Abstract
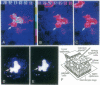
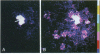
Plasmodesmata are cytoplasmic bridges between plant cells thought to generally allow only the passage of small molecules and metabolites. However, large structures such as plant viruses also move from cell to cell via plasmodesmata. In tobacco mosaic virus (TMV) infection a viral movement protein (TMV-MP) mediates viral spread. Here, a microinjection assay is used to monitor the dynamics of TMV-MP function directly in wild-type plants. The results indicate that TMV-MP interacts with an endogenous plant pathway increasing plasmodesmal size exclusion limit to permit passage of 20-kDa dextrans. Furthermore, TMV-MP influences plasmodesmal size exclusion limit several cells distant from the injection site, indicating either that TMV-MP itself crosses plasmodesmata or that TMV-MP induces a diffusable signal capable of dilating microchannels of plasmodesmata. The region of TMV-MP responsible for increasing plasmodesmal size exclusion limit was mapped to the carboxyl-terminal part of the 268-amino acid residue protein between amino acid residues 126 and 224.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berna A., Gafny R., Wolf S., Lucas W. J., Holt C. A., Beachy R. N. The TMV movement protein: role of the C-terminal 73 amino acids in subcellular localization and function. Virology. 1991 Jun;182(2):682–689. doi: 10.1016/0042-6822(91)90609-f. [DOI] [PubMed] [Google Scholar]
- Citovsky V., Knorr D., Schuster G., Zambryski P. The P30 movement protein of tobacco mosaic virus is a single-strand nucleic acid binding protein. Cell. 1990 Feb 23;60(4):637–647. doi: 10.1016/0092-8674(90)90667-4. [DOI] [PubMed] [Google Scholar]
- Citovsky V., McLean B. G., Zupan J. R., Zambryski P. Phosphorylation of tobacco mosaic virus cell-to-cell movement protein by a developmentally regulated plant cell wall-associated protein kinase. Genes Dev. 1993 May;7(5):904–910. doi: 10.1101/gad.7.5.904. [DOI] [PubMed] [Google Scholar]
- Citovsky V., Wong M. L., Shaw A. L., Prasad B. V., Zambryski P. Visualization and characterization of tobacco mosaic virus movement protein binding to single-stranded nucleic acids. Plant Cell. 1992 Apr;4(4):397–411. doi: 10.1105/tpc.4.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V., Zambryski P. Transport of nucleic acids through membrane channels: snaking through small holes. Annu Rev Microbiol. 1993;47:167–197. doi: 10.1146/annurev.mi.47.100193.001123. [DOI] [PubMed] [Google Scholar]
- Deom C. M., Schubert K. R., Wolf S., Holt C. A., Lucas W. J., Beachy R. N. Molecular characterization and biological function of the movement protein of tobacco mosaic virus in transgenic plants. Proc Natl Acad Sci U S A. 1990 May;87(9):3284–3288. doi: 10.1073/pnas.87.9.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrick P. M., Barker H., Oparka K. J. Increase in Plasmodesmatal Permeability during Cell-to-Cell Spread of Tobacco Rattle Virus from Individually Inoculated Cells. Plant Cell. 1992 Nov;4(11):1405–1412. doi: 10.1105/tpc.4.11.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B., Haudenshield J. S., Hull R. J., Wolf S., Beachy R. N., Lucas W. J. Secondary plasmodesmata are specific sites of localization of the tobacco mosaic virus movement protein in transgenic tobacco plants. Plant Cell. 1992 Aug;4(8):915–928. doi: 10.1105/tpc.4.8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. B., Wu Y., Ku M. S. Turnover of soluble proteins in the wheat sieve tube. Plant Physiol. 1992 Nov;100(3):1433–1441. doi: 10.1104/pp.100.3.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafny R., Lapidot M., Berna A., Holt C. A., Deom C. M., Beachy R. N. Effects of terminal deletion mutations on function of the movement protein of tobacco mosaic virus. Virology. 1992 Apr;187(2):499–507. doi: 10.1016/0042-6822(92)90452-u. [DOI] [PubMed] [Google Scholar]
- Jørgensen K. E., Møller J. V. Use of flexible polymers as probes of glomerular pore size. Am J Physiol. 1979 Feb;236(2):F103–F111. doi: 10.1152/ajprenal.1979.236.2.F103. [DOI] [PubMed] [Google Scholar]
- Lůcas W. J., Wolf S. Plasmodesmata: the intercellular organelles of green plants. Trends Cell Biol. 1993 Sep;3(9):308–315. doi: 10.1016/0962-8924(93)90013-q. [DOI] [PubMed] [Google Scholar]
- McClary J. A., Witney F., Geisselsoder J. Efficient site-directed in vitro mutagenesis using phagemid vectors. Biotechniques. 1989 Mar;7(3):282–289. [PubMed] [Google Scholar]
- Meshi T., Watanabe Y., Saito T., Sugimoto A., Maeda T., Okada Y. Function of the 30 kd protein of tobacco mosaic virus: involvement in cell-to-cell movement and dispensability for replication. EMBO J. 1987 Sep;6(9):2557–2563. doi: 10.1002/j.1460-2075.1987.tb02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Saito T., Imai Y., Meshi T., Okada Y. Interviral homologies of the 30K proteins of tobamoviruses. Virology. 1988 Dec;167(2):653–656. [PubMed] [Google Scholar]
- Wolf S., Deom C. M., Beachy R. N., Lucas W. J. Movement protein of tobacco mosaic virus modifies plasmodesmatal size exclusion limit. Science. 1989 Oct 20;246(4928):377–379. doi: 10.1126/science.246.4928.377. [DOI] [PubMed] [Google Scholar]
- Ziegler-Graff V., Guilford P. J., Baulcombe D. C. Tobacco rattle virus RNA-1 29K gene product potentiates viral movement and also affects symptom induction in tobacco. Virology. 1991 May;182(1):145–155. doi: 10.1016/0042-6822(91)90658-x. [DOI] [PubMed] [Google Scholar]
- le Maire M., Viel A., Møller J. V. Size exclusion chromatography and universal calibration of gel columns. Anal Biochem. 1989 Feb 15;177(1):50–56. doi: 10.1016/0003-2697(89)90012-2. [DOI] [PubMed] [Google Scholar]