Abstract
Rabbit articular chondrocytes synthesize type II collagen [3α1(II)] in vivo and type I collagen [2α1(I)·α2] in monolayer cultures. In suspension culture the nature of phenotype depends on extracellular Ca2+. The relationship of Ca2+ and 3′:5′-cyclic AMP (cAMP) in regulation of collagen synthesis has been investigated. In suspension culture, cAMP levels of chondrocytes increase by 2- to 3-fold and then reach basal values regardless of the presence or absence of extracellular Ca2+. The cells, however, synthesize primarily type II collagen in the absence of CaCl2 in the medium and type I collagen in medium containing 1.8 mM CaCl2. If CaCl2 is added when intracellular cAMP levels are low, the phenotype is type I collagen. These observations minimize the role of cAMP as a second messenger in the chondrocyte culture system. Increasing endogenous cAMP with a phosphodiesterase inhibitor or adding exogenous dibutyryl-cAMP leads the cells to synthesize type I collagen, although this effect is significantly less pronounced if the medium contains ethylene glycol bis(β-aminoethyl ether)-N,N′-tetraacetic acid (EGTA). Increased concentrations of cAMP may mobilize the intracellular calcium pools and activate the cells to switch their phenotypic expression. Prostaglandins E2 and F2α, thought to be involved in rheumatoid arthritis and bone resorption, have no significant effect on cAMP content of chondrocytes and alter their collagen phenotype to a small extent.
Keywords: arthritis, protein synthesis in vitro
Full text
PDF

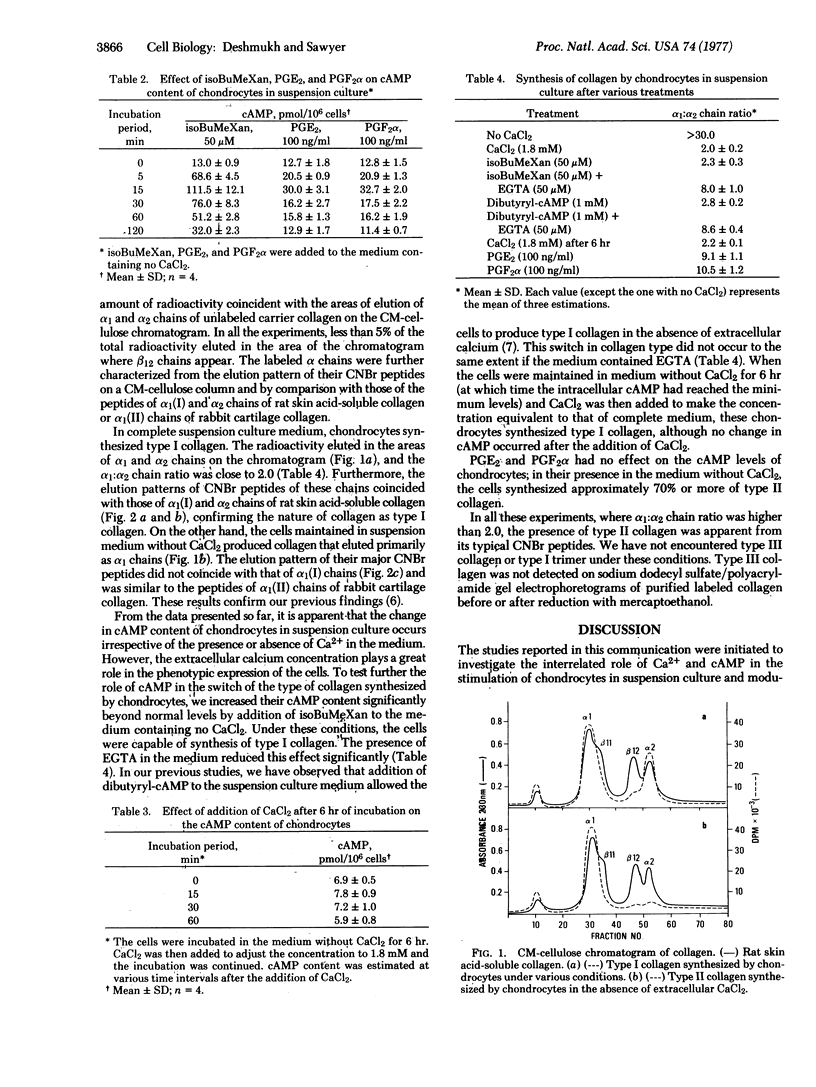
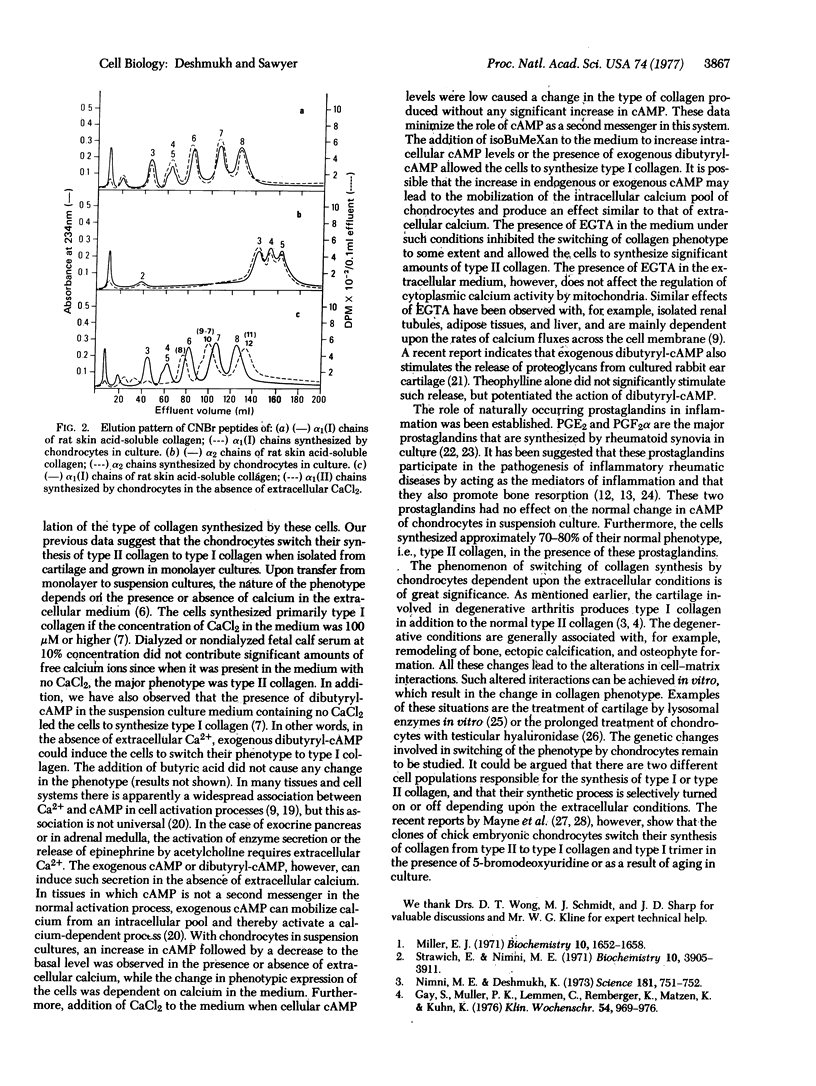

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borle A. B. Calcium metabolism at the cellular level. Fed Proc. 1973 Sep;32(9):1944–1950. [PubMed] [Google Scholar]
- Borle A. B. Cyclic AMP stimulation of calcium efflux from kidney, liver and heart mitochondria. J Membr Biol. 1974;16(3):221–236. doi: 10.1007/BF01872416. [DOI] [PubMed] [Google Scholar]
- Brandt K. D., Palmoski M. Organization of ground substance proteoglycans in normal and osteoarthritic knee cartilage. Arthritis Rheum. 1976 Mar-Apr;19(2):209–215. doi: 10.1002/art.1780190213. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Krane S. M., Russell R. G., Robinson D. R. Production of collagenase and prostaglandins by isolated adherent rheumatoid synovial cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):945–949. doi: 10.1073/pnas.73.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh K., Kline W. G., Sawyer B. D. Role of calcium in the phenotypic expression of rabbit articular chondrocytes in culture. FEBS Lett. 1976 Aug 1;67(1):48–51. doi: 10.1016/0014-5793(76)80868-x. [DOI] [PubMed] [Google Scholar]
- Deshmukh K., Kline W. H. Characterization of collagen and its precursors synthesized by rabbit-articular-cartilage cells in various culture systems. Eur J Biochem. 1976 Oct 1;69(1):117–123. doi: 10.1111/j.1432-1033.1976.tb10864.x. [DOI] [PubMed] [Google Scholar]
- Deshmukh K., Nimni M. E. Characterization of the aldehydes present on the cyanogen bromide peptides from mature rat skin collagen. Biochemistry. 1971 Apr 27;10(9):1640–1647. doi: 10.1021/bi00785a022. [DOI] [PubMed] [Google Scholar]
- Deshmukh K., Nimni M. E. Effects of lysosomal enzymes on the type of collagen synthesized by bovine articular cartilage. Biochem Biophys Res Commun. 1973 Jul 17;53(2):424–431. doi: 10.1016/0006-291x(73)90679-7. [DOI] [PubMed] [Google Scholar]
- Gay S., Müller P. K., Lemmen C., Remberger K., Matzen K., Kühn K. Immunohistological study on collagen in cartilage-bone metamorphosis and degenerative osteoarthrosis. Klin Wochenschr. 1976 Oct 15;54(20):969–976. doi: 10.1007/BF01468947. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg B., Epstein E. H., Jr, Sherr C. J. Precursors of collagen secreted by cultured human fibroblasts. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3655–3659. doi: 10.1073/pnas.69.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D. C., Raisz L. G. Prostaglandins: stimulation of bone resorption in tissue culture. Endocrinology. 1970 Jun;86(6):1436–1440. doi: 10.1210/endo-86-6-1436. [DOI] [PubMed] [Google Scholar]
- Mayne R., Vail M. S., Mayne P. M., Miller E. J. Changes in type of collagen synthesized as clones of chick chondrocytes grow and eventually lose division capacity. Proc Natl Acad Sci U S A. 1976 May;73(5):1674–1678. doi: 10.1073/pnas.73.5.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne R., Vail M. S., Miller E. J. Analysis of changes in collagen biosynthesis that occur when chick chondrocytes are grown in 5-bromo-2'-deoxyuridine. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4511–4515. doi: 10.1073/pnas.72.11.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. J. Isolation and characterization of a collagen from chick cartilage containing three identical alpha chains. Biochemistry. 1971 Apr 27;10(9):1652–1659. doi: 10.1021/bi00785a024. [DOI] [PubMed] [Google Scholar]
- Nimni M., Deshmukh K. Differences in collagen metabolism between normal and osteoarthritic human articular cartilage. Science. 1973 Aug 24;181(4101):751–752. doi: 10.1126/science.181.4101.751. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B., Tenenhouse A. The role of cyclic AMP and calcium in cell activation. CRC Crit Rev Biochem. 1972 Feb;1(1):95–148. doi: 10.3109/10409237209102545. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Jensen P., Lake W., Friedmann N., Goodman D. B. Cyclic nucleotides and cellular calcium metabolism. Adv Cyclic Nucleotide Res. 1975;5:375–394. [PubMed] [Google Scholar]
- Robinson D. R., McGuire M. B., Levine L. Prostaglandins in the rheumatic diseases. Ann N Y Acad Sci. 1975 Jun 13;256:318–329. doi: 10.1111/j.1749-6632.1975.tb36058.x. [DOI] [PubMed] [Google Scholar]
- Robinson D. R., Smith H., McGuire M. B., Levine L. Prostaglandin synthesis by rheumatoid synovium and its stimulation by colchicine. Prostaglandins. 1975 Jul;10(1):67–85. doi: 10.1016/0090-6980(75)90094-5. [DOI] [PubMed] [Google Scholar]
- Robinson D. R., Tashjian A. H., Jr, Levine L. Prostaglandin-stimulated bone resorption by rheumatoid synovia. A possible mechanism for bone destruction in rheumatoid arthritis. J Clin Invest. 1975 Nov;56(5):1181–1188. doi: 10.1172/JCI108195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmei M., Ghosh P., Taylor T. K. N6,O2'-dibutyryl adenosine 3', 5'-monophosphate-stimulated release of proteoglycans from cultured immature rabbit ear cartilage. Biochim Biophys Acta. 1976 Jun 23;437(1):94–105. doi: 10.1016/0304-4165(76)90350-0. [DOI] [PubMed] [Google Scholar]
- Sokoloff L., Malemud C. J., Green W. T., Jr Sulfate incorporation by articular chondrocytes in monolayer culture. Arthritis Rheum. 1970 Mar-Apr;13(2):118–124. doi: 10.1002/art.1780130203. [DOI] [PubMed] [Google Scholar]
- Strawich E., Nimni M. E. Properties of a collagen molecule containing three identical components extracted from bovine articular cartilage. Biochemistry. 1971 Oct 12;10(21):3905–3911. doi: 10.1021/bi00797a017. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Voelkel E. F., Levine L., Goldhaber P. Evidence that the bone resorption-stimulating factor produced by mouse fibrosarcoma cells is prostaglandin E 2 . A new model for the hypercalcemia of cancer. J Exp Med. 1972 Dec 1;136(6):1329–1343. doi: 10.1084/jem.136.6.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]


