growth in Candida albicans. Molecular Microbiology. 2005;56:649–669. doi: 10.1111/j.1365-2958.2005.04576.x. [DOI] [PubMed] [Google Scholar]
- Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, Houillier P, Szpirer J, Szpirer C, Wagner CA, et al. A role for rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature. 2008;456:339–343. doi: 10.1038/nature07518. [DOI] [PubMed] [Google Scholar]
- Brusilow SW, Gordes EH. Ammonia secretion in sweat. American Journal of Physiology. 1968;214:513–517. doi: 10.1152/ajplegacy.1968.214.3.513. [DOI] [PubMed] [Google Scholar]
- Brusilow SW, Gordes EH. Solute and water secretion in sweat. Journal of Clinical Investigation. 1964;43:477–484. doi: 10.1172/JCI104933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreau S, Silandre D, Bourguiba S, Hamden K, Said L, Lambard S, Galeraud-Denis I, Delalande C. Estrogens and male reproduction: a new concept. Brazilian Journal of Medical and Biological Research. 2007;40:761–768. doi: 10.1590/S0100-879X2007000600003. [DOI] [PubMed] [Google Scholar]
- Carreau S, Bouraima-Lelong H, Delalande C. Estrogens – new players in spermatogenesis. Reproductive Biology. 2011;11:174–193. doi: 10.1016/S1642-431X(12)60065-5. [DOI] [PubMed] [Google Scholar]
- Chopra IJ, Tulchinsky DA, Greenway FL. Estrogen–androgen imbalance in hepatic cirrhosis. Annals of Internal Medicine. 1973;79:198–203. doi: 10.7326/0003-4819-79-2-198. [DOI] [PubMed] [Google Scholar]
- Da Silva N, Pisitkun T, Belleannée C, Miller LR, Nelson R, Knepper MA, Brown D, Breton S. Proteomic analysis of V-ATPase-rich cells harvested from the kidney and epididymis by fluorescence-activated cell sorting. American Journal of Physiology. Cell Physiology. 2010;298:C1326–C1342. doi: 10.1152/ajpcell.00552.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eladari D, Cheval L, Quentin F, Bertrand O, Mouro I, Cherif-Zahar B, Cartron JP, Paillard M, Doucet A, Chambrey R. Expression of RhCG, a new putative transporter, along the rat nephron. Journal of the American Society of Nephrology. 2002;13:1999–2008. doi: 10.1097/01.ASN.0000025280.02386.9D. [DOI] [PubMed] [Google Scholar]
- Galvao-Teles A, Anderson DC, Burke CW, Marshall JC, Corker CS, Bown RL, Clark ML. Biologically active androgens and estradiol in men with chronic liver disease. Lancet. 1973;301:173–177. doi: 10.1016/S0140-6736(73)90005-6. [DOI] [PubMed] [Google Scholar]
- Han KH, Mekala K, Babida V, Kim HY, Handlogten ME, Verlander JW, Weiner ID. Expression of the gas transporting proteins, Rh B glycoprotein and Rh C glycoprotein, in the murine lung. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2009;297:L153–L163. doi: 10.1152/ajplung.90524.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handlogten ME, Hong SP, Zhang L, Vander AW, Steinbaum ML, Campbell-Thompson M, Weiner ID. Expression of the ammonia transporter proteins, Rh B glycoprotein and Rh C glycoprotein, in the intestinal tract. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2005;288:G1036–G1047. doi: 10.1152/ajpgi.00418.2004. [DOI] [PubMed] [Google Scholar]
- Haussinger D. Hepatocyte heterogeneity in glutamine and ammonia metabolism and the role of an intercellular glutamine cycle during ureogenesis in perfused rat liver. European Journal of Biochemistry. 1983;133:269–275. doi: 10.1111/j.1432-1033.1983.tb07458.x. [DOI] [PubMed] [Google Scholar]
- Haussinger D, Sies H, Gerok W. Functional hepatocyte heterogeneity in ammonia metabolism. The intercellular glutamine cycle. Journal of Hepatology. 1985;1:3–14. doi: 10.1016/S0168-8278(85)80063-5. [DOI] [PubMed] [Google Scholar]
- Holash JA, Harik SI, Perry G, Stewart PA. Barrier properties of testis microvessels. PNAS. 1993;90:11069–11073. doi: 10.1073/pnas.90.23.11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Pursel VG, Gerrits RJ, Thomas CH. Free amino acid composition of porcine seminal, epididymal and seminal vesicle fluids. Journal of Animal Science. 1972;34:430–434. doi: 10.2527/jas1972.343430x. [DOI] [PubMed] [Google Scholar]
- Jones R. Comparative biochemistry of mammalian epididymal plasma. Comparative Biochemistry and Physiology. B, Comparative Biochemistry. 1978;61:365–370. doi: 10.1016/0305-0491(78)90138-4. [DOI] [PubMed] [Google Scholar]
- Kim HY, Verlander JW, Bishop JM, Cain BD, Han KH, Igarashi P, Lee HW, Handlogten ME, Weiner ID. Basolateral expression of the ammonia transporter family member, Rh C glycoprotein, in the mouse kidney. American Journal of Physiology. Renal Physiology. 2009;296:F545–F555. doi: 10.1152/ajprenal.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knepper MA. Physiology: courier service for ammonia. Nature. 2008;456:336–337. doi: 10.1038/456336a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Verlander JW, Bishop JM, Igarashi P, Handlogten ME, Weiner ID. Collecting duct-specific Rh C glycoprotein deletion alters basal and acidosis-stimulated renal ammonia excretion. American Journal of Physiology. Renal Physiology. 2009;296:F1364–F1375. doi: 10.1152/ajpre-nal.90667.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Verlander JW, Bishop JM, Nelson RD, Handlogten ME, Weiner ID. Effect of intercalated cell-specific Rh C glycoprotein deletion on basal and metabolic acidosis-stimulated renal ammonia excretion. American Journal of Physiology. Renal Physiology. 2010;299:F369–F379. doi: 10.1152/ajprenal.00120.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Verlander JW, Bishop JM, Handlogten ME, Han KH, Weiner ID. Renal ammonia excretion in response to hypokalemia: effects of collecting duct-specific Rh C glycoprotein deletion. American Journal of Physiology. Renal Physiology. 2013;304:F410–F421. doi: 10.1152/ajprenal.00300.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Chen Y, Mo R, Hui CC, Cheng JF, Mohandas N, Huang CH. Characterization of human RhCG and mouse Rhcg as novel nonerythroid Rh glycoprotein homologues predominantly expressed in kidney and testis. Journal of Biological Chemistry. 2000;275:25641–25651. doi: 10.1074/jbc.M003353200. [DOI] [PubMed] [Google Scholar]
- Liu Z, Peng J, Mo R, Hui C, Huang CH. Rh type B glycoprotein is a new member of the Rh superfamily and a putative ammonia transporter in mammals. Journal of Biological Chemistry. 2001;276:1424–1433. doi: 10.1074/jbc.M007528200. [DOI] [PubMed] [Google Scholar]
- Lorenz MC, Heitman J. The MEP2 ammonium permease regulates pseudohyphal differentiation in Saccharomyces cerevisiae. EMBO Journal. 1998;17:1236–1247. doi: 10.1093/emboj/17.5.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak DO, Dang B, Weiner ID, Foskett JK, Westhoff CM. Characterization of transport by the kidney Rh glycoproteins, RhBG and RhCG. American Journal of Physiology. Renal Physiology. 2006;290:F297–F305. doi: 10.1152/ajprenal.00147.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini AM, Matassi G, Raynal V, Andre B, Cartron JP, Cherif-Zahar B. The human rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nature Genetics. 2000;26:341–344. doi: 10.1038/81656. [DOI] [PubMed] [Google Scholar]
- Meistrich M, Hess RA. Assessment of spermatogenesis through staging of seminiferous tubules. In: Carrell DT, Aston KI, editors. Spermatogenesis. New York, NY: Humana Press; 2013. pp. 299–307. [DOI] [PubMed] [Google Scholar]
- Pastor-Soler N, Pietrement C, Breton S. Role of acid/base transporters in the male reproductive tract and potential consequences of their malfunction. Physiology. 2005;20:417–428. doi: 10.1152/physiol.00036.2005. [DOI] [PubMed] [Google Scholar]
- Paunescu TG, Silva ND, Marshansky V, McKee M, Breton S, Brown D. Expression of the 56-kDa B2 subunit isoform of the vacuolar H+-ATPase in proton-secreting cells of the kidney and epididymis. American Journal of Physiology. Cell Physiology. 2004;287:C149–C162. doi: 10.1152/ajpcell.00464.2003. [DOI] [PubMed] [Google Scholar]
- Quentin F, Eladari D, Cheval L, Lopez C, Goossens D, Colin Y, Cartron JP, Paillard M, Chambrey R. RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. Journal of the American Society of Nephrology. 2003;14:545–554. doi: 10.1097/01.ASN.0000050413.43662.55. [DOI] [PubMed] [Google Scholar]
- Raeside JI, Christie HL, Renaud RL, Sinclair PA. The boar testis: the most versatile steroid producing organ known. Society of Reproduction and Fertility Supplement. 2006;62:85–97. [PubMed] [Google Scholar]
- van Straaten HW, He Y, van Duist MM, Labruyere WT, Vermeulen JL, van Dijk PJ, Ruijter JM, Lamers WH, Hakvoort TB. Cellular concentrations of glutamine synthetase in murine organs. Biochemistry and Cell Biology. 2006;84:215–231. doi: 10.1139/o05-170. [DOI] [PubMed] [Google Scholar]
- Svechnikov K, Landreh L, Weisser J, Izzo G, Colon E, Svechnikova I, Soder O. Origin, development and regulation of human Leydig cells. Hormone Research in Pædiatrics. 2010;73:93–101. doi: 10.1159/000277141. [DOI] [PubMed] [Google Scholar]
- Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins, RhBG and RhCG, in mouse kidney. American Journal of Physiology. Renal Physiology. 2003;284:F323–F337. doi: 10.1152/ajprenal.00050.2002. [DOI] [PubMed] [Google Scholar]
- Weiner ID. Expression of the non-erythroid Rh glycoproteins in mammalian tissues. Transfusion Clinique et Biologique. 2006;13:159–163. doi: 10.1016/j.tracli.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner ID, Hamm LL. Molecular mechanisms of renal ammonia transport. Annual Review of Physiology. 2007;69:317–340. doi: 10.1146/annurev.physiol.69.040705.142215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner ID, Verlander JW. Molecular physiology of the Rh ammonia transport proteins. Current Opinion in Nephrology and Hypertension. 2010;19:471–477. doi: 10.1097/MNH.0b013e32833bfa4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner ID, Verlander JW. Role of NH3 and transporters in renal acid–base transport. American Journal of Physiology. Renal Physiology. 2011;300:F11–F23. doi: 10.1152/ajprenal.00554.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner ID, Verlander JW. Renal ammonia metabolism and transport. Comprehensive Physiology. 2013;3:201–220. doi: 10.1002/cphy.c120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner ID, Miller RT, Verlander JW. Localization of the ammonium transporters, Rh B glycoprotein and Rh C glycoprotein in the mouse liver. Gastroenterology. 2003;124:1432–1440. doi: 10.1016/S0016-5085(03)00277-4. [DOI] [PubMed] [Google Scholar]
- Winkler FK. Amt/MEP/Rh proteins conduct ammonia. Pflügers Archives. 2005;451:701–707. doi: 10.1007/s00424-005-1511-6. [DOI] [PubMed] [Google Scholar]