Abstract
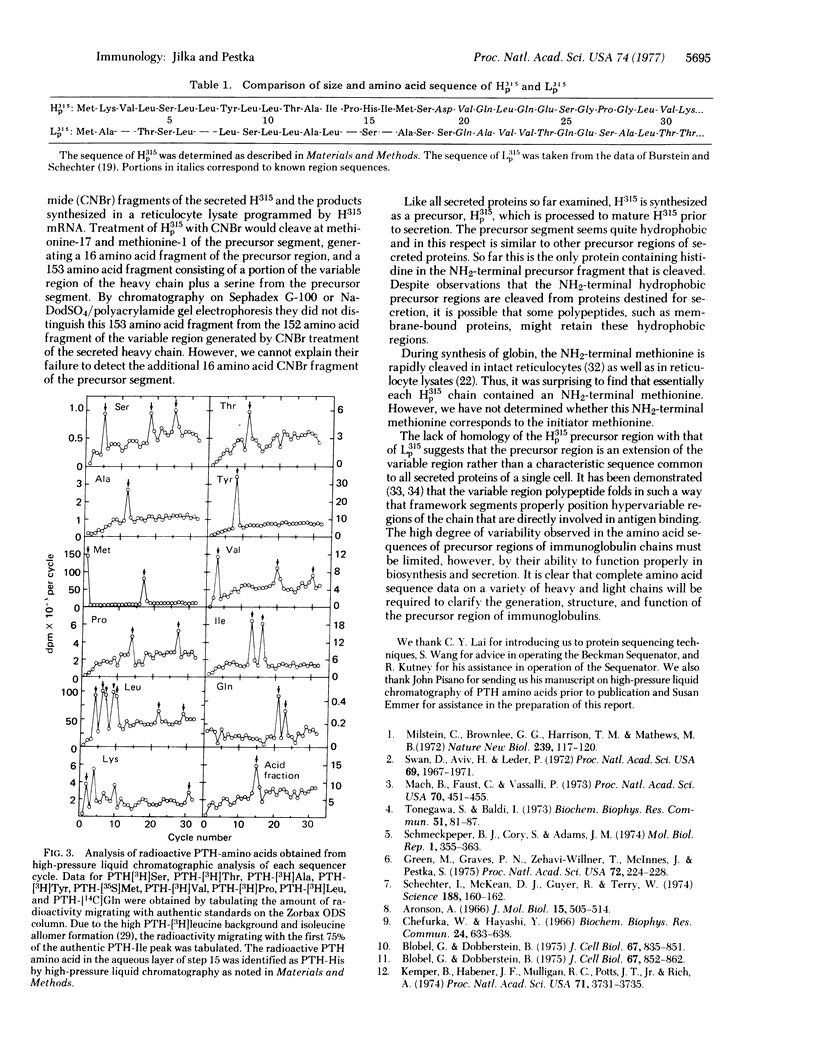
Partially purified mRNA coding for the MOPC-315 heavy immunoglobulin alpha chain was translated in a reticulocyte lysate containing 20 labeled amino acids. Radiolabeled MOPC-315 heavy chain precursor protein, purified by preparative gel electrophoresis and immunoprecipitation, was sequenced by Edman degradation. The labeled phenylthiohydantoin amino acid obtained in each cycle was identified and quantitated by high-pressure liquid chromatography. The precursor sequence of 18 amino acids, Met-Lys-Val-Leu-Ser-Leu-Leu-Tyr-Leu-Leu-Thr-Ala-Ile-Pro-His-Ile-Met-Ser, preceded the sequence corresponding to the NH2 terminus of the mature secreted heavy chain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. Adsorption of polysomes to bacterial membranes. J Mol Biol. 1966 Feb;15(2):505–514. doi: 10.1016/s0022-2836(66)80124-9. [DOI] [PubMed] [Google Scholar]
- Bedard D. L., Huang R. C. Initiation and translation in vitro of mRNA for MOPC 315 immunoglobulin heavy chain and characterization of translation product. J Biol Chem. 1977 Apr 25;252(8):2592–2598. [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burstein Y., Schechter I. Amino acid sequence of the NH2-terminal extra piece segments of the precursors of mouse immunoglobulin lambda1-type and kappa-type light chains. Proc Natl Acad Sci U S A. 1977 Feb;74(2):716–720. doi: 10.1073/pnas.74.2.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein Y., Schechter I. Amino acid-sequence variability at the N-terminal extra piece of mouse immunoglobulin light-chain precursors of the same and different subgroups. Biochem J. 1976 Jul 1;157(1):145–151. doi: 10.1042/bj1570145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein Y., Schechter I. Glutamine as a precursor to N-terminal pyrrolid-2-one-5-carboxylic acid in mouse immunoglobulin lambda-type light chains. Amino acid-sequence variability at the N-terminal extra piece of lambda-type light-chain precursors. Biochem J. 1977 Aug 1;165(2):347–354. doi: 10.1042/bj1650347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. J., Keim P., Steiner D. F. Cell-free synthesis of rat preproinsulins: characterization and partial amino acid sequence determination. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1964–1968. doi: 10.1073/pnas.73.6.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefurka W., Hayashi Y. The effect of trypsin on rough endoplasmic membranes. Biochem Biophys Res Commun. 1966 Sep 8;24(5):633–638. doi: 10.1016/0006-291x(66)90370-6. [DOI] [PubMed] [Google Scholar]
- Francis S. H., Leslie R. G., Hood L., Eisen H. N. Amino-acid sequence of the variable region of the heavy (alpha) chain of a mouse myeloma protein with anti-hapten activity. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1123–1127. doi: 10.1073/pnas.71.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Graves P. N., Zehavi-Willner T., McInnes J., Pestka S. Cell-free translation of immunoglobulin messenger RNA from MOPC-315 plasmacytoma and MOPC-315 NR, a variant synthesizing only light chain. Proc Natl Acad Sci U S A. 1975 Jan;72(1):224–228. doi: 10.1073/pnas.72.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housman D., Jacobs-Lorena M., Rajbhandary U. L., Lodish H. F. Initiation of haemoglobin synthesis by methionyl-tRNA. Nature. 1970 Aug 29;227(5261):913–918. doi: 10.1038/227913a0. [DOI] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Mulligan R. C., Potts J. T., Jr, Rich A. Pre-proparathyroid hormone: a direct translation product of parathyroid messenger RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3731–3735. doi: 10.1073/pnas.71.9.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach B., Faust C., Vassalli P. Purification of 14S messenger RNA of immunoglobulin light chain that codes for a possible light-chain precursor. Proc Natl Acad Sci U S A. 1973 Feb;70(2):451–455. doi: 10.1073/pnas.70.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R. A., Gorski J., McKean D. J. Partial amino acid sequence of rat pre-prolactin. Biochem J. 1977 Jan 1;161(1):189–192. doi: 10.1042/bj1610189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Padlan E. A., Davies D. R. Variability of three-dimensional structure in immunoglobulins. Proc Natl Acad Sci U S A. 1975 Mar;72(3):819–823. doi: 10.1073/pnas.72.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Schechter I., Burstein Y. Identification of N-terminal methionine in the precursor of immunoglobulin light chain. Initiation of translation of messenger ribonucleic acid in plants and animals. Biochem J. 1976 Mar 1;153(3):543–550. doi: 10.1042/bj1530543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I. Partial amino acid sequence of the precursor of immunoglobulin light chain programmed by messenger RNA in vitro. Science. 1975 Apr 11;188(4184):160–162. doi: 10.1126/science.803715. [DOI] [PubMed] [Google Scholar]
- Schmeckpeper B. J., Cory S., Adams J. M. Translation of immunoglobulin mRNAs in a wheat germ cell-free system. Mol Biol Rep. 1974 Mar;1(6):355–363. doi: 10.1007/BF00309570. [DOI] [PubMed] [Google Scholar]
- Shields D., Blobel G. Cell-free synthesis of fish preproinsulin, and processing by heterologous mammalian microsomal membranes. Proc Natl Acad Sci U S A. 1977 May;74(5):2059–2063. doi: 10.1073/pnas.74.5.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchanek G., Kindås-Mügge I., Kreil G. Translation of honeybee promelittin messenger RNA. Formation of a larger product in a mammalian cell-free system. Eur J Biochem. 1975 Dec 1;60(1):309–315. doi: 10.1111/j.1432-1033.1975.tb21005.x. [DOI] [PubMed] [Google Scholar]
- Swan D., Aviv H., Leder P. Purification and properties of biologically active messenger RNA for a myeloma light chain. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1967–1971. doi: 10.1073/pnas.69.7.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S., Baldi I. Electrophoretically homogeneous myeloma light chain mRNA and its translation in vitro. Biochem Biophys Res Commun. 1973 Mar 5;51(1):81–87. doi: 10.1016/0006-291x(73)90510-x. [DOI] [PubMed] [Google Scholar]
- Tucker P., Pestka S. De novo synthesis and glycosylation of the MOPC-46B mouse immunoglobulin light chain in cell-free extracts. J Biol Chem. 1977 Jul 10;252(13):4474–4486. [PubMed] [Google Scholar]
- Wilson D. B., Dintzis H. M. Protein chain initiation in rabbit reticulocytes. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1282–1289. doi: 10.1073/pnas.66.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T., Kabat E. A. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med. 1970 Aug 1;132(2):211–250. doi: 10.1084/jem.132.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman C. L., Appella E., Pisano J. J. Advances in the analysis of amino acid phenylthiohydantoins by high performance liquid chromatography. Anal Biochem. 1976 Sep;75(1):77–85. doi: 10.1016/0003-2697(76)90057-9. [DOI] [PubMed] [Google Scholar]