Abstract
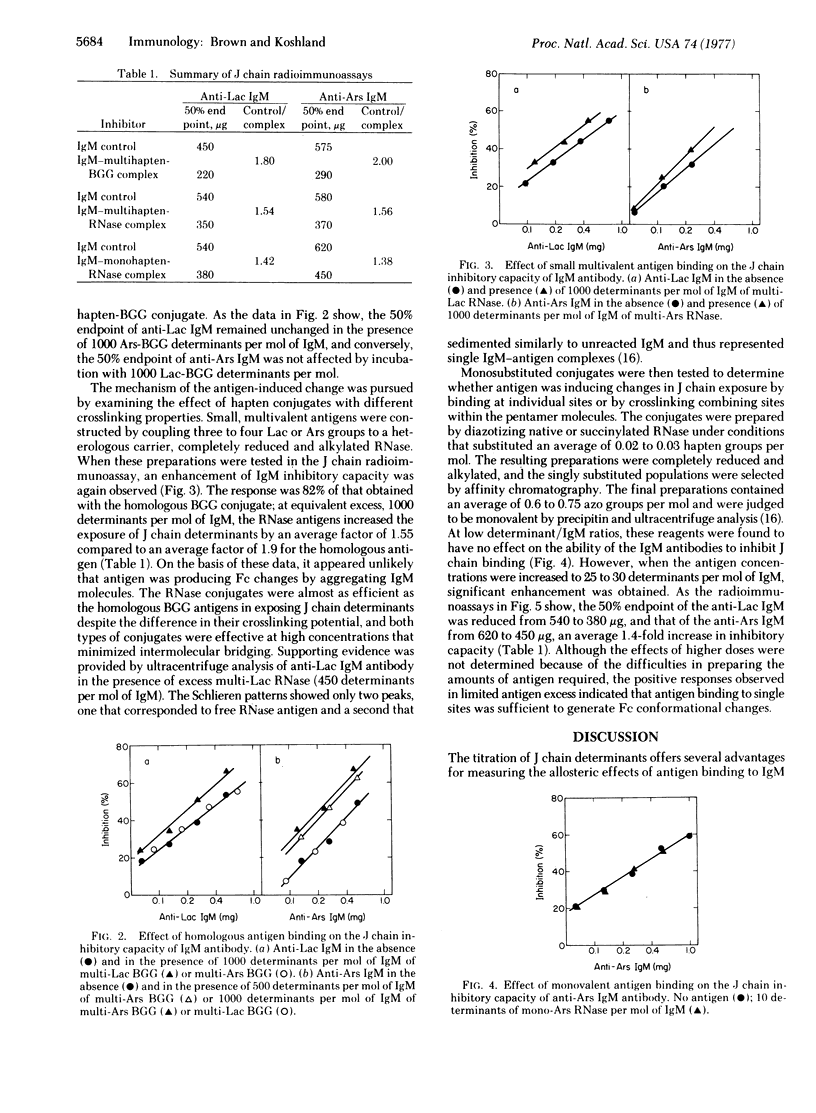
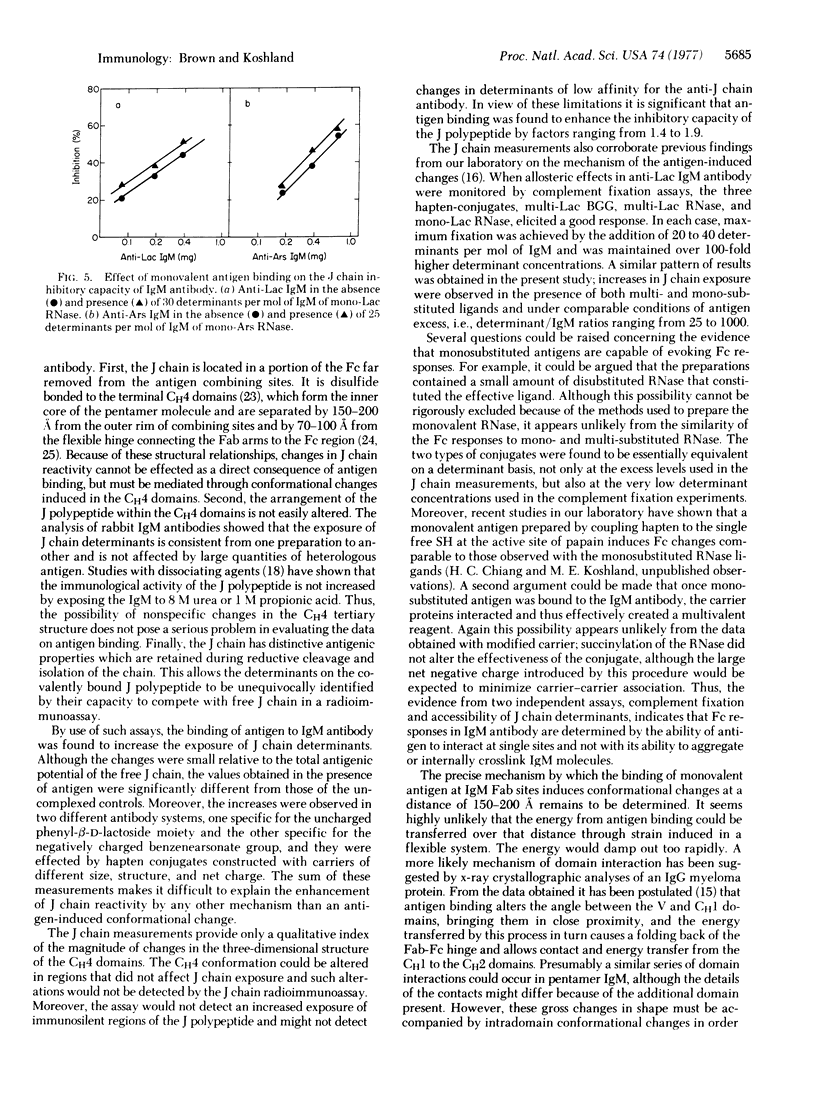
The effects of antigen binding on IgM antibody conformation were investigated by measuring the immunological reactivity of the Fc-bound J polypeptide. For such measurements anti-azophenyl-beta-D-lactoside and anti-azobenzenearsonate IgM antibodies were examined in a J chain radioimmunoassay before and after complexing with various hapten-conjugates. The assays showed that (i) the accessibility of J chain determinants is very limited in uncomplexed IgM and (ii) their accessibility is significantly enhanced in the presence of an excess of specific antigen. In both antibody systems, enhanced J chain exposure was achieved with the homologous multi-hapten-substituted antigen (1.9-fold), with a small multivalent antigen in which three to four hapten groups were coupled to a heterologous carrier (1.55-fold), and with monohapten-substituted antigen (1.4-fold). Because the J chain is located in the terminal CH4 Fc domains, these data provide direct evidence that a change in Fc conformation is induced by the binding of antigen to the distant Fab combining sites. Moreover, the data indicate that the changes in J chain exposure do not depend on crosslinking by antigen, but can be induced by the interaction of antigen at individual IgM combining sites.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. C., Koshland M. E. Activation of antibody Fc function by antigen-induced conformational changes. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5111–5115. doi: 10.1073/pnas.72.12.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathou R. E., Werner T. C. Hapten stabilization of antibody conformation. Biochemistry. 1970 Aug 4;9(16):3149–3155. doi: 10.1021/bi00818a006. [DOI] [PubMed] [Google Scholar]
- Colman P. M., Deisenhofer J., Huber R. Structure of the human antibody molecule Kol (immunoglobulin G1): an electron density map at 5 A resolution. J Mol Biol. 1976 Jan 25;100(3):257–278. doi: 10.1016/s0022-2836(76)80062-9. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Colman P. M., Epp O., Huber R. Crystallographic structural studies of a human Fc fragment. II. A complete model based on a Fourier map at 3.5 A resolution. Hoppe Seylers Z Physiol Chem. 1976 Dec;357(10):1421–1434. doi: 10.1515/bchm2.1976.357.2.1421. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Colman P. M., Huber R., Haupt H., Schwick G. Crystallographic structural studies of a human Fc-fragment. I. An electron-density map at 4 A resolution and a partial model. Hoppe Seylers Z Physiol Chem. 1976 Mar;357(3):435–445. doi: 10.1515/bchm2.1976.357.1.435. [DOI] [PubMed] [Google Scholar]
- Feinstein A., Munn E. A., Richardson N. E. The three-dimensional conformation of M and A globulin molecules. Ann N Y Acad Sci. 1971 Dec 31;190:104–121. doi: 10.1111/j.1749-6632.1971.tb13526.x. [DOI] [PubMed] [Google Scholar]
- Feldmann M. Induction of immunity and tolerance in vitro by hapten protein conjugates. I. The relationship between the degree of hapten conjugation and the immunogenicity of dinitrophenylated polymerized flagellin. J Exp Med. 1972 Apr 1;135(4):735–753. doi: 10.1084/jem.135.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N. M. Electron microscopy of the immunoglobulins. Adv Immunol. 1969;11:1–30. doi: 10.1016/s0065-2776(08)60476-9. [DOI] [PubMed] [Google Scholar]
- Holowka D. A., Strosberg A. D., Kimball J. W., Haber E., Cathou R. E. Changes in intrinsic circular dichroism of several homogeneous anti-type 3 pneumococcal antibodies on binding of a small hapten. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3399–3403. doi: 10.1073/pnas.69.11.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R., Deisenhofer J., Colman P. M., Matsushima M., Palm W. Crystallographic structure studies of an IgG molecule and an Fc fragment. Nature. 1976 Dec 2;264(5585):415–420. doi: 10.1038/264415a0. [DOI] [PubMed] [Google Scholar]
- Hyslop N. E., Jr, Dourmashkin R. R., Green N. M., Porter R. R. The fixation of complement and the activated first component (C1) of complement by complexes formed between antibody and divalent hapten. J Exp Med. 1970 Apr 1;131(4):783–802. doi: 10.1084/jem.131.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T. IgE and reaginic hypersensitivity. Ann N Y Acad Sci. 1971 Dec 31;190:443–456. doi: 10.1111/j.1749-6632.1971.tb13554.x. [DOI] [PubMed] [Google Scholar]
- Jaton J. C., Huser H., Braun D. G., Givol D., Pecht I., Schlessinger J. Conformational changes induced in a homogeneous anti-type III pneumococcal antibody by oligosaccharides of increasing size. Biochemistry. 1975 Dec 2;14(24):5312–5315. doi: 10.1021/bi00695a014. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Vaerman J. P., Heremans J. F. J-chain determinants in polymeric immunoglobulins. Eur J Immunol. 1973 Apr;3(4):185–191. doi: 10.1002/eji.1830030402. [DOI] [PubMed] [Google Scholar]
- Koshland M. E., Davis J. J., Fujita N. J. Evidence for multiple gene control of a single polypeptide chain: the heavy chain of rabbit immunoglobulin. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1274–1281. doi: 10.1073/pnas.63.4.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland M. E. Structure and function of the J chain. Adv Immunol. 1975;20:41–69. doi: 10.1016/s0065-2776(08)60206-0. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J., Schrohenloher R. E. Site of attachment of J chain to human immunoglobulin M. Nature. 1974 Jun 14;249(458):650–652. doi: 10.1038/249650a0. [DOI] [PubMed] [Google Scholar]
- Metzger H. Effect of antigen binding on the properties of antibody. Adv Immunol. 1974;18:169–207. doi: 10.1016/s0065-2776(08)60310-7. [DOI] [PubMed] [Google Scholar]
- Morrison S. L., Koshland M. E. Characterization of the J chain from polymeric immunoglobulins (IgA-IgM-immunological specificity-primary structure). Proc Natl Acad Sci U S A. 1972 Jan;69(1):124–128. doi: 10.1073/pnas.69.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Steinberg I. Z., Givol D., Hochman J., Pecht I. Antigen-induced conformational changes in antibodies and their Fab fragments studied by circular polarization of fluorescence. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2775–2779. doi: 10.1073/pnas.72.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal D. M., Taurog J. D., Metzger H. Dimeric immunoglobulin E serves as a unit signal for mast cell degranulation. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2993–2997. doi: 10.1073/pnas.74.7.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde C. E., 3rd, Koshland M. E. Molecular size and shape of the J chain from polymeric immunoglobulins. Biochemistry. 1973 Aug 14;12(17):3218–3224. doi: 10.1021/bi00741a012. [DOI] [PubMed] [Google Scholar]


