Abstract
Methyl salicylate (MeSa) is a stress hormone released by plants under attack by pathogens or herbivores . MeSa has been shown to attract predatory insects of herbivores and repel pests. The molecules and neurons underlying animal response to MeSa are not known. Here we found that the nematode Caenorhabditis elegans exhibits a strong avoidance response to MeSa, which requires the activities of two closely related neuropeptide receptors NPR-1 and NPR-2. Molecular analyses suggest that NPR-1 expressed in the RMG inter/motor neurons is required for MeSa avoidance. An NPR-1 ligand FLP-18 is also required. Using a rescuing npr-2 promoter to drive a GFP transgene, we identified that NPR-2 is expressed in multiple sensory and interneurons. Genetic rescue experiments suggest that NPR-2 expressed in the AIZ interneurons is required for MeSa avoidance. We also provide evidence that the AWB sensory neurons might act upstream of RMGs and AIZs to detect MeSa. Our results suggest that NPR-2 has an important role in regulating animal behavior and that NPR-1 and NPR-2 act on distinct interneurons to affect C. elegans avoidance response to MeSa.
Keywords: C. elegans, methyl salicylate, npr-1, npr-2, interneuron
PLANTS emit odorants that can affect animal behaviors. The identification of the molecules and neurons regulating these behaviors remains a central task for understanding an animal’s nervous system.
Methyl salicylate (MeSa) is a volatile stress hormone released by plants when infected by pathogens (Park et al. 2007) or attacked by herbivores (van den Boom et al. 2004). Besides enhancing the systemic acquired resistance of the affected plants, MeSa could be sensed by adjacent plants as a warning signal for the infection (Park et al. 2007).
Ecological experiments also uncovered interesting effects of MeSa on animal behaviors. For example, MeSa is released by some plants as a pest repellent (Hardie et al. 1994; Jayasekara et al. 2005) and as an attractant for beneficial insects as well (James 2003; De Boer and Dicke 2004; James and Price 2004; Zhu and Park 2005). It is not clear what molecules and neural mechanisms determine whether an animal is attracted to or repelled by MeSa.
Caenorhabditis elegans has been an efficient model for studying the molecular and neural mechanisms underlying odorant-elicited behaviors (Bargmann 2006, 2012). Specifically, the detailed description of the neural connections by reconstructing serial-section electron microscopic pictures of the animals (White et al. 1986) provides a unique map for dissecting the neural correlates of each individual behavior. A combination of the neural connection diagram with molecular analyses will likely eventually lead to a systematic understanding of the neural regulation of a behavior, from key regulatory molecules to signaling integration in neural circuits. Several examples of such efforts include the dissection of a hub-and-spoke circuit that controls C. elegans social behavior (Macosko et al. 2009), the thermotaxis circuit (Kimata et al. 2012), the circuit that generates long-lasting roaming and dwelling states (Flavell et al. 2013), the mechanosensation circuit (Chalfie et al. 1985), and the behavioral quiescence circuit (Choi et al. 2013).
We found that C. elegans strongly avoids MeSa, suggesting that this animal can be used for studying the molecular and neuronal mechanisms underlying the behavioral effects of MeSa. In this study we identified multiple genes important for the behavior and analyzed in detail how neuropeptide receptors NPR-1 and NPR-2 and neuropeptide FLP-18 act in different neurons to affect the avoidance behavior.
Materials and Methods
Strains are listed in Supporting Information, File S1.
MeSa avoidance assay
C. elegans avoidance to MeSA was performed using a previously described odortaxis assay (Bargmann et al. 1993) with modifications. Synchronized young adults were washed with M9 twice and H2O once and placed on the midline of a 9-cm NGM plate without food. On the assay plate, 2 μl ethanol and 2 μl MeSa (Sigma, cat. no. M2047-100ML), respectively, were spotted in a small area (<3 mm in diameter and 0.5 cm from the periphery) at opposite ends. One microliter of NaN3 (1.0 M) was spotted at these sites to paralyze animals that locomoted nearby. The plate was loosely sealed with paraffin membrane and kept in a 20° incubator for 4 hr (the standard exposure time in our study). The number of animals on the MeSa side (A) and the ethanol side (B) was scored under a dissecting microscope. The MeSa avoidance index is calculated as the ratio of (B minus A) divided by (B plus A). Animals climbing up the side of the plate were excluded from the analysis. A positive avoidance index indicates that the animals avoid MeSa while a negative index indicates that the animals are attracted to MeSa. A total of 30–200 animals were tested in each assay and the experiments were repeated at least three times for each strain. We found that 2 μl ethanol alone had no obvious effects on the behaviors of wild-type, npr-1, and npr-2 animals (J. Luo and L. Ma, unpublished observations).
Molecular biology
To construct the npr-1p::npr-1::GFP transgene, a PCR-amplified full-length npr-1 genomic DNA (gDNA) together with an npr-1 promoter (2 kb upstream of the npr-1 start codon) was subcloned to the pPD95.79 vector in-frame with GFP using XmaI/AgeI restriction sites.
To construct the flp-18p::flp-18 transgene, a PCR-amplified full-length flp-18 gDNA with a flp-18 promoter (1.7 kb upstream of the flp-18 start codon) and a 3′ UTR fragment (0.8 kb downstream of the flp-18 stop codon) was subcloned to the pMD18-T vector (Sino Biological).
To construct the npr-2p::npr-2::GFP transgene, a PCR-amplified full-length npr-2 gDNA was subcloned to the pPD95.79 vector in-frame with GFP using BamHI/AgeI restriction sites. An npr-2 promoter (2 kb upstream of the start codon of npr-2) was subcloned to the pPD95.79::npr-2 backbone using PstI/BamHI restriction sites.
We inserted a PCR-amplified full-length npr-1 gDNA fragment to pPD95.79 in-frame with GFP using XmaI/AgeI restriction sites. The resulting pPD95.79::npr-1 and pPD95.79::npr-2 (see above) were used as backbones for constructing transgenes for neuron-specific rescue experiments.
For control transgenes, we tested myo-3p::GFP alone and myo-3p::GFP with npr-1p::GFP, flp-18p::GFP or npr-2p::GFP. We found no obvious effects of these transgenes on the MeSa avoidance responses in wild-type, flp-18(gk3063), npr-1(ky13), and npr-2(ok419) animals.
Neuron-specific promoters are listed in Table S1 (for npr-1 transgenes), Table S2 (for flp-18 transgenes), and Table S3 (for npr-2 transgenes). Promoters were amplified from wild-type animals using primers listed in Table S4.
Transgene experiments
Germline transgene experiments were performed as described (Mello et al. 1991). Transgene mixtures contain 5–10 ng/μl transgene and 20 ng/μl pPD95.86::GFP (myo-3p::GFP) plasmid (which expresses GFP in body-wall muscles) as co-injection marker.
Identification of npr-2-expressing neurons
Two transgenic lines expressing GFP under control of the 2 kb rescuing npr-2 promoter were generated. GFP-positive neurons were identified using a ×100 DIC/fluorescent objective of a Leica 5000B inverted microscope (Figure S2A) or a Leica TCS SP5 II laser confocal microscope (see Figure 4C) and compared to anatomical and morphological characteristics described in WormAtlas (www.wormatlas.org). We used DiI-stained sensory neurons (Tong and Burglin 2010) as landmarks to facilitate the verification of the identities of npr-2-expressing neurons (Figure S2A).
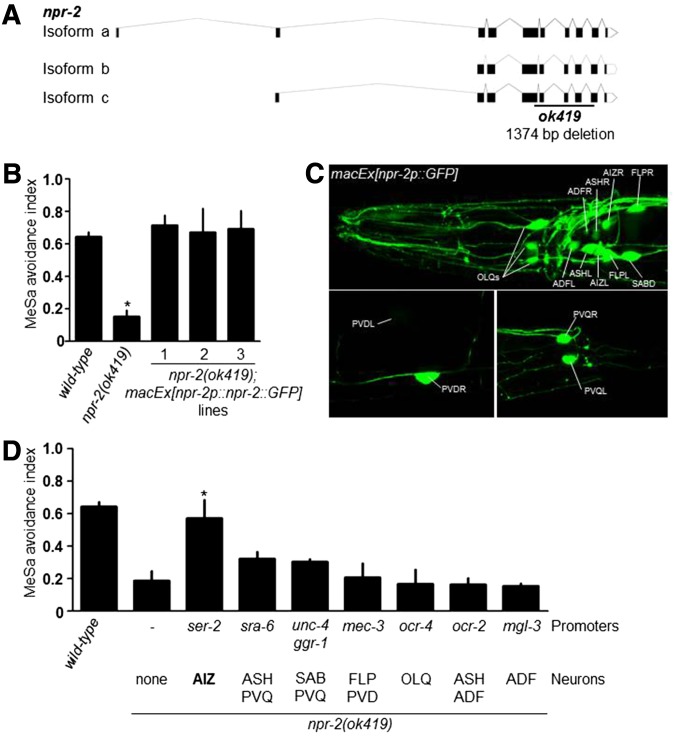
Figure 4.
npr-2 is expressed in multiple neurons and functions in the AIZ interneurons to regulate the MeSa avoidance response. (A) npr-2 gene structure (designed using the Exon-Intron Graphic Maker software at www.wormweb.org based on gene sequence information at www.wormbase.org). The ok419 mutation deletes a region including exon 5 (partial), exons 6, 7, and 8, and exon 9 (partial) of npr-2 isoform a. (B) MeSa avoidance response of npr-2(ok419) mutants expressing an npr-2::GFP transgene under control of an endogenous npr-2 promoter. Statistics: different from wild type. Error bars: standard errors. *P < 0.01 (Bonferroni correction after one-way ANOVA). (C) A GFP transgene under control of the endogenous npr-2 promoter is expressed in multiple sensory neurons and interneurons. (D) MeSa avoidance responses of npr-2(ok419) mutants expressing an npr-2::GFP transgene under control of different neuron-specific promoters. Target neurons are indicated. Three lines were assayed for each transgene. Statistics: different from npr-2(ok419). Error bars: standard errors. *P < 0.05 (Bonferroni correction after one-way ANOVA).
Statistical analysis
A two-tailed unpaired Student’s t-test was used for single comparison. The Bonferroni correction after one-way ANOVA was used for multiple comparisons. fat-4(wa14), odr-4(n2144), egl-4(n478), odr-3(n2150), and che-1(e1034) mutants were found to be significantly different from wild-type animals in the MeSa avoidance response by Student t-test at the error rate of 1% (J. Luo and L. Ma, unpublished observations). In addition, fat-4(wa14), odr-4(n2144), egl-4(n478), and che-1(e1034) were found to be significantly different from wild type based on the Benjamini–Hochberg procedure (also called false discovery rate method) (reviewed by Fay and Gerow 2013) at the false discovery rate of 1%.
Results
C. elegans exhibits a strong avoidance response to MeSa
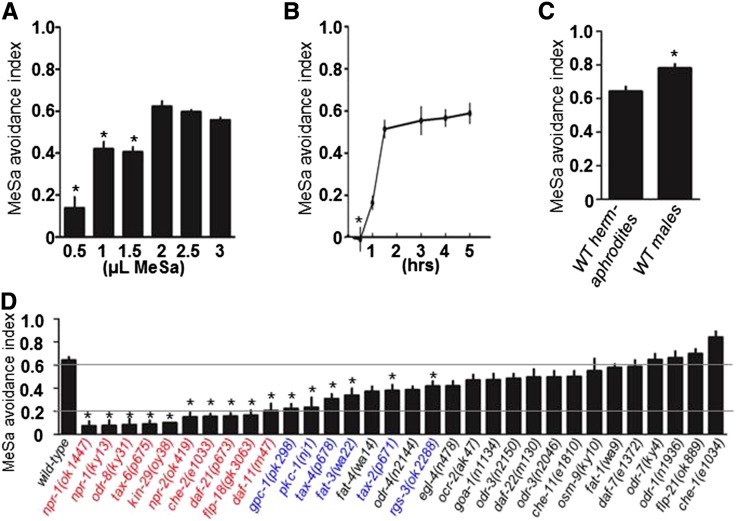
To understand the molecular mechanism underlying the biological effect of MeSa on animal behaviors, we examined how C. elegans responds to different doses of MeSa using a previously described odortaxis assay (Bargmann et al. 1993). After an 8-hr exposure to MeSa, wild-type animals exhibit a dose-dependent avoidance response, reaching a maximal response at 2 μl MeSa or higher doses (Figure 1A). Using 2 μl MeSa as the standard dose, we found that wild-type animals exhibit a gradually increased avoidance as the MeSa exposure time increases, reaching a maximal response between 1.5 and 5 hr (Figure 1B). Animals sense MeSa as a volatile because MeSa spotted on the inside of the Petri dish lid that did not contact the agar medium also caused a strong avoidance response (Figure S1A). We found that wild-type males have a slightly stronger MeSa avoidance response compared to hermaphrodites (Figure 1C), implying that sex might affect an animal’s response to MeSa. In plants MeSa is converted to the biologically active defense hormone salicylic acid by the SA-binding protein 2 (SABP2, a MeSa esterase) to induce systemic acquired resistance (Kumar and Klessig 2008). We failed to identify a homolog of SABP2 in the C. elegans genome (BLAST, www.wormbase.org), suggesting that MeSa hydrolysis might not be required for the avoidance response.
Figure 1.
C. elegans exhibits a strong avoidance response to MeSa that requires activities of multiple genes. (A) Wild-type animals exhibited dose-dependent avoidance responses after 8-hr exposure to MeSa. Statistics: different from the index of 2 μl MeSa. Error bars: standard errors. *P < 0.01 (Bonferroni correction after one-way ANOVA). (B) Wild-type animals exhibited a maximal avoidance response after 1.5- to 5-hr exposure to 2 μl MeSa. Statistics: different from the index at 4 hr. Error bars: standard errors. *P < 0.01 (Bonferroni correction after one-way ANOVA). (C) MeSa avoidance response of wild-type male animals. Statistics: different from hermaphrodites. Error bars: standard errors. *P < 0.05 (Student’s t-test). (D) MeSa avoidance responses of C. elegans behavioral mutants. Three groups of mutants were identified in the screen: mutants with strong (index ≤ 0.2, red), moderate (0.2 < index < 0.6, blue) and no apparent defects (black). Statistics: different from wild type. Error bars: standard errors. *P < 0.01 (Bonferroni correction after one-way ANOVA).
A screen for genes required for the MeSa avoidance behavior
To identify genes required for MeSa avoidance, we screened 32 C. elegans mutants with previously described or putative behavioral defects, in which 30 distinct genes were affected (Figure 1D). We identified mutants with strong (index ≤ 0.2, red), moderate (0.2 < index < 0.6, blue), or no apparent (index > 0.6, black) defects (Figure 1D). Nine genes with strong effects on MeSa avoidance include the neuropeptide receptor genes npr-1 (de Bono and Bargmann 1998) and npr-2 (de Bono and Bargmann 1998), the ER-associated Ufm1-specific protease 2 gene odr-8 (Dwyer et al. 1998; Chen et al. 2014), the calcineurin A subunit gene tax-6 (Kuhara et al. 2002), the serine/threonine kinase gene kin-29 (Lanjuin and Sengupta 2002), the WD40 domain protein-encoding gene che-2 (Lewis and Hodgkin 1977; Fujiwara et al. 1999), the HSP90 chaperone gene daf-21 (Vowels and Thomas 1994; Birnby et al. 2000), the neuropeptide gene flp-18 (Rogers et al. 2003), and the receptor guanylyl cyclase gene daf-11 (Vowels and Thomas 1994; Birnby et al. 2000).
We also identified six genes with moderate effects on the MeSa avoidance response (Figure 1D, blue). These genes encode a G protein gamma-subunit (gpc-1) (Jansen et al. 2002), a protein kinase C (pkc-1) (Okochi et al. 2005; Sieburth et al. 2007), two subunits of a cGMP-gated channel (tax-4 and tax-2) (Coburn and Bargmann 1996; Komatsu et al. 1996), a delta-6 fatty acid desaturase (fat-3) (Watts and Browse 2002), and a regulator of G protein signaling (rgs-3) (Ferkey et al. 2007).
The neuropeptide receptor gene npr-1 is expressed in the RMG neurons to regulate the MeSa avoidance behavior
npr-1 acts in the RMG inter/motor neurons at the center of a hub-and-spoke neural circuit (the RMG circuit hereafter) to regulate C. elegans social feeding behavior (de Bono and Bargmann 1998; Macosko et al. 2009) and is a key regulatory gene for ethanol adaptation (Davies et al. 2004), pathogen susceptibility (Styer et al. 2008; Reddy et al. 2009), and behavioral quiescence (Choi et al. 2013). npr-2 is the closest paralog of npr-1 (de Bono and Bargmann 1998) with previously unknown functions in animal behaviors. We postulate that a detailed analysis of npr-1 and npr-2 in regulating the MeSa avoidance behavior might provide novel insights into functions of neuropeptide receptors.
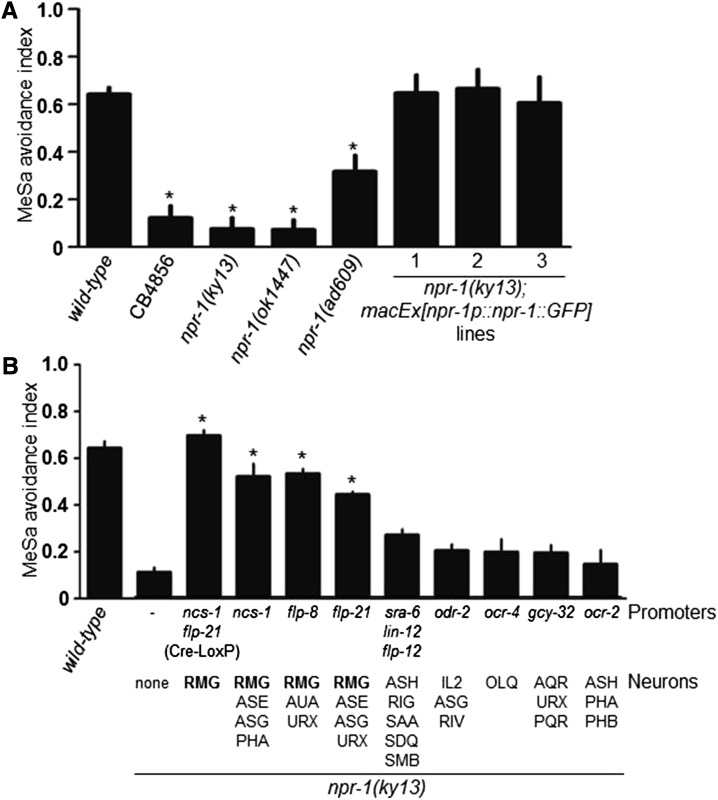
To verify the role of npr-1 in the MeSa avoidance response, we tested the Hawaiian C. elegans strain CB4856 that carries a hypomorphic allele of npr-1 (de Bono and Bargmann 1998) and three npr-1 loss-of-function mutants, npr-1(ky13), npr-1(ad609) (de Bono and Bargmann 1998), and npr-1(ok1447) (Stawicki et al. 2013). We found that all strains exhibited apparent MeSa avoidance defects (Figure 2A), in which CB4856, npr-1(ky13), and npr-1(ok1447) animals had similarly strong defects while npr-1(ad609) animals had a moderate defect. An npr-1::GFP transgene under control of a 2-kb npr-1 endogenous promoter completely rescued the defective MeSa avoidance of npr-1(ky13) mutants (Figure 2A), demonstrating that npr-1 is essential for the behavior. The moderate effect of npr-1(ad609) on the MeSa avoidance response implies that NPR-1(ad609) might retain a residual activity for mediating this behavior. Alternatively an unknown mutation in the npr-1(ad609) strain might have modified the MeSa avoidance behavior.
Figure 2.
npr-1 expressed in the RMG inter/motor neurons is required for C. elegans avoidance response to MeSa. (A) MeSa avoidance responses of different npr-1 mutants and npr-1(ky13) mutants expressing an npr-1::GFP transgene under control of an endogenous npr-1 promoter. Statistics: different from wild type. Error bars: standard errors. *P < 0.01 (Bonferroni correction after one-way ANOVA). (B) MeSa avoidance responses of npr-1(ky13) mutants expressing an npr-1::GFP transgene under control of neuron-specific promoters. Target neurons are listed. Three lines were assayed for each transgene. Statistics: different from npr-1(ky13). Error bars: standard errors. *P < 0.01 (Bonferroni correction after one-way ANOVA).
To identify npr-1-expressing neurons involved in the behavior, we performed transgene rescue experiments using previously described neuron-specific promoters (Table S1). npr-1 transgene expression in the RMG neurons could significantly rescue the defective MeSa avoidance of npr-1(ky13) mutants (Figure 2B), while transgene expression in npr-1-expressing neurons other than RMGs failed to rescue (Figure 2B). Using a Cre-LoxP transgene combination (Macosko et al. 2009) to express an npr-1 transgene specifically in RMGs, we found that the defective MeSa avoidance of npr-1(ky13) mutants was also completely rescued (Figure 2B). Therefore, npr-1 likely acts in RMGs to regulate the MeSa avoidance.
The neuropeptide FLP-18 is required for the MeSa avoidance behavior
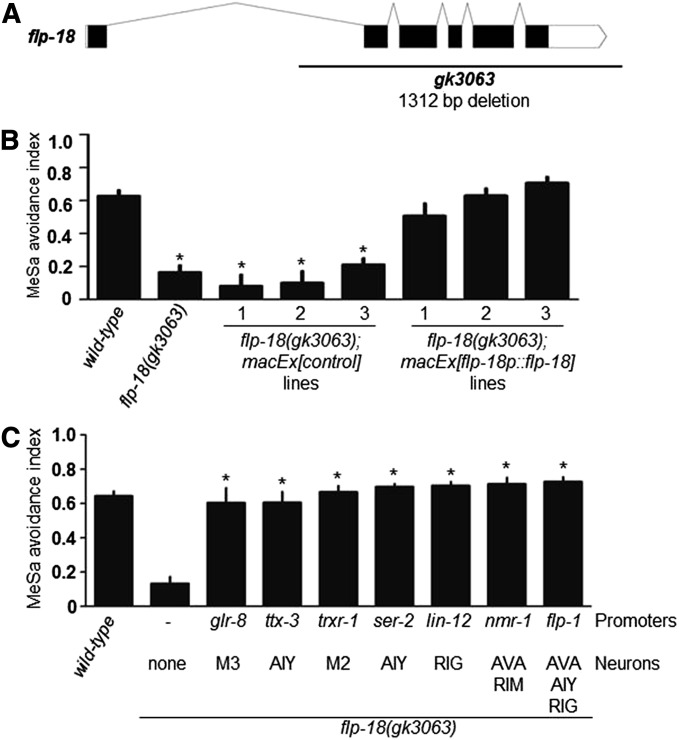
flp-18 and flp-21 were previously shown to encode FMRFamide-related ligands for NPR-1 (Kubiak et al. 2003; Rogers et al. 2003). We found that gk3063 (Figure 3A), a deletion mutation of flp-18 caused a strong MeSa avoidance defect (Figure 1D and Figure 3B). This defect could be rescued by a flp-18 transgene (Figure 3B). To test whether FLP-18 expressed by different neurons (Rogers et al. 2003) might differentially affect the MeSa avoidance, we expressed a flp-18 transgene under control of various neuron-specific promoters (Table S2). We found that each transgene rescued the defective MeSa avoidance of flp-18(gk3063) mutants to a level similar to that of wild type (Figure 3C), suggesting indistinguishable roles for FLP-18-expressing neurons in mediating the MeSa avoidance. Different from flp-18, a flp-21(ok889) deletion mutation did not obviously affect the MeSa avoidance (Figure 1D). Therefore FLP-18 activity might be specifically required for the behavior.
Figure 3.
flp-18 is required for C. elegans avoidance response to MeSa. (A) flp-18 gene structure (designed using the Exon-Intron Graphic Maker software at www.wormweb.org based on gene sequence information at www.wormbase.org) and the gk3063 deletion mutation. (B) MeSa avoidance responses of flp-18(gk3063) mutants expressing a myo-3p::GFP control transgene or a flp-18p::flp-18 transgene. Three lines were assayed for each transgene. Statistics: different from wild type. Error bars: standard errors. *P < 0.01 (Bonferroni correction after one-way ANOVA). (C) MeSa avoidance response of flp-18(gk3063) mutants expressing a flp-18 transgene in different flp-18-expressing neurons. Three lines were assayed for each transgene. Statistics: different from flp-18(gk3063). Error bars: standard errors. *P < 0.01 (Bonferroni correction after one-way ANOVA).
The neuropeptide receptor gene npr-2 acts in the AIZ interneurons to regulate the MeSa avoidance behavior
Among mutants exhibiting strong MeSa avoidance defects (Figure 1D) is a deletion mutant (ok419) (Figure 4A) of the neuropeptide receptor gene npr-2 (de Bono and Bargmann 1998). npr-2(ok419) mutants were previously found to have increased intestinal fat storage (Cohen et al. 2009). However behavioral defects have not been described. A transgene expressing an NPR-2::GFP fusion protein under control of a 2-kb endogenous npr-2 promoter rescued the defective MeSa avoidance in npr-2(ok419) mutants (Figure 4B), suggesting that npr-2 is required for the behavior.
To identify npr-2-expressing cells, we generated transgenic animals expressing GFP (Figure 4C) under control of the rescuing npr-2 promoter and identified a total of 15–17 GFP-positive neurons that likely express npr-2 (Figure 4C and Figure S2A). These neurons include sensory neurons OLQs (2–4), ASHL/R, ADFL/R, FLPL/R, and PVDL/R and interneurons AIZL/R, SABD, and PVQL/R (Figure 4C, Figure S2A, and Figure S2B). The expressivity of the npr-2p::GFP transgene in each class of neurons ranges from 62.5% for the AIZ interneurons and 100% for the FLP sensory neurons (Figure S2B). The variable penetrance of GFP expression in these neurons might be caused by transgene mosaicism or variable GFP expression intensity.
In C. elegans, FLPs and PVDs are the sensory neurons that mediate harsh touch stimuli (Way and Chalfie 1989). npr-2(ok419) mutants exhibited a grossly normal response to harsh touch (J. Luo and L. Ma, unpublished observations), suggesting that npr-2 might not be essential for harsh touch sensation.
To identify npr-2-expressing neurons involved in the MeSa avoidance, we performed neuron-specific transgene rescue experiments. We tested eight promoters (Table S3) that drive transgene expression in each or a combination of the npr-2-expressing neurons. Only a ser-2 promoter that was previously shown to drive expression in the AIZ interneurons (Tsalik et al. 2003) but not in any other npr-2-expressing neurons could strongly rescue the defective MeSa avoidance (Figure 4D), while other promoters (Table S3) that do not drive transgene expression in AIZs had no apparent rescuing effects (Figure 4D). Hence npr-2 might act in the AIZ interneurons to regulate the MeSa avoidance.
npr-1 loss-of-function mutants exhibit social feeding (de Bono and Bargmann 1998), a behavior regulated by the RMG circuit (Macosko et al. 2009). We found that npr-2(ok419) mutants did not exhibit an obvious social feeding (Figure S1B).
The AWB sensory neurons might be required for detecting MeSa
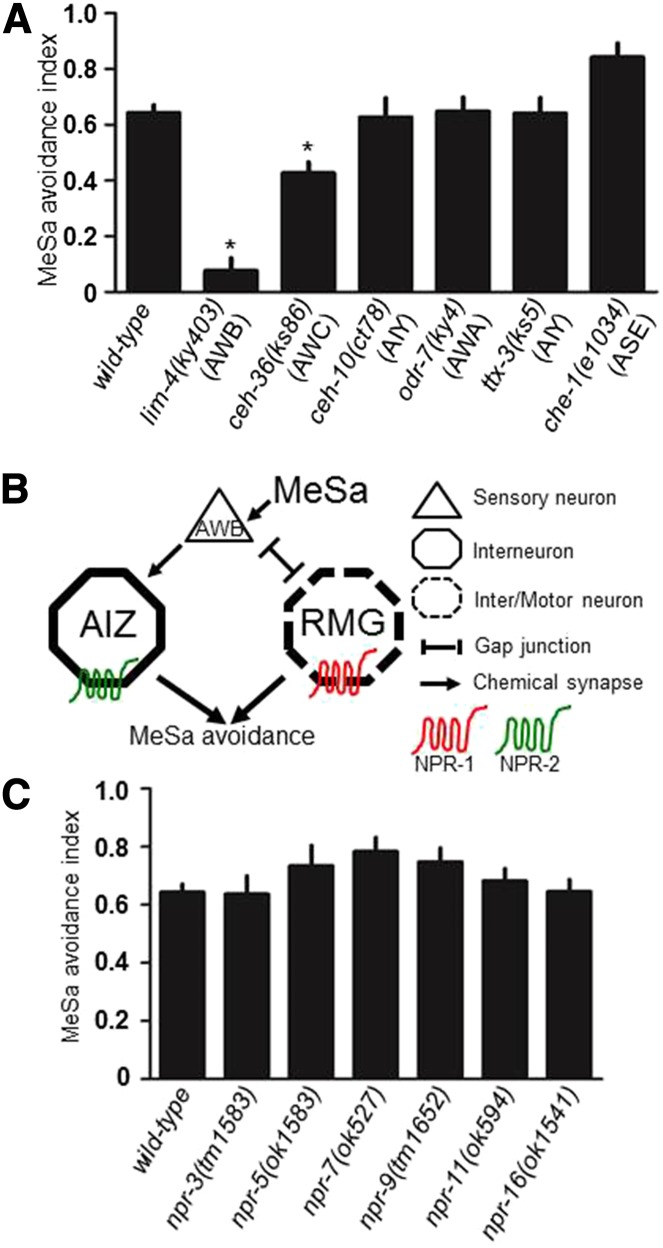
In C. elegans the AWB sensory neurons primarily detect repulsive volatiles (Bargmann 2006), raising the question of whether AWBs are responsible for sensing MeSa. To test this, we examined the lim-4(ky403) mutants, in which the AWB neurons are transformed to the AWC cell fate (Sagasti et al. 1999), and other mutants with different sensory neuron fate transformations (Hobert 2010). The results showed that only the lim-4(ky403) mutants are strongly defective in the MeSa avoidance (Figure 5A), implying that AWBs might be the sensory neurons primarily responsible for MeSa detection. We examined the C. elegans wiring diagram (White et al. 1986) (www.wormweb.org) and found that AWBs synapse onto AIZs and connect with RMGs by a gap junction (Figure 5B). Such a wiring diagram implies a neural pathway that consists of AWBs, AIZs, and RMGs for mediating C. elegans avoidance response to MeSa.
Figure 5.
The AWB sensory neurons might be required for detecting MeSa. (A) MeSa avoidance responses of terminal selector gene mutants. Affected neurons are indicated. Statistics: different from wild type. Error bars: standard errors. *P < 0.01 (Bonferroni correction after one-way ANOVA). (B) A simplified wiring diagram of the neurons that likely mediate the MeSa avoidance response. Neuron classes, NPR-1 (red) and NPR-2 (green) are indicated. (C) MeSa avoidance responses of deletion mutants of six other GPCRs.
Finally we tested six other neuropeptide receptors and found that none was required for the MeSa avoidance (Figure 5C). NPR-5 and NPR-4 (not available in this study) were previously found to be FLP-18 receptors as well (Cohen et al. 2009). That npr-5 mutants were not apparently defective in the MeSa avoidance supports the notion the FLP-18 might act though NPR-1 or other receptors to regulate this behavior.
Discussion
In this study we provide evidence that C. elegans exhibits a strong avoidance response to the plant stress hormone methyl salicylate, which requires activities of two neuropeptide receptor genes npr-1 and npr-2. We propose a model that the AWB sensory neurons act upstream to detect MeSa and transmit the odorant signals to NPR-1-expressing RMG inter/motor neurons and NPR-2-expressing AIZ interneurons. Our study suggests a novel function of NPR-2 in regulating C. elegans response to natural odorants.
MeSa has a myriad of effects on plants and animals. As a volatile, MeSa is released by plants to elicit systemic acquired resistance upon pathogen infection (Park et al. 2007), to attract predators of herbivores (James 2003; De Boer and Dicke 2004; James and Price 2004; Zhu and Park 2005) and to repel pests (Hardie et al. 1994; Jayasekara et al. 2005). MeSa is widely used as a refreshing odorant in food (Lewis 1989) and hygiene products (Lachenmeier et al. 2013). MeSa is also an ingredient in over-the-counter topical creams for the relief of musculoskeletal aches and pains (Chan 1996a,b; Higashi et al. 2010). Excess intake of MeSa could be life threatening probably due to severe, rapid-onset salicylate poisoning (Chan 1996a,b; Davis 2007). The broad effects of MeSa on animals warrant a detailed study of the underlying biological mechanisms.
How sensory neurons detect MeSa is unclear. MeSa was shown to have both stimulatory and inhibitory effects on human transient receptor potential V1 (TRPV1), in which the inhibitory effect of MeSa on capsaicin-induced TRPV1 activation was proposed to underlie the analgesic effects of MeSa (Ohta et al. 2009). Five genes (osm-9, ocr-1, ocr-2, ocr-3, and ocr-4) encode TRPV channels in the C. elegans genome (Tobin et al. 2002) and it appears that none is expressed in the AWB sensory neurons (Colbert et al. 1997; Tobin et al. 2002). In addition, osm-9 and ocr-2 mutants had grossly normal MeSa avoidance responses (Figure 1D). Therefore, TRPV channels might not be the MeSa receptors in AWBs. Recently EpOR1, a seven-transmembrane odorant receptor expressed in antennae sensory neurons of the tortricid moth Epiphyas postvittana, was shown to exhibit high sensitivity to MeSa when expressed in the insect sf9 cells (Jordan et al. 2009). We failed to identify a C. elegans protein similar to EpOR1 (BLAST, www.wormbase.org). C. elegans has >1000 G protein-coupled receptors, among which >500 might function as chemosensory receptors (Bargmann 2006). A future survey of AWB-specific GPCRs that are required for the MeSa avoidance response might lead to the identification of a MeSa receptor in C. elegans.
NPR-1 regulates C. elegans social feeding behavior (de Bono and Bargmann 1998; Macosko et al. 2009), oxygen sensing (Cheung et al. 2005), acute response to ethanol (Davies et al. 2004), pathogen susceptibility (Styer et al. 2008; Reddy et al. 2009), and behavioral quiescence (Choi et al. 2013). We found that RMG-expressed NPR-1 is required for C. elegans avoidance to MeSa, verifying the key role of the RMG inter/motor neurons in NPR-1-regulated behaviors (Macosko et al. 2009; Choi et al. 2013). As a close paralog of NPR-1 (de Bono and Bargmann 1998), NPR-2 acts in the AIZ interneurons to regulate C. elegans avoidance to MeSa (Figure 4). AIZs also have a well-defined function in mediating cryophilic migration (Mori and Ohshima 1995; Kimata et al. 2012) and are involved in chemotaxis to NaCl (Iino and Yoshida 2009) and aversive olfactory learning (Ha et al. 2010). These findings suggest that AIZs might function like RMGs as an integrating site for various sensory stimuli. If so, NPR-2 might be a regulator of these behaviors.
Besides npr-1, npr-2, and flp-18, we identified 12 other genes required for the MeSa avoidance response (Figure 1D and Figure S1C). These genes encode a G protein gamma-subunit (gpc-1) (Jansen et al. 2002) and a G protein beta-subunit-like (che-2) (Fujiwara et al. 1999), an Ufm1 protease subunit required for GPCR maturation (odr-8) (Dwyer et al. 1998; Chen et al. 2014), an AMP/SNF kinase essential for GPCR expression (kin-29) (Lanjuin and Sengupta 2002), a protein kinase C involved in neuropeptide secretion (pkc-1) (Okochi et al. 2005; Sieburth et al. 2007), and five factors downstream of G protein signaling (daf-11, tax-4, tax-2, fat-3, and rgs-3) (Coburn and Bargmann 1996; Komatsu et al. 1996; Birnby et al. 2000; Watts and Browse 2002; Bargmann 2006; Ferkey et al. 2007). tax-6 encodes a calcineurin A protein (Kuhara et al. 2002) that genetically interacts with G proteins (Lee et al. 2004), while daf-21 encodes an HSP90 chaperone that functions similarly to daf-11 in the dauer pathway (Vowels and Thomas 1994; Birnby et al. 2000). Therefore G protein signaling is likely essential for the MeSa avoidance response, consistent with our findings that NPR-1, NPR-2, and FLP-18 play critical roles in this behavior and implying that an unknown GPCR might be the chemosensory receptor for MeSa.
In short, we found that NPR-1 and NPR-2 regulate C. elegans avoidance response to MeSa. We identified a neuronal pathway from the AWB sensory neurons to the RMG inter/motor neurons and AIZ interneurons as a possible regulatory pathway for this behavior. Future identification of the MeSa receptor and dissection of the detailed neural circuit will provide novel insight into the effects of MeSa on animals.
Acknowledgments
We thank Nikhil Bhatla and Dengke Ma for suggestions; Yanling Teng, Fei Wang, and Yaoyu Zhong for strain maintenance and technical support; Andy Fire for providing pPD95.79 and pPD95.86 plasmids; and Shohei Mitani (National BioResource Project::C. elegans) for providing the npr-3(tm1583) strain. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). This study was supported by a National Key Basic Research Program of China grant (2011CB510005), a National Natural Science Foundation of China grant (31371253), and a Ministry of Education grant (20130162110057) to L.M.
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.114.172239/-/DC1.
Communicating editor: M. V. Sundaram
Literature Cited
- Bargmann C. I., 2006. Chemosensation in C. elegans (October 25, 2006), WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.123.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann C. I., 2012. Beyond the connectome: how neuromodulators shape neural circuits. BioEssays 34: 458–465. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Hartwieg E., Horvitz H. R., 1993. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74: 515–527. [DOI] [PubMed] [Google Scholar]
- Birnby D. A., Link E. M., Vowels J. J., Tian H., Colacurcio P. L., et al. , 2000. A transmembrane guanylyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in Caenorhabditis elegans. Genetics 155: 85–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., Sulston J. E., White J. G., Southgate E., Thomson J. N., et al. , 1985. The neural circuit for touch sensitivity in Caenorhabditis elegans. J. Neurosci. 5: 956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T. Y., 1996a Medicated oils and severe salicylate poisoning: quantifying the risk based on methyl salicylate content and bottle size. Vet. Hum. Toxicol. 38: 133–134. [PubMed] [Google Scholar]
- Chan T. Y., 1996b Potential dangers from topical preparations containing methyl salicylate. Hum. Exp. Toxicol. 15: 747–750. [DOI] [PubMed] [Google Scholar]
- Chen C., Itakura E., Weber K. P., Hegde R. S., de Bono M., 2014. An ER complex of ODR-4 and ODR-8/Ufm1 specific protease 2 promotes GPCR maturation by a Ufm1-independent mechanism. PLoS Genet. 10: e1004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung B. H., Cohen M., Rogers C., Albayram O., de Bono M., 2005. Experience-dependent modulation of C. elegans behavior by ambient oxygen. Curr. Biol. 15: 905–917. [DOI] [PubMed] [Google Scholar]
- Choi S., Chatzigeorgiou M., Taylor K. P., Schafer W. R., Kaplan J. M., 2013. Analysis of NPR-1 reveals a circuit mechanism for behavioral quiescence in C. elegans. Neuron 78: 869–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn C. M., Bargmann C. I., 1996. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron 17: 695–706. [DOI] [PubMed] [Google Scholar]
- Cohen M., Reale V., Olofsson B., Knights A., Evans P., et al. , 2009. Coordinated regulation of foraging and metabolism in C. elegans by RFamide neuropeptide signaling. Cell Metab. 9: 375–385. [DOI] [PubMed] [Google Scholar]
- Colbert H. A., Smith T. L., Bargmann C. I., 1997. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J. Neurosci. 17: 8259–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A. G., Bettinger J. C., Thiele T. R., Judy M. E., McIntire S. L., 2004. Natural variation in the npr-1 gene modifies ethanol responses of wild strains of C. elegans. Neuron 42: 731–743. [DOI] [PubMed] [Google Scholar]
- Davis J. E., 2007. Are one or two dangerous? Methyl salicylate exposure in toddlers. J. Emerg. Med. 32: 63–69. [DOI] [PubMed] [Google Scholar]
- De Boer J. G., Dicke M., 2004. The role of methyl salicylate in prey searching behavior of the predatory mite phytoseiulus persimilis. J. Chem. Ecol. 30: 255–271. [DOI] [PubMed] [Google Scholar]
- de Bono M., Bargmann C. I., 1998. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell 94: 679–689. [DOI] [PubMed] [Google Scholar]
- Dwyer N. D., Troemel E. R., Sengupta P., Bargmann C. I., 1998. Odorant receptor localization to olfactory cilia is mediated by ODR-4, a novel membrane-associated protein. Cell 93: 455–466. [DOI] [PubMed] [Google Scholar]
- Fay D. S., Gerow K., 2013. A biologist’s guide to statistical thinking and analysis (July 9, 2013), WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.159.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferkey D. M., Hyde R., Haspel G., Dionne H. M., Hess H. A., et al. , 2007. C. elegans G protein regulator RGS-3 controls sensitivity to sensory stimuli. Neuron 53: 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell S. W., Pokala N., Macosko E. Z., Albrecht D. R., Larsch J., et al. , 2013. Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell 154: 1023–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara M., Ishihara T., Katsura I., 1999. A novel WD40 protein, CHE-2, acts cell-autonomously in the formation of C. elegans sensory cilia. Development 126: 4839–4848. [DOI] [PubMed] [Google Scholar]
- Ha H. I., Hendricks M., Shen Y., Gabel C. V., Fang-Yen C., et al. , 2010. Functional organization of a neural network for aversive olfactory learning in Caenorhabditis elegans. Neuron 68: 1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie J., Isaacs R., Pickett J. A., Wadhams L. J., Woodcock C. M., 1994. Methyl salicylate and (-)-(1R,5S)-myrtenal are plant-derived repellents for black bean aphid,Aphis fabae Scop. (Homoptera: Aphididae). J. Chem. Ecol. 20: 2847–2855. [DOI] [PubMed] [Google Scholar]
- Higashi Y., Kiuchi T., Furuta K., 2010. Efficacy and safety profile of a topical methyl salicylate and menthol patch in adult patients with mild to moderate muscle strain: a randomized, double-blind, parallel-group, placebo-controlled, multicenter study. Clin. Ther. 32: 34–43. [DOI] [PubMed] [Google Scholar]
- Hobert O., 2010. Neurogenesis in the nematode Caenorhabditis elegans (October 4, 2010), WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.12.2, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino Y., Yoshida K., 2009. Parallel use of two behavioral mechanisms for chemotaxis in Caenorhabditis elegans. J. Neurosci. 29: 5370–5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D. G., 2003. Field evaluation of herbivore-induced plant volatiles as attractants for beneficial insects: methyl salicylate and the green lacewing, Chrysopa nigricornis. J. Chem. Ecol. 29: 1601–1609. [DOI] [PubMed] [Google Scholar]
- James D. G., Price T. S., 2004. Field-testing of methyl salicylate for recruitment and retention of beneficial insects in grapes and hops. J. Chem. Ecol. 30: 1613–1628. [DOI] [PubMed] [Google Scholar]
- Jansen G., Weinkove D., Plasterk R. H., 2002. The G-protein gamma subunit gpc-1 of the nematode C.elegans is involved in taste adaptation. EMBO J. 21: 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasekara T. K., Stevenson P. C., Hall D. R., Belmain S. R., 2005. Effect of volatile constituents from Securidaca longepedunculata on insect pests of stored grain. J. Chem. Ecol. 31: 303–313. [DOI] [PubMed] [Google Scholar]
- Jordan M. D., Anderson A., Begum D., Carraher C., Authier A., et al. , 2009. Odorant receptors from the light brown apple moth (Epiphyas postvittana) recognize important volatile compounds produced by plants. Chem. Senses 34: 383–394. [DOI] [PubMed] [Google Scholar]
- Kimata T., Sasakura H., Ohnishi N., Nishio N., Mori I., 2012. Thermotaxis of C. elegans as a model for temperature perception, neural information processing and neural plasticity. Worm 1: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu H., Mori I., Rhee J. S., Akaike N., Ohshima Y., 1996. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron 17: 707–718. [DOI] [PubMed] [Google Scholar]
- Kubiak T. M., Larsen M. J., Nulf S. C., Zantello M. R., Burton K. J., et al. , 2003. Differential activation of “social” and “solitary” variants of the Caenorhabditis elegans G protein-coupled receptor NPR-1 by its cognate ligand AF9. J. Biol. Chem. 278: 33724–33729. [DOI] [PubMed] [Google Scholar]
- Kuhara A., Inada H., Katsura I., Mori I., 2002. Negative regulation and gain control of sensory neurons by the C. elegans calcineurin TAX-6. Neuron 33: 751–763. [DOI] [PubMed] [Google Scholar]
- Kumar D., Klessig D. F., 2008. The search for the salicylic acid receptor led to discovery of the SAR signal receptor. Plant Signal. Behav. 3: 691–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachenmeier D. W., Monakhova Y. B., Markova M., Kuballa T., Rehm J., 2013. What happens if people start drinking mouthwash as surrogate alcohol? A quantitative risk assessment. Food Chem. Toxicol. 51: 173–178. [DOI] [PubMed] [Google Scholar]
- Lanjuin A., Sengupta P., 2002. Regulation of chemosensory receptor expression and sensory signaling by the KIN-29 Ser/Thr kinase. Neuron 33: 369–381. [DOI] [PubMed] [Google Scholar]
- Lee J., Jee C., Song H. O., Bandyopadhyay J., Lee J. I., et al. , 2004. Opposing functions of calcineurin and CaMKII regulate G-protein signaling in egg-laying behavior of C.elegans. J. Mol. Biol. 344: 585–595. [DOI] [PubMed] [Google Scholar]
- Lewis J. A., Hodgkin J. A., 1977. Specific neuroanatomical changes in chemosensory mutants of the nematode Caenorhabditis elegans. J. Comp. Neurol. 172: 489–510. [DOI] [PubMed] [Google Scholar]
- Lewis R. J., 1989. Food Additives Handbook, Van Nostrand Reinhold, New York. [Google Scholar]
- Macosko E. Z., Pokala N., Feinberg E. H., Chalasani S. H., Butcher R. A., et al. , 2009. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature 458: 1171–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V., 1991. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10: 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I., Ohshima Y., 1995. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature 376: 344–348. [DOI] [PubMed] [Google Scholar]
- Ohta T., Imagawa T., Ito S., 2009. Involvement of transient receptor potential vanilloid subtype 1 in analgesic action of methylsalicylate. Mol. Pharmacol. 75: 307–317. [DOI] [PubMed] [Google Scholar]
- Okochi Y., Kimura K. D., Ohta A., Mori I., 2005. Diverse regulation of sensory signaling by C. elegans nPKC-epsilon/eta TTX-4. EMBO J. 24: 2127–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. W., Kaimoyo E., Kumar D., Mosher S., Klessig D. F., 2007. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318: 113–116. [DOI] [PubMed] [Google Scholar]
- Reddy K. C., Andersen E. C., Kruglyak L., Kim D. H., 2009. A polymorphism in npr-1 is a behavioral determinant of pathogen susceptibility in C. elegans. Science 323: 382–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C., Reale V., Kim K., Chatwin H., Li C., et al. , 2003. Inhibition of Caenorhabditis elegans social feeding by FMRFamide-related peptide activation of NPR-1. Nat. Neurosci. 6: 1178–1185. [DOI] [PubMed] [Google Scholar]
- Sagasti A., Hobert O., Troemel E. R., Ruvkun G., Bargmann C. I., 1999. Alternative olfactory neuron fates are specified by the LIM homeobox gene lim-4. Genes Dev. 13: 1794–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth D., Madison J. M., Kaplan J. M., 2007. PKC-1 regulates secretion of neuropeptides. Nat. Neurosci. 10: 49–57. [DOI] [PubMed] [Google Scholar]
- Stawicki T. M., Takayanagi-Kiya S., Zhou K., Jin Y., 2013. Neuropeptides function in a homeostatic manner to modulate excitation-inhibition imbalance in C. elegans. PLoS Genet. 9: e1003472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styer K. L., Singh V., Macosko E., Steele S. E., Bargmann C. I., et al. , 2008. Innate immunity in Caenorhabditis elegans is regulated by neurons expressing NPR-1/GPCR. Science 322: 460–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin D., Madsen D., Kahn-Kirby A., Peckol E., Moulder G., et al. , 2002. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron 35: 307–318. [DOI] [PubMed] [Google Scholar]
- Tong Y. G., Burglin T. R., 2010. Conditions for dye-filling of sensory neurons in Caenorhabditis elegans. J. Neurosci. Methods 188: 58–61. [DOI] [PubMed] [Google Scholar]
- Tsalik E. L., Niacaris T., Wenick A. S., Pau K., Avery L., et al. , 2003. LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev. Biol. 263: 81–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boom C. E., van Beek T. A., Posthumus M. A., de Groot A., Dicke M., 2004. Qualitative and quantitative variation among volatile profiles induced by Tetranychus urticae feeding on plants from various families. J. Chem. Ecol. 30: 69–89. [DOI] [PubMed] [Google Scholar]
- Vowels J. J., Thomas J. H., 1994. Multiple chemosensory defects in daf-11 and daf-21 mutants of Caenorhabditis elegans. Genetics 138: 303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts J. L., Browse J., 2002. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 99: 5854–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way J. C., Chalfie M., 1989. The mec-3 gene of Caenorhabditis elegans requires its own product for maintained expression and is expressed in three neuronal cell types. Genes Dev. 3: 1823–1833. [DOI] [PubMed] [Google Scholar]
- White J. G., Southgate E., Thomson J. N., Brenner S., 1986. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 314: 1–340. [DOI] [PubMed] [Google Scholar]
- Zhu J., Park K. C., 2005. Methyl salicylate, a soybean aphid-induced plant volatile attractive to the predator Coccinella septempunctata. J. Chem. Ecol. 31: 1733–1746. [DOI] [PubMed] [Google Scholar]