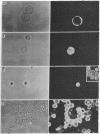
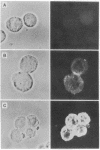
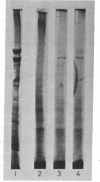
Abstract
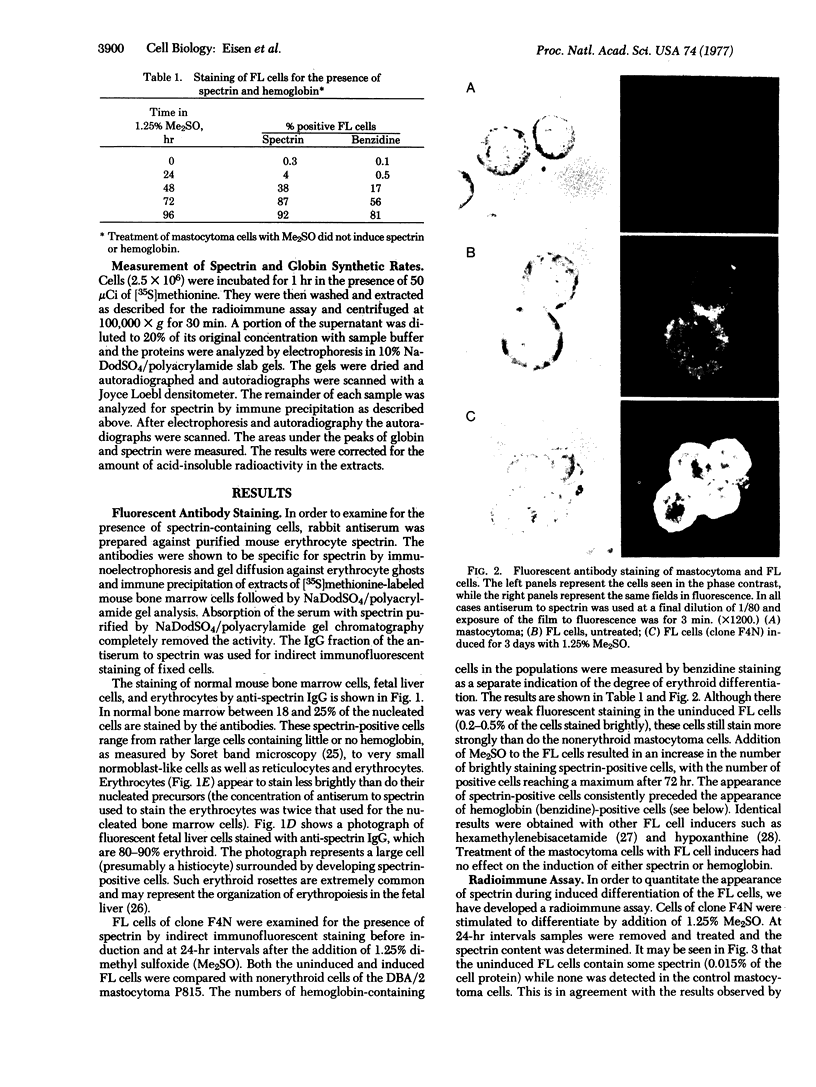
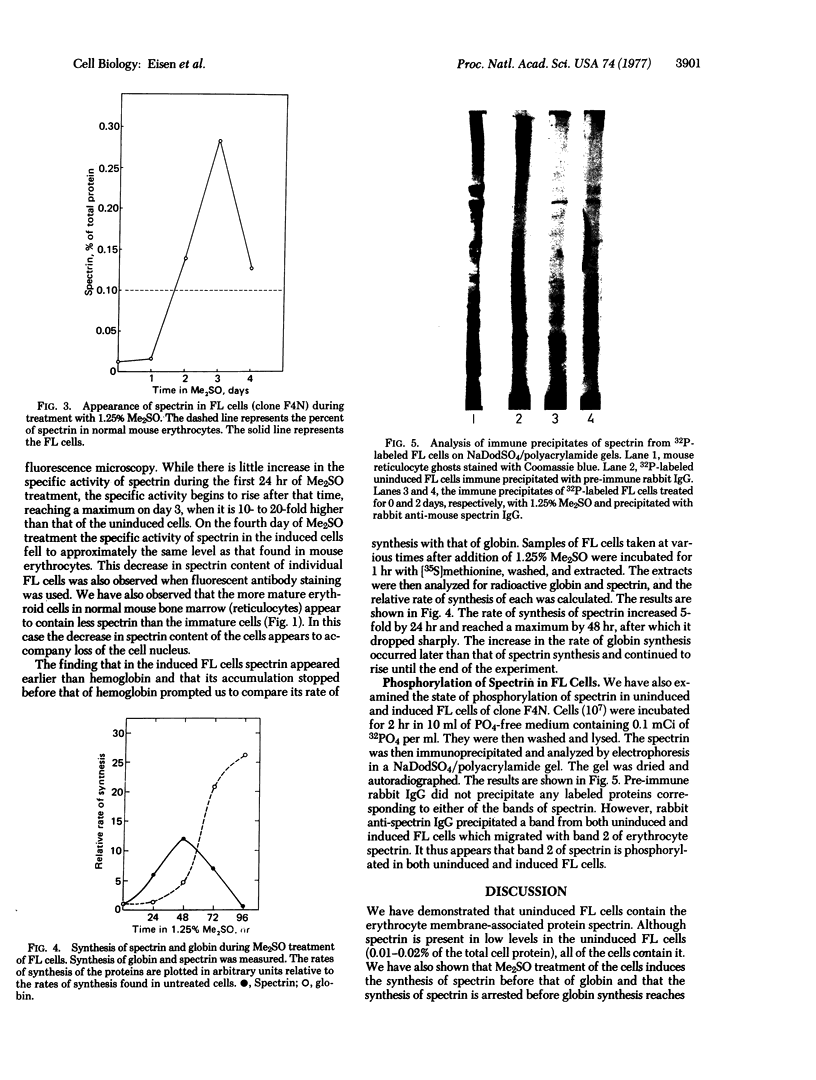
The presence and accumulation in murine erythroleukemic cells transformed by Friend virus of the erythrocyte membrane-associated protein spectrin has been investigated. Spectrin was present in the uninduced cells and was induced 10- to 20-fold in dimethyl sulfoxide-treated differentiating cells. The intracellular concentration of spectrin reached a peak on the third day of dimethyl sulfoxide treatment, after which it fell to levels found in mouse erythrocytes. We also found that the small subunit of spectrin was phosphorylated in the cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt-Jovin D. J., Ostertag W., Eisen H., Klimek F., Jovin T. M. Studies of cellular differentiation by automated cell separation. Two model systems: Friend virus-transformed cells and Hydra attenuata. J Histochem Cytochem. 1976 Jan;24(1):332–347. doi: 10.1177/24.1.1254928. [DOI] [PubMed] [Google Scholar]
- Avruch J., Fairbanks G. Phosphorylation of endogenous substrates by erythrocyte membrane protein kinases. I. A monovalent cation-stimulated reaction. Biochemistry. 1974 Dec 31;13(27):5507–5514. doi: 10.1021/bi00724a009. [DOI] [PubMed] [Google Scholar]
- Chang H., Langer P. J., Lodish H. F. Asynchronous synthesis of erythrocyte membrane proteins. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3206–3210. doi: 10.1073/pnas.73.9.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgsaeter A., Branton D. Intramembrane particle aggregation in erythrocyte ghosts. I. The effects of protein removal. J Cell Biol. 1974 Dec;63(3):1018–1036. doi: 10.1083/jcb.63.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Friend C., Patuleia M. C., De Harven E. Erythrocytic maturation in vitro of murine (Friend) virus-induced leukemic cells. Natl Cancer Inst Monogr. 1966 Sep;22:505–522. [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaedicke G., Abedin Z., Dube S. K., Kluge N., Neth R., Steinheider G., Weimann B. J., Ostertag W. Control of globin synthesis during DMSO-induced differentiation of mouse erythroleukemic cells in culture. Hamatol Bluttransfus. 1974;14:278–287. [PubMed] [Google Scholar]
- Gusella J. F., Housman D. Induction of erythroid differentiation in vitro by purines and purine analogues. Cell. 1976 Jun;8(2):263–269. doi: 10.1016/0092-8674(76)90010-6. [DOI] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Ikawa Y., Furusawa M., Sugano H. Erythrocyte membrane-specific antigens in Friend virus-induced leukemia cells. Bibl Haematol. 1973;39:955–967. doi: 10.1159/000427928. [DOI] [PubMed] [Google Scholar]
- Keppel F., Allet B., Eisen H. Appearance of a chromatin protein during the erythroid differentiation of Friend virus-transformed cells. Proc Natl Acad Sci U S A. 1977 Feb;74(2):653–656. doi: 10.1073/pnas.74.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamelin J. P., Lisowska-Bernstein B., Matter A., Ryser J. E., Vassalli P. Mouse thymus-independent and thymus-derived lymphoid cells. I. Immunofluorescent and functional studies. J Exp Med. 1972 Nov 1;136(5):984–1007. doi: 10.1084/jem.136.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T., Steers E., Jr Selective solubilization of a protein component of the red cell membrane. Science. 1968 Jan 12;159(3811):203–204. doi: 10.1126/science.159.3811.203. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- NOMARSKI G., BESSIS M. [Examination and microphotography of cells containing hemoglobin with the aid of interferential filters transmitting Soret's band]. Rev Hematol. 1959 Oct-Nov;14:399–404. [PubMed] [Google Scholar]
- Ostertag W., Melderis H., Steinheider G., Kluge N., Dube S. Synthesis of mouse haemoglobin and globin mRNA in leukaemic cell cultures. Nat New Biol. 1972 Oct 25;239(95):231–234. doi: 10.1038/newbio239231a0. [DOI] [PubMed] [Google Scholar]
- Pinder J. C., Bray D., Gratzer W. B. Actin polymerisation induced by spectrin. Nature. 1975 Dec 25;258(5537):765–766. doi: 10.1038/258765a0. [DOI] [PubMed] [Google Scholar]
- Ross J., Ikawa Y., Leder P. Globin messenger-RNA induction during erythroid differentiation of cultured leukemia cells. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3620–3623. doi: 10.1073/pnas.69.12.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Singer S. J. On the mechanism of ATP-induced shape changes in human erythrocyte membranes. I. The role of the spectrin complex. J Cell Biol. 1977 Jun;73(3):638–646. doi: 10.1083/jcb.73.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Levy J., Terada M., Breslow R., Rifkind R. A., Marks P. A. Induction of erythroid differentiation in murine virus infected eythroleukemia cells by highly polar compounds. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1003–1006. doi: 10.1073/pnas.72.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Detmers P. Actin in erythrocyte ghosts and its association with spectrin. Evidence for a nonfilamentous form of these two molecules in situ. J Cell Biol. 1975 Sep;66(3):508–520. doi: 10.1083/jcb.66.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]