Abstract
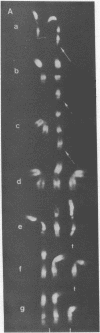
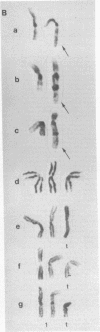
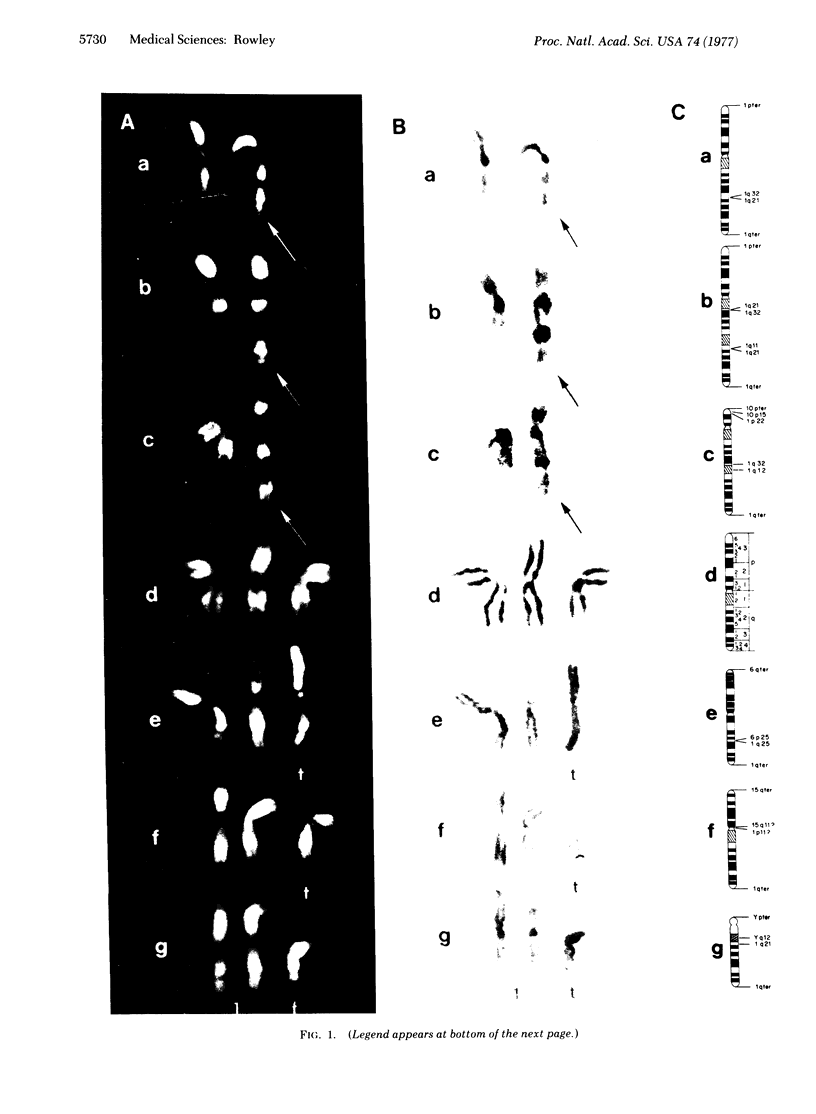
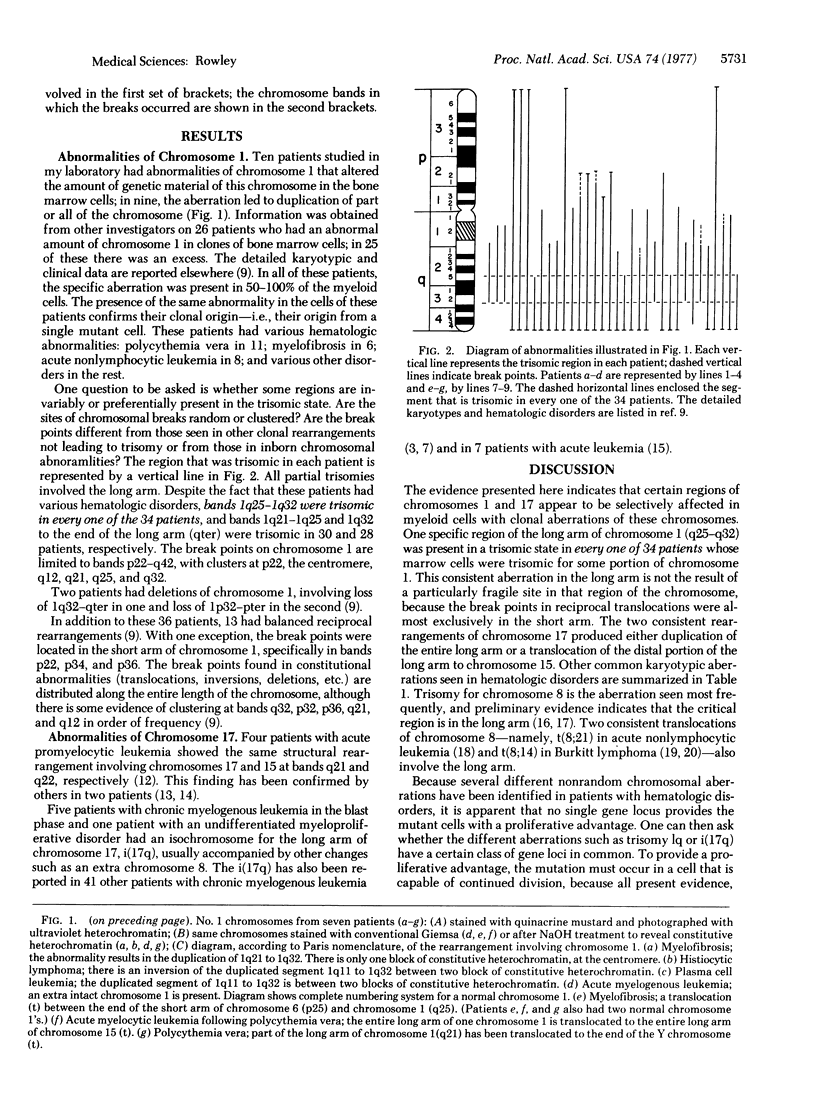
In clonal aberrations leading to an excess or partial excess of chromosome 1, trisomy for bands 1q25-1q32 was noted in the myeloid cells from all of 34 patients who had various disorders such as acute leukemia, polycythemia vera, and myelofibrosis. This was not the result of a particularly fragile site in that region of the chromosome because the break points in reciprocal translocations that involve it occurred almost exclusively in the short arm. Two consistent rearrangements that have been observed in chromosome 17 produced either duplication of the entire long arm or a translocation of the distal portion of the long arm to chromosome 15. The nonrandom chromosomal changes found in hematologic disorders can now be correlated with the gene loci on these chromosomes or chromosomal segments. Seventy-five genes related to various metabolic enzymes have been mapped; it may be significant that chromosomes carrying gene loci related to nucleic acid metabolism are more frequently involved in hematologic disorders (and other malignancies as well) than are gene loci related to intermediary or carbohydrate metabolism. Furthermore, the known virus-human chromosome associations are closely correlated with the chromosomes affected in hematologic disorders. If one of the effects of carcinogens (including viruses) is to activate genes that regulate host cell DNA synthesis, and if translocations or duplications of specific chromosomal segments produce the same effect, then either of these mechanisms might provide the affected cell with a proliferative advantage.
Keywords: gene mapping, virus interaction, hematologic disorders
Full text
PDF
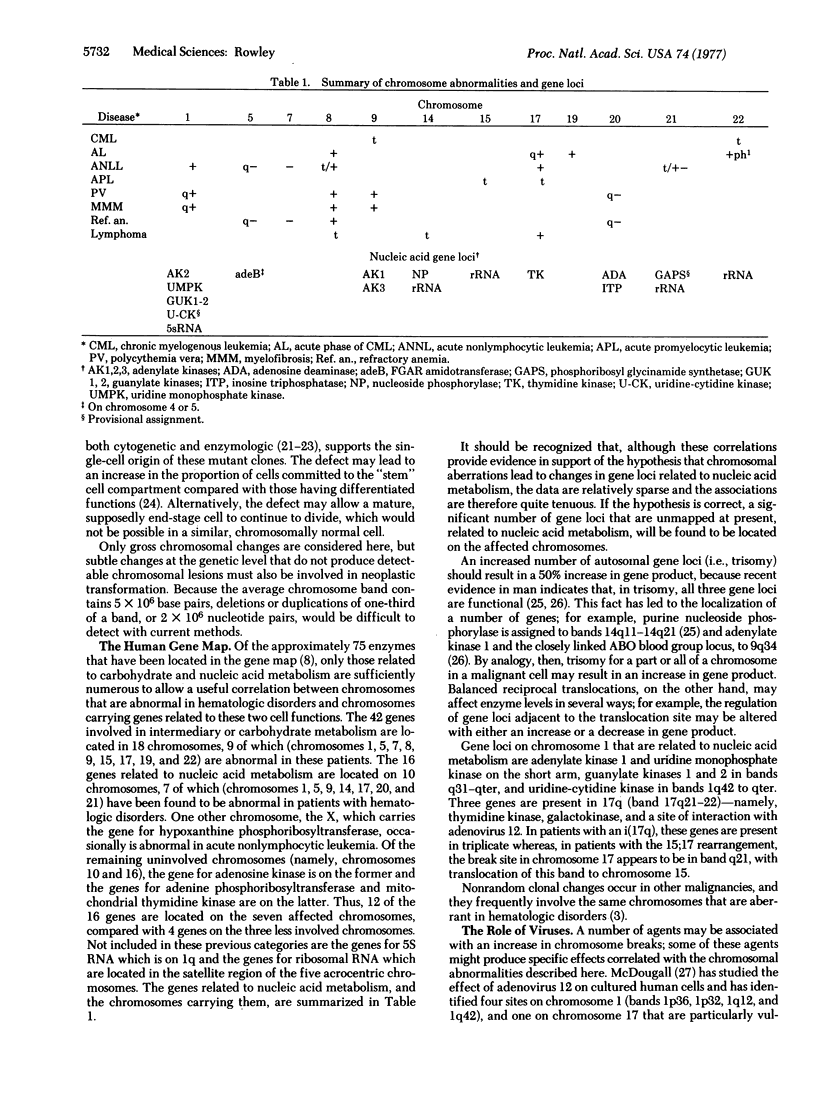

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azumi J. I., Sachs L. Chromosome mapping of the genes that control differentiation and malignancy in myeloid leukemic cells. Proc Natl Acad Sci U S A. 1977 Jan;74(1):253–257. doi: 10.1073/pnas.74.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brynes R. K., Golomb H. M., Desser R. K., Recant W., Reese C., Rowley J. Acute monocytic leukemia. Cytologic, histologic, cytochemical, ultrastructural, and cytogenetic observations. Am J Clin Pathol. 1976 Apr;65(4):471–482. doi: 10.1093/ajcp/65.4.471. [DOI] [PubMed] [Google Scholar]
- Caspersson T., Zech L., Johansson C., Modest E. J. Identification of human chromosomes by DNA-binding fluorescent agents. Chromosoma. 1970;30(2):215–227. doi: 10.1007/BF00282002. [DOI] [PubMed] [Google Scholar]
- Codish S. D., Paul B. Reversible appearance of a specific chromosome which suppresses malignancy. Nature. 1974 Dec 13;252(5484):610–612. doi: 10.1038/252610a0. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Koprowski H. Assignment of gene(s) for cell transformation to human chromosome 7 carrying the simian virus 40 genome. Proc Natl Acad Sci U S A. 1975 May;72(5):1658–1660. doi: 10.1073/pnas.72.5.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPaolo J. A., Popescu N. C. Relationship of chromosome changes to neoplastic cell transformation. Am J Pathol. 1976 Dec;85(3):709–738. [PMC free article] [PubMed] [Google Scholar]
- Engel E., McKee L. C., Flexner J. M., McGee B. J. 17 long arm isochromosome. A common anomaly in malignat blood disorders. Ann Genet. 1975 Mar;18(1):56–60. [PubMed] [Google Scholar]
- Ferguson-Smith M. A., Aitken D. A., Turleau C., de Grouchy J. Localisation of the human ABO: Np-1: AK-1 linkage group by regional assignment of AK-1 to 9q34. Hum Genet. 1976 Sep 10;34(1):35–43. doi: 10.1007/BF00284432. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J., Martin G. M., Klein G., Clifford P., Singh S. Evidence for a clonal origin of head and neck tumors. Int J Cancer. 1972 Jan 15;9(1):133–142. doi: 10.1002/ijc.2910090116. [DOI] [PubMed] [Google Scholar]
- Gahrton G., Lindsten J., Zech L. Clonal origin of the Philadelphia chromosome from either the paternal or the maternal chromosome number 22. Blood. 1974 Jun;43(6):837–840. [PubMed] [Google Scholar]
- George D. L., Francke U. Gene dose effect: regional mapping of human nuceloside phosphorylase on chromosome 14. Science. 1976 Nov 19;194(4267):851–852. doi: 10.1126/science.824731. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Gallo R. C. RNA processing and RNA tumor virus origin and evolution. Science. 1975 May 23;188(4190):802–811. doi: 10.1126/science.47650. [DOI] [PubMed] [Google Scholar]
- Kaiser-McCaw B., Epstein A. L., Kaplan H. S., Hecht F. Chromosome 14 translocation in African and North American Burkitt's lymphoma;. Int J Cancer. 1977 Apr 15;19(4):482–486. doi: 10.1002/ijc.2910190408. [DOI] [PubMed] [Google Scholar]
- Klein G., Bregula U., Wiener F., Harris H. The analysis of malignancy by cell fusion. I. Hybrids between tumour cells and L cell derivatives. J Cell Sci. 1971 May;8(3):659–672. doi: 10.1242/jcs.8.3.659. [DOI] [PubMed] [Google Scholar]
- Linder D., Gartler S. M. Glucose-6-phosphate dehydrogenase mosaicism: utilization as a cell marker in the study of leiomyomas. Science. 1965 Oct 1;150(3692):67–69. doi: 10.1126/science.150.3692.67. [DOI] [PubMed] [Google Scholar]
- McCaw B. K., Hecht F., Harnden D. G., Teplitz R. L. Somatic rearrangement of chromosome 14 in human lymphocytes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2071–2075. doi: 10.1073/pnas.72.6.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall J. K. Adenovirus-induced chromosome aberrations in human cells. J Gen Virol. 1971 Jul;12(1):43–51. doi: 10.1099/0022-1317-12-1-43. [DOI] [PubMed] [Google Scholar]
- McDougall J. K., Kucherlapati R., Ruddle F. H. Localization and induction of the human thymidine kinase gene by adenovirus 12. Nat New Biol. 1973 Oct 10;245(145):172–175. doi: 10.1038/newbio245172a0. [DOI] [PubMed] [Google Scholar]
- McKusick V. A., Ruddle F. H. The status of the gene map of the human chromosomes. Science. 1977 Apr 22;196(4288):390–405. doi: 10.1126/science.850784. [DOI] [PubMed] [Google Scholar]
- Mitelman F., Levan G. Clustering of aberrations to specific chromosomes in human neoplasms. II. A survey of 287 neoplasms. Hereditas. 1976 Jun 14;82(2):167–174. doi: 10.1111/j.1601-5223.1976.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Nowell P. C. Preleukemia. Cytogenetic clues in some confusing disorders. Am J Pathol. 1977 Nov;89(2):459–476. [PMC free article] [PubMed] [Google Scholar]
- Rowley J. D., Golomb H. M., Dougherty C. 15/17 translocation, a consistent chromosomal change in acute promyelocytic leukaemia. Lancet. 1977 Mar 5;1(8010):549–550. doi: 10.1016/s0140-6736(77)91415-5. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Nonrandom chromosomal abnormalities in hematologic disorders of man. Proc Natl Acad Sci U S A. 1975 Jan;72(1):152–156. doi: 10.1073/pnas.72.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley J. D., Potter D. Chromosomal banding patterns in acute nonlymphocytic leukemia. Blood. 1976 May;47(5):705–721. [PubMed] [Google Scholar]
- Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971 Oct 30;2(7731):971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Sumner A. T., Evans H. J., Buckland R. A. New technique for distinguishing between human chromosomes. Nat New Biol. 1971 Jul 7;232(27):31–32. doi: 10.1038/newbio232031a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Hayashi M., Rabinowitz Z., Sachs L. Chromosomal control of malignancy in tumours from cells transformed by polyoma virus. Int J Cancer. 1973 May;11(3):555–566. doi: 10.1002/ijc.2910110307. [DOI] [PubMed] [Google Scholar]
- Zech L., Haglund U., Nilsson K., Klein G. Characteristic chromosomal abnormalities in biopsies and lymphoid-cell lines from patients with Burkitt and non-Burkitt lymphomas. Int J Cancer. 1976 Jan 15;17(1):47–56. doi: 10.1002/ijc.2910170108. [DOI] [PubMed] [Google Scholar]