Abstract
A historical perspective on estradiol’s enhancement of cognitive function is presented, and research, primarily in animals, but also in humans, is reviewed. Data regarding the mechanisms underlying the enhancements are discussed. Newer studies showing rapid effects of estradiol on consolidation of memory through membrane interactions and activation of inter-cellular signaling pathways are reviewed as well as studies focused on traditional genomic mechanisms. Recent demonstrations of intra-neuronal estradiol synthesis and possible actions as a neurosteroid to promote memory are discussed. This information is applied to the critical issue of the current lack of effective hormonal (or other) treatments for cognitive decline associated with menopause and aging. Finally, the critical period hypothesis for estradiol effects is discussed along with novel strategies for hormone/drug development. Overall, the historical record documents that estradiol positively impacts some aspects of cognitive function, but effective therapeutic interventions using this hormone have yet to be realized.
Keywords: Aging, Cognition, Estradiol, Hormone replacement therapy, Memory, Estrogen receptors
Introduction
Evidence, accumulated over the past 25 years, shows that estradiol modulates cognitive function in animals and humans. Modulation begins in utero when estrogens direct sexual differentiation of various brain regions controlling reproduction and some cognitive functions. Gonadal hormone influences on the nervous system, including both estrogens and androgens, continue through adolescence and reach their zenith at adulthood when gonadal hormone secretions reach the highest levels. With aging, levels of circulating estrogens plummet and appear to contribute to age-related declines in learning and memory function. These hormone-dependent effects on cognition over the lifespan have been attributed to classic, genomic mechanisms — hormone binding to specific receptors, alterations in gene transcription and the initiation of organ-specific effects in target areas in the brain and peripheral organs. More recently, it has also been established that gonadal hormones, including estradiol, can act at membrane receptors to activate intra-cellular signaling mechanisms which alter cellular function. Moreover, there is increasing documentation that gonadal hormones are neurosteroids, that is, estrogens and androgens are synthesized locally in brain areas and thereby rapidly alter cognition and other neural functions. The physiological functions and implications for this mode of estrogen action are unknown at this time but present a novel area for investigation. Finally, in a more global way, estradiol also acts indirectly to maintain cognitive functions because it exerts neurotrophic effects within the brain. Thus, gonadal hormones, estradiol in particular, have a rich repertoire of possible mechanisms for influencing cognition.
This review provides a brief overview of studies establishing estrogen’s role in promoting cognition primarily in animal models but also in humans, and it discusses some data regarding the mechanisms underlying these influences. Newer studies demonstrating how estrogens rapidly alter learning and memory are discussed. The need for exploiting this information is emphasized as it may provide an entirely novel area for therapeutic interventions for memory loss. The failure of current hormone replacement therapy in post-menopausal women is used to highlight the importance of implementing basic research combined with translational studies in order to develop new treatments and strategies for memory loss.
Overall, gonadal hormones exert diverse and multi-dimensional influences on some aspects of cognition which are pervasive throughout the lifespan and provide fundamental regulation over higher order neural function, i.e., the intellectual/cognitive realms of life. Accumulating, but still controversial, evidence suggests that estradiol, synthesized within the hippocampus and prefrontal cortex, may also contribute to the normal process(es) of memory consolidation. Since the review covers such widespread topics, each cannot be discussed in depth, but the reader is directed to current reviews. The review concludes that research on estrogens which act as potent hormones, neurosteroids and neuromodulators should be expanded in order to take advantage of already demonstrated cognitive enhancements, to extend positive hormonal effects throughout the entire lifespan and to determine how estrogens may be of value in mitigating neurodegenerative diseases. More provocatively, since intra-neuronally derived estrogens may be involved in the normal process(es) of memory consolidation, it is argued that further research may lead to a better understanding of the fundamental processes of learning and memory. The path for achieving these goals is to forge a strong and more informative translation from animal to human studies and to integrate newer information on estradiol’s neurosteroid and neuromodulator effects with its traditional genomic effects in order to develop new target drugs. While enormous progress has been made in the last twenty-five years, the breadth and depth of new developments concerning neural mechanisms for estradiol action suggest that researchers will continue to be engaged in this area of neuroendocrine research for the near and far future.
Defining cognition
Cognitive function, put in its most basic context, is the ability to learn, retain, and recall information. In humans, it also represents a complex, multidimensional set of intellectual functions like judgment and evaluation. Thus, in a broader context, cognition includes all mental abilities and processes related to knowledge including, but not limited to, attention, memory, reasoning, comprehension and language production. Since this review focuses primarily on animals, but also includes human research, cognition is used in its more basic context. In addition, since effects of the gonadal steroid, estradiol, are considered, learning and memory (information recall) is the cognitive function which comprises much of the discussion because it has been studied the most in relation to estrogen’s effects. Nonetheless, because effects of estradiol have been demonstrated in a number of cognitive domains in humans and in a number of different learning and memory paradigms in animals, cognition or cognitive function will be used for general discussion. It should be noted that such usage does not indicate that estradiol influences all aspects of cognitive functioning. Moreover, some effects of estradiol may be mediated by non-cognitive processes (see Dohanich, 2002; Luine and Dohanich, 2008; Luine, in press and the section Adult cognition for further discussion of these issues). When specific studies and experiments are described, the exact cognitive process investigated and effects found will be given.
Areas and mechanisms for gonadal hormone action
In 1998, Hormones and Behavior devoted an entire issue to the topic of Estrogen Effects on Cognition Across The Lifespan (Hormones and Behavior 34, 1998) which gave credence to the newly developing concept that hormones, especially estrogens, could modulate cognitive function. Historically, estradiol had been recognized to act within the brain to regulate sexual behavior and ovulation in the late sixties (Everett, 1965; Gorski, 1971), and this research area contributed greatly in the founding of the journal. Currently, the role of gonadal hormones in brain functioning has expanded, and many higher order brain functions such as mood, psychiatric disorders and some social behaviors, as well as cognition are now known to be impacted (Watson et al., 2010; Gillies and McArthue, 2010). Thus, hormonal influences on cognition no longer seem unusual, and, indeed, gonadal hormone effects on neural functions are pervasive.
Cognitive effects of estradiol are mediated at sites and/or neural systems in the cerebral cortex, basal forebrain, hippocampus and striatum that regulate higher order neural function. The most investigated areas for memory in general, and for hormonal effects on memory specifically, are the medial prefrontal cortex and hippocampus. Cognitive effects of estradiol, similar to effects on reproduction, are mediated through binding to classical nuclear receptors which are found in two distinct forms, estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ), in these brain areas (Fig. 1). Both receptors are ligand dependent transcription factors and through interactions at specific sites on DNA (EREs, estrogen response elements), initiate a cascade of intra-cellular reactions that alter protein synthesis and culminate in the unique physiological responses of each estrogen target tissue. These genomic actions generally result in long lasting and sustained effects on neural function and most likely underlie estrogen effects during development when sex-dependent functions and sex differences in neural functions are programmed, although recent studies indicate that epigenetic mechanisms may also contribute to sexual differentiation (McCarthy and Nugent, 2013). At adulthood, functional changes which occur during the menstrual and estrous cycles, pregnancy, menopause and aging are also dependent on genomic mechanisms. While changes in cognition appear to depend on genomic mechanisms, current research suggests that other mechanisms such as binding to membrane receptors may also be important. This line of work began with the demonstration that estrogen receptors were present outside of the cell nucleus in membranes of cell bodies, spines and presynaptic terminals in a number of brain regions including the medial PFC and the hippocampus (Srivastava et al., 2013b). Binding of estrogens to membrane receptors initiates rapidly mediated hormonal effects (sec to min) through activation of signaling pathways (Fig. 2). Thus, estrogen can alter memory, and other functions, through mechanisms that are rapid or slow in onset.
Fig. 1.
Schematic of genomic mechanism for enhancing memory by estradiol. Circulating estradiol enters the cell nucleus where it can bind to two types of receptors, ERα or ERβ. The complexes act as nuclear transcription factors by binding to an ERE (estrogen response element) and stimulating gene transcription which leads to increases in cellular proteins that, by increasing neural transmission and function, enhance cognitive function.
Fig. 2.
Schematic of rapid membrane receptor mediated mechanisms for enhancing memory by estradiol. Circulating estradiol can bind to various receptors in the cell membrane. At this time, the exact receptors and details of the binding have not been described, but evidence for activation of the pathways shown have been obtained (see text for details and references). Binding to the receptors results in activation of protein kinases which phosphorylate other proteins. These proteins then cause increases in synthesis of specific proteins through either phosphorylation of the transcription factor cAMP-response element-binding protein (CREB) located on DNA in the cell nucleus or through mRNAs located in the cytoplasm in dendrites or other areas. These actions result in structural changes at existing synapses as well as synaptogenesis that enhance memory formation, consolidation, storage and retrieval. Abbreviations: ERK, extracellular signal regulated kinase; PKA, cAMP-dependent protein kinase; P13K/Akt, phosphatidylinositol3-kinase/Akt pathway; m pathway; mTOR, targets of rapamycin. Schematic adapted from Frick (2012) and Giese and Mizuno (2013).
Developmental effects of gonadal hormones on cognition
Animal studies
Influences of gonadal hormones on cognition begin during in utero development when the testes of males actively secrete testosterone, which directly masculinizes the male external genitalia. In the central nervous system, testosterone is aromatized to estradiol, and it is this locally produced estradiol which programs the expression of male-like behaviors including, most importantly, sexual behavior. Estradiol also underlies the expression of some non-sexual behaviors like aggression, activity and motor abilities and some aspects of learning and memory (Watson et al., 2010; Gillies and McArthue, 2010). These organizational events by estradiol leave long lasting imprints which are manifested in sex differences at adulthood and which in some (but not all) cases can be additionally modulated by circulating estrogens and androgens. Sex behaviors are strikingly different between the sexes: rodent males exhibit mounting and little lordosis while rodent females exhibit lordosis and little mounting. Sex differences in non-sexual behaviors, including cognition can be less dimorphic/different, but male rodents outperform females on tasks requiring spatial memory, the radial arm maze (Williams et al., 1990; Luine and Rodriguez, 1994) Morris water maze and object placement (Beck and Luine, 2002; Bisagno et al., 2003). Fig. 3 illustrates this sex difference: on the radial arm, maze female mice make approximately twice as many errors as males in completing the task, and in object placement, female rats significantly discriminate only at a one hour inter-trial delay while males discriminate with 1–4 h inter-trial delays.
Fig. 3.
Sex differences in spatial memory tasks. A. Radial arm maze – Bars show the total errors made in completion of the task by adult male and female mice. **P < 0.01, Student’s t-test. Data from an unpublished study by Kneavel, Christakos and Luine. B. Object placement task-Bars represent the mean time ± SEM exploring the object at the old and new locations for male and female rats in the recognition trial (T2) at inter-trial delays of 1,2, and 4 h. ANOVA showed a significant difference in time spent at the locations and a significant interaction, sex × object. **P < 0.01, **P < 0.01, *P < 0.05 (paired t-test) within each group. Data adapted from Bisagno et al. (2003).
Sex differences are also prominent in the strategies by which cognitive tasks are accomplished in rodents. For example, in learning to obtain rewards on the T-maze, two strategies can be utilized. One, a place or spatial strategy, involves learning to go to a specific place (arm), while a response strategy, which involves always turning the same direction, can also be used. Korol et al. (2004) reported that female rats were more likely to use a place strategy (but could alter strategy based on estrus cycle state) while Packard and McGaugh (1996) reported that 90% of male rats use a response strategy. In solving the spatial memory task, radial arm maze, female and male rats also often apply different strategies which are reflected in males having a faster rate and accuracy in learning the task, but over time both sexes reach the same level of performance (Williams and Meck, 1991; Tropp and Markus, 2001). Females appear to construct a detailed cognitive map of all cues around the maze which makes acquisition of the task slower but allows for sustained, accurate performance even when cues are removed. Males, on the other hand, map only a few cues and apply a “vectoring approach,” use of the geometric properties of the shape of the environment in relation to only a few cues. Hence males rapidly map and acquire the task, but male performance plummets when the environment is even minimally altered. Similar strategy differences between the sexes and effects of hormones have also been noted in the water maze (Korol, 2004; Daniel and Lee, 2004).
An important question, which has not been widely investigated in relation to these sex differences in cognition, is whether the differences are due to gonadal hormones acting during development (organizing/programming effect) to establish long lasting neuroanatomical, chemical and/or functional sex differences. Alternatively, sex differences in cognitive function may arise from differences in circulating hormones in the sexes that act on neurons to influence their function. In addition, the difference may depend on both organizational and activational effects of hormones, and genes on the Y chromosome may also contribute. Little research has addressed these issues but Williams and colleagues investigated the influence of neonatal castration and estradiol treatment to females on radial maze performance and found that the treatments generally mimicked the demonstrated sex differences of adult rats (Williams et al., 1990; Williams and Meck, 1991). Neonatal exposure to testosterone also modifies sex differences in radial arm and Morris water maze performance and hippocampal morphology (Roof, 1993; Isgor and Sengelaub, 1998). Thus, results suggest a strong organizing effect of estradiol, derived from testosterone, on adult sex differences in the acquisition and performance of spatial memory tasks.
Human studies
Some gender differences in cognition are also apparent. A similar pattern as in animals exists where most men outperform most women in tasks requiring spatial contexts (Hampson, 1990). On the other hand, most women are superior to men in aspects of verbal abilities including spelling, grammar, and rate of speech acquisition (Hampson, 2002). The same relationship, females superior to men, exists in tests of perceptual speed and accuracy, and women also exhibit better short-term memory (Hampson, 2002). Such tasks cannot be assessed in rodents, and non-human primates have not been investigated. Like rodents, it is unknown whether the differences are due to organizational or activational hormone effects. Gender differences in spatial abilities are present in children (Kerns and Berenbaum, 1991) and become larger at puberty (Voyer et al., 1995) suggesting both kinds of hormonal influences. Women with Congenital Adrenal Hyperplasia (CAH), a condition where testosterone is produced in large amounts by the adrenal glands beginning in utero, provide an opportunity to assess organizational effects of testosterone and estradiol (derived from the testosterone) on cognitive function (activational effects of hormones cannot usually be assessed because medical treatments effectively lower androgens). Some, but not all, studies suggest that CAH women develop a more male-typical cognitive pattern, especially with regard to spatial memory, and this observation thus provides some evidence for perinatal organizing effects on cognition in humans (Malouf et al., 2006; Mueller et al., 2008; see Berenbaum and Beltz, 2011 for a review).
Sex differences in cognitive function in both rodents and human are usually small in magnitude, with the largest differences usually constituting a little more than one standard deviation unit, and some studies have failed to demonstrate such differences. Thus, while an “average” female may score higher than an “average” male on a particular test, there is considerable overlap in performance, and some men will score higher than some women.
An important aspect of the programming of sex differences during development is that these differences may contribute to the development and patterns of adult cognitive loss seen in some neurodegenerative and psychiatric diseases. Females have more Alzheimer’s disease and less Parkinson’s disease than males and develop Parkinson’s later than males. Schizophrenia develops earlier in males, and males present with more negative symptoms and possibly more cognitive deficits than females (Gillies and McArthue, 2010). Sex differences in these diseases as well as responses to drug treatments have initiated the gender medicine movement. Gender medicine posits that we need to do away with the assumption that men and women are similarly affected by diseases and treatments. Further emphasizing the importance of sex in biological systems, the National Institutes of Health (N.I.H.) has recently unveiled policies to ensure that preclinical research funded by the US NIH includes both females and males (Clayton and Collins, 2014). Clearly, most current research does not include both sexes, and we have only scratched the surface on the extent to which sex contributes to biological functions, especially cognition.
In conclusion, further studies of sex differences in cognition and the role of possible organizing effects of gonadal hormones on cognition are necessary to determine to determine the extent to which this period of development makes contributions to normal cognitive function and effects of neurodegenerative disease. It should also be noted that adolescence is a period where hormones may contribute to long lasting, sexually dimorphic patterns of behavioral performance (Schulz et al., 2009), and this period needs further investigation in relation to the setting down of sex-dependent, cognitive attributes. Answers to these questions are important because they could frame the type and nature of successful hormonal treatments given in adulthood.
Adult cognition
Introduction and caveats
Research conducted in many laboratories has documented cognitive changes in female rodent models in relation to gonadal hormone status, and these changes include effects related to ovariectomy, estrous cyclicity, pregnancy, multiparity, estrapause and aging (Dohanich, 2002; Luine, 2006, 2008; Luine and Frankfurt, 2012a,b). Studies presented in the 1998 issue and somewhat later utilized primarily spatial memory tasks such as radial arm maze, Morris water maze and T-maze to show that estradiol administration improved performance of ovariectomized rats (Luine and Rodriguez, 1994; Luine et al., 1998; Daniel et al., 1997; Dohanich et al., 1994; Dohanich, 2002). Differences in performance of tasks were also found across the estrous cycle (Frye et al., 2007; Walf et al., 2006; Frick and Berger-Sweeney, 2001; Frye, 1995; Warren and Juraska, 1997). It should be emphasized that reported changes are small and not all studies reported positive effects, but at this time, the preponderance of studies report enhancements of learning and memory by estradiol. It is also important to note whether studies examined learning vs memory as differential effects of estrogens have been reported on these aspects of cognition (Dohanich, 2002). Many issues have contributed to reported failures of estradiol in enhancing cognitive function. It is important to review these issues because “failures” contribute to the notion held by some scientists that estrogens do not exert modulation of cognition. In addition, knowledge of potential pitfalls is helpful in designing future, successful experiments.
Experimental design issues in assessing hormone effects
Type of “estrogen”
As indicated previously, a preponderance of studies show that gonadal hormones enhance cognitive function, but many studies report no or marginal effects. First, some confusion has arisen over the use of the general term estrogen(s), as compared to the specific hormone, 17β-estradiol (Blaustein, 2008). Several types of estrogens are synthesized and secreted by the ovaries, commonly referred to as E1 (estrone), E2 (17α and 17β-estradiol) and E3 (estriol). 17β-Estradiol is the most prevalent and most potent female gonadal hormone followed by estrone and estriol, at least as indicated by binding affinity to ERα and potency in inducing uterine growth (MacLusky, 1997; Folmar et al., 2002). Most animal studies utilize 17β-estradiol or 17β-estradiol–benzoate (EB). EB provides longer and more sustained increases in serum 17β-estradiol because the benzoate moiety protects estradiol from rapid degradation. Use of 17β-estradiol or EB leads to different effective doses and time courses for effects (Scharfman et al., 2007). Some studies have also used the synthetic estrogen, diethylstilbestrol (DES), which is more potent than any endogenous estrogen (MacLusky, 1997; Luine et al., 2003). Long term, sustained levels of estradiol can be achieved by use of Silastic capsules packed with pure hormone or diluted with cholesterol. Estradiol is slowly released, and constant levels can be maintained for one to six months (Brown et al., 1990; Rodgers et al., 2010). A rapidly metabolized, water-soluble form of estradiol which is encapsulated in 2-hydroxypropyl-β-cyclodextrin (estradiol–cyclodextrin) does not enter cells and can be used can be used for assessing effects of estradiol mediated through binding to membrane receptors (Frick, 2012).
Many studies in women have not utilized the above described estrogens but instead used the most commonly available and prescribed Hormone-Replacement Therapy (HRT) formulation, Premarin, which consists of Conjugated Equine Estrogens (CEE; trade name Premarins) (Hersh et al., 2004). These conjugated estrogens are primarily a mixture of sodium estrone sulfate and sodium equilin sulfate with other components in lesser amounts including sodium sulfate conjugates of 17α- and 17β-dihydroequilin, 17α-es-tradiol (isomeric form of estradiol which binds poorly to estrogen receptors) and trace amounts of 17β-estradiol. The presence of equilin compounds reflects that Premarins were originally obtained from the urine of pregnant horses. Currently, these estrogens are obtained from other natural sources. After absorption and metabolism of CEEs, the resulting primary circulating hormones are estrone and, after estrone’s conversion, 17β-estradiol, as well as equilin, but only the levels of estrone and equilin have been reported (Bhavnani, 2003; Sitruk-Ware, 2002). These three metabolites are hypothesized to be primarily responsible for the estrogenic effects of CEE (Sitruk-Ware, 2002), although there are other estrogens and related metabolites that could also exert effects (Kuhl, 2005). Most of these estrogenic compounds have not been extensively investigated in animals or humans, but effects of individual components as compared to CEE appear to differ as estrone impaired (Engler-Cjoirazzo, 2012), dehydroestrone enhanced and equiline had no effect on memory tasks in middle aged rats (Talboom et al., 2010) while low dose Premarin impaired learning and memory retention but medium and high doses enhanced it in middle aged rats (Engler-Ehiurazzi, 2011; see Acosta et al., 2013 for further discussion). In women with a uterus, Premarin (as well as estradiol) must be given with a progestin, such as Medroxyprogesterone acetate, because uterine cancer can develop with chronic estrogen treatment. As with Premarin, Medroxyprogesterone acetate is not an endogenous progestin. In general, fewer positive effects on cognition in women have been noted after CEE treatments as compared to treatment with estradiol itself (see the section Human studies and the critical period hypothesis). Unfortunately, some studies using CEE (referred to as estrogen replacement studies, ERT), which failed to see cognitive enhancements, were erroneously assumed to have used estradiol.
Treatment dosage and timing
The estradiol dose given is critical because many drug/hormone effects on cognition do not follow a traditional sigmoidal shaped dose–response curve but instead show an inverted U-shape with both lower and higher doses being ineffective or even inhibitory (Inagaki et al., 2010). Thus, experiments which include only one dose, or even two doses, of hormone may not show positive effects or even inhibitory effects. The duration, days vs weeks, or type of treatment, injection vs sustained release capsules, are important as different effects have been obtained, even on the same cognitive tests (Luine et al., 1998; Fernandez and Frick, 2004; Galea et al., 2001, 2002). The delay period between ovariectomy and the initiation of estrogen treatment in animal models (McLaughlin et al., 2008) or after menopause in humans also critically impacts on the effectiveness of estrogens (see the section Human studies and the critical period hypothesis). At present, it appears that the longer the interval between cessation of circulating gonadal hormones and initiation of treatment, the smaller the response, and this observation holds for both ovariectomy paradigms and naturally occurring estrapause in rats and may extend to women.
Life history
For animal studies, the life history of the subjects maybe important and can interact with or interfere with treatments. Environmental enrichment mitigates or obliterates some estrogenic effects on cognition (Daniel et al., 1999), especially in aged rodents (Gresack and Frick, 2004). Diet can contribute to effects in rodents because phytoestrogens (plant-derived estrogens with weak potencies) are present in rat chow. Long-term maintenance of ovariectomized rats on chow containing high phytoestrogen levels or treatment with phytoestrogens is associated with better performance of some memory tasks as compared to ovariectomized rats receiving chow with minimal phytoestrogens (Luine et al., 2006; Pan et al., 2010). Thus, ceiling effects could be present when environmentally enriched or phytoestrogen exposed rats receive chronic estrogen treatments. Dietary phytoestrogens may also be critical in aging studies since aged females have low circulating estradiol. Thus, feeding chow high in phytoestrogens may provide maintenance or enhancing effects of estrogens. Multi-parity, having had multiple pregnancy, birth and lactation bouts, mitigates age-related declines in spatial memory, and thus such subjects may also respond differently when given estradiol treatments as compared to age-matched virgins (Macbeth et al., 2008; Gatewood et al., 2005). Thus, many factors interact with hormonal effects and appear to minimize or obscure the effects and need to be taken into account when executing experiments.
Issues of life experiences have not been well addressed in women, but some studies show that women having had multiple pregnancies may show enhancements in certain aspects of memory (Macbeth and Luine, 2010). Moreover, some clinical data suggest that estrogen treatment may be most effective in women with less education (Mathews et al., 1996, 1999) or who show normal to below average verbal memory performance (Tierney et al., 2013) which effects may be similar to the ceiling effect of environmental enrichment in rats.
Inter-actions between the HPG and HPA axes
Further complicating studies on estrogen effects are possible interactions of the hypothalamic-pituitary-gonadal axis (HPG) with the hypothalamic-pituitary-adrenal (HPA) axis. The HPA is activated with stress and resultant release of corticosterone (in rats) or cortisol (in humans) can interfere or alter responses to gonadal hormones. In rats, depending on the intensity or duration of stress and the sex and age of the subject, memory can be impaired or enhanced (Luine et al., 2007). Interactions between the HPA and HPG axis are critical because many learning and memory tasks in animals rely on negative (shock or swimming until finding a hidden platform) or positive (food or water after privation) reinforcements which introduce stresses to the subjects. In this regard, recognition memory tasks, which engender little stress, show robust memory enhancements by estrogen in reports from a number of different laboratories (Luine, in press) while stress encountered during performance of some tasks which have stressful components such as the water maze, radial arm maze and avoidance paradigms may interfere with or override results (Beck and Luine, 1999; Englemann et al., 2006).
Indirect effects on psychological performance parameters
A final consideration is whether estradiol acts on mnemonic processes to enhance memory or whether the enhancements reflect indirect effects on psychological performance parameters. Hormones can influence performance parameters like affect (arousal, anxiety, mood, motivation), regulatory mechanisms (thirst, hunger, body weight, composition, temperature), sensory-perception (vision, audition, olfaction, gustation, touch, attention, proprioception, nociception) and motor ability (activity, balance, skill) (Luine and Dohanich, 2008). These performance parameters are present in both animal and human tests and could thereby enhance or impair performance. Human experiments usually include other instruments to assess these parameters and the extent to which they contribute to main effects, and thus, these complications are usually ruled out but could still remain as possible mediators of estrogenic effects. Accounting for performance variables in animal experiments is more difficult, but many investigators have tested them (Luine and Dohanich, 2008; Luine et al., 2003; Inagaki, 2010) or have utilized testing paradigms which preclude effects on performance parameters (Luine, in press; see the section Post-training trial injections and memory enhancement).
Does estradiol influence cognition?
Does the above list of potential caveats indicate that estrogenic influences on cognition may occur only in circumscribed situations and be only marginal in real time? Data on this question is presented in the next section, but the reader is referred to Fig. 4 where performance on the spatial learning and memory task, eight arm radial arm maze, is shown in adult male and female rats, adults receiving chronic stress and aged (25 months old) rats. Sex differences are expected, and they are obvious; males, regardless of age, perform better than females. The response to stress is different in the sexes; males are impaired after stress while females perform better. Moreover, it is overwhelmingly clear that old female rats are severely compromised in their cognitive abilities as compared to all groups. Thus, both gonadal and adrenal hormones appear to impact cognition, and old female rats, with very low circulating levels of estrogens, are severely compromised. Whether lack of estrogen is a main contributor to age-related learning and memory losses in females is unknown, but this figure certainly begs the question. It is also known that spatial reference memory on the Morris water maze declines remarkably faster with aging in female rats, 12 months old, than male rats, 18 months old (Markowska, 1999) or in female mice, 17 months old, than male mice, 18 months old (Frick et al., 2000).
Fig. 4.
Radial arm maze performance in rats across sex, age and after stress. Bars are the average ± SEM of the choice where subjects made the first mistake (Larger numbers indicate better performance). Young males and females (2–3 months) served as controls or received daily restraint for 21 days. Old rats were 22–24 months old. Chronic stress impairs young male performance but enhances female performance. Both aged males and females are impaired as compared to young controls, and aged females show the worst performance of all the groups. Data are combined form a number of studies and are for illustrative purposes only as no statistical tests were performed. See the original studies for details and statistical analyses.
Data from Luine and Rodriguez (1994), Luine et al. (1990, 1994), and Bowman et al. (2001). Reprinted by permission from Luine (2006).
Estradiol enhancements of cognition — chronic effects
Animal studies
The results of extensive research, using a variety of learning and memory tasks, show that estradiol enhances cognition (Dohanich, 2002; Frick, 2009; Luine, 2008). The balance of studies show estrogen dependent enhancements in performance during the acquisition phase (Daniel et al., 1997; Luine et al., 1998; Luine and Rodriguez, 1994), but when subjects have acquired (learned) how to complete/solve the tasks, estradiol may no longer enhance performance (Fader et al., 1998, 1999; Luine et al., 1998). Effects of estradiol on response learning, mediated by the striatum, have not been well studied, but it may impair this type of learning in females (Zurkovsky et al., 2011; Davis et al., 2005).
When memory is assessed in spatial and non-spatial tasks (using and remembering within trial information), consistent enhancements by estradiol are seen (reviewed in Luine, 2008). An example of estradiol’s ability to enhance memory is shown in Fig. 5 where two recognition memory tasks were evaluated, one is object recognition and the other is object place recognition. In both tasks, rats explore two identical objects in an open field in the training or sampling trial, and then after an inter-trial delay of 4 h, a new object replaces one of the objects (object recognition) or a one object is moved to a new location (object placement). If rats spend more time exploring the new object or the object in the new location, it is considered that they remember the old object/location. We gave estradiol benzoate (EB) or vehicle to ovariectomized rats via SC injection (50 μg/kg) for two days and assessed object recognition or object placement memory 48 h after the last estradiol injection (Jacome et al., 2010). As shown in Fig. 5A, treatments did not alter exploration of objects in the sample trial. The lack of differences in exploring the objects suggests that estradiol did not alter the psychological performance parameters of overall activity, anxiety for object exploration or novelty seeking behavior. In contrast, during the recognition trials (Fig. 5B), estradiol treated rats had significantly higher exploration ratios than vehicle treated rats, approximately 0.70 vs. 0.50 (exploration ratio is time exploring new/time exploring old + new so a higher ratio indicates better memory). Thus, estradiol treatment appeared to enhance memory. Nonetheless, it might still be argued that changes in performance parameters may have contributed. We therefore tested the same estradiol treatment regimen for activity and anxiety effects on the open field and for anxiety effects on the elevated plus maze. No differences between vehicle and estradiol treated rats were noted suggesting that estradiol, at least at the dose and duration given, enhanced memory (Jacome et al., 2010). Localized infusion of estradiol directly into the hippocampus is sufficient to enhance both recognition memory (Bouleware, 2013) and spatial memory in the water maze (Packard, 1998) which also argues against effects on non-mnemonic parameters.
Fig. 5.
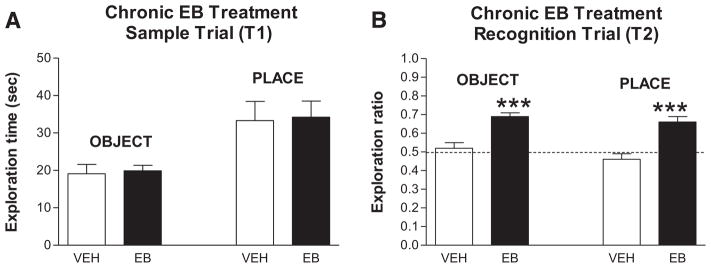
Effects of chronic estradiol on recognition memory in rats. A. Sample Trial (T1) — Exploration times ± SEM for object and place tests are shown in vehicle- and EB-treated subjects. No significant differences. B. Recognition trial (T2) — Trial was given 4 h post-T1 and entries are the ratios (new/old + new) of time spent exploring each object or objects in each location for vehicle- and EB-treated subjects. Dotted line at 0.5 indicates spending the same amount of time exploring new and old objects or locations. ***P < 0.001 (paired t-test within each group of old vs new).
Figure reprinted with permission from Neurobiol. Learn. Mem. (Jacome et al., 2010).
Chronic effects of estrogens appear to occur mainly through genomic mediation, and the receptor(s) mediating the changes in recognition memory were assessed using the ERα specific agonist PPT and the ERβ specific agonists DPN and C19. Given in the same regimen as estradiol, only the ERβ agonists enhanced object recognition and object placement (Jacome et al., 2010). Similar results have been reported by others (Liu et al., 2008; Walf et al., 2008); however, some other studies indicate that both receptors may mediate changes in memory (Frye et al., 2007; Hammond et al., 2009; Boulware, 2013). Thus, it remains unclear at this time whether estrogen binding to one or both of the estrogen receptors mediate chronic changes in memory function.
Consistent with estradiol’s enhancing effects in ovariectomized females, ovariectomy is associated with a loss in the ability to significantly discriminate in recognition memory tasks (Wallace et al., 2006). Interestingly, object recognition is affected before object placement, 1 vs 4 weeks following ovariectomy which may reflect differences in amount or kind of estrogen receptors in the PFC vs the hippocampus or the ability of the hippocampus to synthesize estrogens in order to maintain memory function (see the section Synthesis of estradiol within brain areas).
Human studies
Effects of estrogens in humans generally reflect effects seen in animals but are less consistent. Assessed over the menstrual cycle, women do better on cognitive tests favoring females (verbal fluency, fine motor skills, perceptual speed) when estrogens are high but perform better on cognitive skills favoring males, spatial ability, when estrogens are low (Hampson, 1990; Hampson and Morley, 2013; reviewed in Sherwin, 2012). A recent study in young, cycling women (Hampson and Morley, 2013) showed that when their salivary estradiol levels were high, 7.3 pmol/L, they had fewer working memory errors on a spatial working memory task than when their levels were low, 2.2 pmol/L (Fig. 6A). Moreover, salivary estradiol significantly correlated with the number of working memory errors (Fig. 6B). On the other hand, another recent study reported no changes in immediate or working memory, delayed recall, verbal learning or verbal fluency across the menstrual cycle (Mihalj et al., 2014). Still, postmenopausal women (with presumably low estradiol levels) performed significantly worse than pre-and perimenopausal women on delayed verbal memory tasks and significantly worse than perimenopausal women on phenomic verbal fluency tasks (Weber et al., 2013). In terms of estrogen treatment paradigms, young women with a surgical menopause who were treated with estradiol maintained performance on tests of verbal memory whereas scores decreased significantly in placebo treated women (Sherwin, 1988). Most estrogen treatment studies in females have been conducted after menopause and are discussed in the section Human studies and the critical period hypothesis.
Fig. 6.

Relationship of salivary estradiol to working memory errors in young women. A. The mean number of working memory errors (WME) observed on a spatial working memory task from Trial 1 to Trial 3 (T1—T3) and in the delay condition (Delay) for women tested at low estradiol (Group I, n = 18), women tested at high estradiol (Group II, n = 21), and male controls (Males, n = 31). Bars represent SEM. A. Scatterplot showing the association between salivary estradiol concentrations after log transformation to normalize the distribution and the total number of working memory errors on the spatial working memory task. With all females in the sample included (n = 39), a correlation of r = −.40 was found.
Figures reproduced with permission from Hampson and Morley (2013).
Estradiol enhancements of cognition — acute effects
Background
During the 1990s, evidence accumulated that receptor(s) for estrogens were also present outside of the cell nucleus in membranes of cell bodies and in axons, spines, presynaptic terminals, and near post-synaptic neurotransmitter receptors (Blaustein, 1992; Milner et al., 2001; Towart et al., 2003). Brain regions mediating reproductive function like the hypothalamus as well as regions mediating cognition, including the medial PFC and the hippocampus, were shown to contain these non-nuclear receptors. Thus, our lab and others began investigating whether estrogens might more rapidly activate sex behavior or learning and memory than in previous studies where injections of 17β-estradiol benzoate or Silastic capsules of 17β-estradiol were given for several days or weeks.
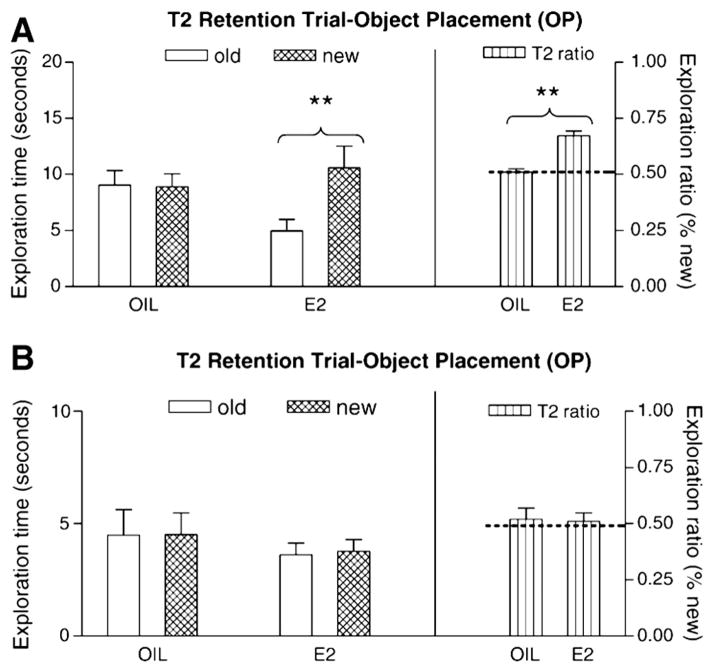
In initial studies of recognition memory, 17α- or 17β-estradiol (15 μg/kg) was given to ovariectomized subjects 30 min before exploration of objects in T1, and the T2 recognition/retention trial was given 4 h later (Luine et al., 2003). Ovariectomized rats did not significantly discriminate between the objects, but both 17α- and 17β-estradiol treated subjects significantly discriminated between the old and the new objects and the old and new locations (no differences in exploration were seen in T1). 17α-Estradiol binds to membrane receptors so it was not surprising that it also enhanced memory (MacLusky, 1997). DES also enhanced OR and OP in the same pre-T1 paradigm (Luine et al., 2003). Thus, a variety of estrogens rapidly enhanced recognition memory, 4.5 h after injection. Moreover, the effective doses after acute treatments were much lower than in studies where estrogens were given chronically, 50 μg/kg/day (Jacome et al., 2010) vs 15 μg/kg dose (Luine et al., 2003). Supporting these novel behavioral findings, inhibitory avoidance, a task relying on conditioned learning, was also enhanced by a similar dose (10 μg) of 17β-estradiol within 4 h (Rhodes and Frye, 2004).
Post-training trial injections and memory enhancement
In subsequent studies, we and others adopted a post-training protocol for assessing memory as advanced by McGaugh (1989). There are two important advantages for this protocol: psychological performance parameters and learning are largely eliminated. Thus, memory can be investigated. In this protocol, treatments are given after subjects acquire information such as searching for the submerged platform in water maze tasks or having explored objects in recognition tasks (T1 Trial). Following the acquisition or training trial, new information requires consolidation, and drugs or hormones could influence memory storage processes during this immediate period following training. Studies by the McGaugh laboratory showed that rats receiving injections of drugs like amphetamine or noradrenergic agonists had better retention from the training trial (1989). Moreover, the post-training injections were time dependent, only treatments given within 1–2 h following the training/sampling trials enhanced memory, and those given later in the inter-trial delay did not. The important attribute of post-training protocols is that enhancements after immediate, but not delayed, post-trial injections show that the enhancing effects on memory do not derive from non-mnemonic effects during learning since the treatments are given after the learning trial. Several labs, using post-training injections in recognition memory tasks, reported that estradiol enhances consolidation of memory when given immediately following T1 but not when given between 45 min and 2 h after T1, depending on the dose of estradiol and the inter-trial delay between T1 and T2 (Packard, 1998; Luine et al., 2003; Lewis et al., 2008; Fernandez et al., 2009; Inagaki et al., 2010, 2013; Jacome et al., 2010; Luine, in press).
Fig. 7 shows that S.C. 17-β-estradiol (20 μg/kg) enhances object placement when given immediately following the sample trial, but not when given 45 min after the sample trial. Thus, estradiol enhances consolidation of memory. The dose–response curves for post-T1 estrogen effects are inverted U curves which is consistent with other membrane mediated effects (Inagaki, 2010; Watson et al., 2010). Other studies show that immediate, post-sample trial enhancements of object recognition in ovariectomized mice are present 48 h following estradiol (see Frick, 2012). In addition, 17β-estradiol–cyclodextrin (does not pass through cell membranes) applied directly to the dorsal hippocampus immediately following T1 enhances object memory (Fernandez et al., 2008; Lewis et al., 2008) or place memory (Boulware et al., 2012) suggesting that estrogenic activation within the hippocampus is sufficient to enhance memory for objects or places in a post-sample trial paradigm. Packard et al. (1998) had earlier reported that post-training injections of estradiol, either IP or intra-hippocampal, enhanced Morris water maze performance 24 h later.
Fig. 7.
Immediate, but not delayed, post-sample trial estradiol injections enhance object placement memory. A. Injections of 20 ug/kg of 17β-estradiol immediately after T1 enhanced object placement memory 4 h later. Left panel: exploration times around objects during T1. Middle panel: time spent exploring objects at old and new locations during T2. Right panel: exploration ratios during T2. Dashed lines at 0.5 indicate chance level performance. Entries are means ± SEM. **P < 0.01. B. Injections of 17β-estradiol 45 min after T1 did not enhance memory. Panels as in A.
Figure reprinted by permission from Ingaki et al., 2010.
Thus, recent studies show that estrogens can rapidly enhance consolidation of memory in recognition memory tasks as well as in spatial memory dependent water mazes and inhibitory avoidance. These effects open whole new areas for investigation and are consistent with information that the hippocampus may synthesize estradiol de novo (see the section Synthesis of estradiol within brain areas). However, it is unknown whether such rapid effects of estrogens occur in women. Nonetheless, this mechanism of estradiol action may provide the basis for developing drugs that act as estrogens but not through classic, genomic mechanisms. This idea is further explored in the section Novel strategies for estradiol replacement/treatments.
Neural mechanisms for chronic and acute effects of estrogens
Background
Ample evidence exists that estrogens cause morphologic and neurochemical changes in many areas of the brain when given acutely or chronically to animals or humans. Chronic treatments alter the major neuronal systems of the brain including, but not limited to, cholinergic, monoaminergic, GABAergic and glutaminergic neurons in both animals and humans (Gibbs, 2010; McEwen and Alves, 1999). Cholinergic neural systems have been the most extensively investigated. Cholinergic cell bodies originate in the vertical and horizontal diagonal bands and n. basalis areas within the basal forebrain and project to the hippocampus and frontal cortex. On the behavioral side, estrogen treatments which enhance learning and memory in rodents are associated with increases in cholinergic neurochemicals, synthetic enzymes, receptors, or RNA for the chemicals (Luine, 1985; Dohanich et al., 1994; Daniel and Dohanich, 2001; Gibbs, 2010). Cholinergic antagonists also block or interfere with performance of learning and memory tasks and with estrogen’s enhancements in their performance in rodents and humans (Fader et al., 1998, 1999; Dohanich, 2002; Dumas et al., 2008, 2012). In humans, for example, post-menopausal women received three months of oral 17β-estradiol or placebo and then underwent a visual verbal N-backtest of working memory while undergoing functional MRI. Immediately prior to the session, subjects were administered the antimuscarinic drug scopolamine, the antinicotinic drug mecamylamine or placebo. Results showed that estradiol modulated antimuscarinic and antinicotinic-induced brain activity and provided further evidence that estradiol affects cholinergic system regulation of cognition-related brain activation (Dumas et al., 2012). Thus, as illustrated for the cholinergic system, chronic estradiol treatments up regulate neuronal systems which in turn then enhance cognition.
Dendritic spine density
Increasing evidence shows that the general mechanism(s) underlying learning and memory involve dendritic remodeling. In the hippocampus, the acquisition of new memories in a conditioning paradigm is associated with increases in dendritic spine density in the CA1 region of both male (Jedlicka et al., 2008; Leuner et al., 2003) and female rats (Beltran-Campos et al., 2011). In addition, existing spines undergo structural alterations that result in long term potentiation (Jedlicka et al., 2008; Morgado-Bernal, 2011). The functional implications of these alterations in spine density are best understood in the hippocampus because increases in dendritic spine density and/or spine sizes are associated with long term potentiation (Muller et al., 2000, LTP [81,86]. Overall, it appears that memories require alterations in dendritic spines that then alter LTP (Matsuzaki et al., 2011). In relation to estrogen effects, Vedder et al., 2013 recently showed that treatment for two days enhanced novel object recognition memory with the same time course as increases in CA1 LTP magnitude, and both effects required an increase in NMDA receptors. With regard to hippocampal spines and memory, it is notable that chronic treatment with estradiol was shown to increase hippocampal dendritic spine density 25 years ago (see McEwen and Alves, 1999 for review) and that it is now abundantly clear that dendritic spines are extremely responsive to both acute and chronic changes in circulating estrogen. For ex., fluctuations of approximately 30% in dendritic spine density on neurons in the ventromedial hypothalamus nucleus (important for female sexual behavior) (Frankfurt et al., 1990) and pyramidal cells in the CA1 region of the hippocampus occur during the 4–5 day estrous cycle (Woolley and McEwen, 1992). Similar results have been obtained in the monkey for CA1 (Hajszan and Leranth, 2010; Parducz et al., 2006) and the PFC (Tang et al., 2004; Hajszan et al., 2007) where estradiol has been shown to increase both dendritic spine and spine synapse density. Moreover, we have demonstrated that ovariectomized rats, treated with estradiol for two days, show enhanced place memory in an object placement task and have an approximately 10% higher spine density in basal CA1 dendritic spines than ovariectomized rats (Luine and Frankfurt, 2013). Li et al. (2004) reported that 1 μg of estradiol for 5 days, a dose which enhances object placement, also increases mushroom spine density in CA1 of ovariectomized mice. Velázquez-Zamora et al. (2012) showed that estradiol increased spine density in layer III of the PFC at the same dose which enhanced allocentric working memory in the Y-maze. Consistent with estradiol administration to ovariectomy rats enhancing memory, ovariectomy is associated with a significant decline in recognition memory (object recognition and placement) and 17–55% lower spine density in the PFC and CA1 as compared to intact rats (Wallace et al., 2006; Beltran-Campos et al., 2011). Aged rats showing impaired recognition memory in comparison with young rats have approximately 16% lower spine density in PFC and CA1 (Wallace et al., 2008; Luine et al., 2011). Thus, over the lifespan of female rats, both memory function and spine density in CA1 region of the hippocampus and the medial PFC fluctuate, but whether the dendritic changes are causal to the memory changes has not been demonstrated (see Luine and Frankfurt, 2012b for further discussion).
Rapid effects of estradiol on morphology and neurochemistry have also been demonstrated. Within 30–40 min after injection, estradiol increases post-synaptic synapse density by 62% (MacLusky et al., 2005) and spine density by 29% in basal dendritic spines in CA1 (Inagaki et al., 2012; Phan et al., 2012). Fig. 8 is a photomicrograph showing CA1 spine density in ovariectomized rats 30 min after receiving either vehicle or 20 μg estradiol. Increases in PFC spine density are also noted within the same time frame in both apical, 16%, and basal, 27%, trees (Inagaki et al., 2012) and in arcuate nucleus (Christensen et al., 2011). Changes in all areas are maintained for 4 h after estradiol administration. Khan et al. (2013) recently reported rapid increases in spines in the somatosensory cortex, an area believed to contribute to working memory, as well as the PFC. As indicated above, spine changes are at a dose and time when memory consolidation is known to occur (Luine and Frankfurt, 2012a). As with chronic estradiol treatments, whether relationships between the variables are causal requires further investigation.
Fig. 8.

Photomicrograph of estradiol effects on secondary basal dendrites in CA1. A. Spine density is shown at 30 min following injection of vehicle. Arrows indicate spines on the secondary basal dendrites at ×100. B. Spine density is shown at 30 min following injections of 20 ug/kg of 17β-estradiol. Arrows indicate spines on the secondary basal dendrites at ×100. Reprinted with permission from Inagaki et al. (2012).
Cell signaling
Our understanding of how the brain acquires, consolidates and retrieves information has advanced rapidly in the last twenty years. Several lines of evidence support the critical role of signaling cascades and protein kinases in the process (Giese and Mizuno, 2013 and see Fig. 2). There is evidence that estradiol activates many of these pathways. In the hippocampus, the initial event in activating intracellular signaling which is responsible for memory consolidation appears to be the activation of glutamate receptors (mGluR), primarily MGluR1. These events are responsible for triggering LTP induction and memory formation (see Sweat, 2010 for review). It has recently been shown that estradiol rapidly activates in vivo mGluR signaling in the PFC and hippocampus (Meitzen and Mermelstein, 2011; Khan et al., 2013; Boulware, 2013) and several kinases (see below). ERs have been identified on membranes of dendrites and spines, in presynaptic terminals and near post-synaptic receptors, and estradiol binding to these ERs initiates rapid activation of intracellular signaling cascades and immediate early genes which are critical for memory consolidation (Srivastava et al., 2013b). These effects are well described by in vitro and in vivo experiments, but the receptor(s) responsible are still unclear. Both ERα and ERβ have been localized to membranes in the hippocampus and PFC (Milner et al., 2001, 2005; Bean et al., 2014; Khan et al., 2013), and estradiol also binds to a G-Protein coupled receptor (GPR30) which is expressed at high levels in the hippocampus (Brailoiu et al., 2007). However, the role of this receptor remains unclear because rapid enhancements in recognition memory occur at levels of estradiol which are below its binding affinity for GPR30, and the binding affinity of various estrogens for GPR30 are not consistent with their effects on memory (Inagaki et al., 2012). However, GPR30 may collaborate with an ER to promote cell signaling (Langer et al., 2010).
In relation to the previous discussion of increased spines as a mechanism for enhancing memory consolidation, estrogen rapidly activates several signaling cascades involved in the formation of spines and in regulating spine/synapse size (McEwen et al., 2012; Srivastava, 2012; Kramar et al., 2013). Acting through membrane receptors, estradiol increases the phosphorylation of RAS/Raf, MEK, ERK and P13K pathways which in turn increase phosphorylation of the transcription factor cAMP response element-binding protein (CREB) (Fig. 2). CREB decreases transcription of Cofilin, which allows for increased polymerization of actin polymers for assembly of spines and for increased transcription of actin, and RhoA, which is necessary for filopodial extension and spine formation (Kramar et al., 2009; Luine and Frankfurt, 2012a,b). Phosphorylation of P13K also leads to increases in AkT and 4E-BP1 which enhances PSD-95 formation, an integral framework for synapse formation (McEwen et al., 2012). Enhanced ERK/MAPK signaling by estradiol may also enhance memory consolidation by activation of epigenetic processes such as histone acetylation and DNA methylation that enhance the expression of genes and synthesis of proteins (Fortress and Frick, 2014). Specifically, a recent in vivo study showed that estrogen treatment increased dendritic spine number in the PFC as well as spinophilin expression (Khan et al., 2013). Follow up in vitro studies showed that estradiol enhanced AMPA GluR1 receptor expression and excitatory glutamatergic synapse formation through increased ERK and Akt activation. Similar effects have been reported in the hippocampus using an in vivo approach; localized infusion of estradiol into CA1 enhanced recognition memory in an ERK dependent manner, and an mGluR1 antagonist blocked memory enhancements by estradiol, PPT (ERα agonist), or DPN (ERβ agonist) showing that ER/mGluR signaling can mediate estradiol enhanced memory consolidation in the hippocampus (Boulware et al., 2013). Other mechanisms for enhanced memory consolidation in the hippocampus include estradiol-dependent increases of brain derived neurotrophic factor (BDNF) through activation of an estrogen response element on the BDNF gene, or through interactions with BDNF’s membrane receptor (TrKB), or through other growth factor receptors in membranes (Luine and Frankfurt, 2013; Srivastava et al., 2013a). Growth factor receptor activation includes phosphorylation of P13K and AkT with activation of mTOR which can directly increase protein synthesis or interact with ERK to alter transcription (Luine and Frankfurt, 2013; Srivastava et al., 2013a). Elegant behavioral and molecular studies by the Frick lab (reviewed in Frick, 2012) have put these observations together to show that systemic or intra-hippocampal estradiol enhances object and place memory consolidation in rats and that this effect is associated with rapid activations of NMDA receptors and multiple cell signaling cascades.
Thus, ample molecular and morphological evidence shows that estradiol causes both acute and chronic changes in a number of brain areas important for cognition. Important questions remaining are whether these effects are similar across the lifespan, from development through adulthood and aging, or whether they change. Most of the above studies were conducted in adult rodents. Differences in how estrogen interacts with and alters neural function over the lifespan can have an enormous impact for well-being as discussed in the next sections.
Synthesis of estradiol within brain areas
Evidence has accumulated that significant concentrations of estradiol are produced locally in discrete regions of the brain by the enzyme aromatase from androgen precursors such as testosterone and/or directly from cholesterol and that these levels are higher than in the circulation. Recently, Kato et al. (2013) reported that the concentration of 17β-estradiol was 4 nM in the hippocampus of a female rat during proestrus while circulating concentrations were only 0.1 nM. In addition, estradiol remained present in the hippocampus following ovariectomy. The in situ production of estradiol indicates that it may act as a neurosteroid as well as a gonadal steroid, and some evidence shows that it may be a neuromodulator (Balthazart and Ball, 2006; Saldanha et al., 2011). This work opens up a completely new area of investigation concerning the role of locally synthesized estrogens in neural function. Brain-synthesized estradiol was first measured in birds and has been recognized as a mediator of neural functions in regions of a number of avian species (Boon et al., 2010; Saldanha et al., 2011). In addition, in vitro studies report that hippocampal estradiol synthesis is important for the maintenance of hippocampal spines, synapses and synaptic proteins in cultures from rats (Hojo et al., 2004), and a recent in vivo study showed that hippocampal spine density correlated with hippocampal estradiol concentrations over the estrus cycle (Kato et al., 2013). Aromatase has been detected in human brain via immunocytochemistry, gene expression and in vivo PET scan studies (Biegon et al., 2010), but whether neurally derived estradiol plays a role in development or in neuromodulation is unexplored.
An important question for the present review is whether neurally derived estrogens contribute to learning and memory. Support for this idea comes from earlier studies showing that stimulation of hippocampal slices with NMDA significantly increases estradiol synthesis (Hojo et al., 2004) and that forebrain estradiol levels in songbirds are rapidly regulated by voltage-gated calcium channels (Remage-Healey et al., 2011). Direct support for hippocampal derived estradiol contributing to memory came in a recent zebra finch study which showed that local inhibition of hippocampal aromatization impaired spatial memory performance in an ecologically valid food-finding task (Bailey et al., 2013). Neural estradiol may also contribute to sex differences in cognitive responses to stress. Several laboratories have shown that chronic stress impairs spatial memory in male, but not female, rats (Luine et al., 2007), an effect which was attributed to neuroprotective effects of estrogens which are present in greater amounts in females than males. Surprisingly, ovariectomized rats showed preserved spatial memory on either the radial arm or Y maze following chronic stress (Bowman et al., 2002; McLaughlin et al., 2005). The resilience post-ovariectomy was, at the time of these experiments, attributed to possible enduring, organizational effects of estradiol on neural function.
A recent study has provided evidence for the possibility that neural synthesis of estradiol may mediate the resilience of females to stress and has extended the observation to the PFC (Wei et al., 2014). Male, but not female, rats were impaired by chronic stress on a temporal order recognition memory (TORM) task mediated by the prefrontal cortex through glutamate receptors. When aromatase was blocked and the females were stressed, TORM was impaired suggesting that estradiol synthesized in the PFC conferred stress resilience on the females. Thus, work in the stress field provides some evidence that de novo brain estradiol may contribute to normal memory formation. This provocative idea was previously advanced by Frick (2012) who speculated that estradiol, which is synthesized and released in response to specific learning events in the hippocampus, may facilitate normal learning and memory processes. Since estradiol has now been reported to be some forty times higher in the female rat hippocampus than in the circulation (Kato et al., 2013), estradiol does appear to be a neurosteroid in search of a function(s). This concept is further discussed in the section Novel strategies for estradiol replacement/treatments.
Cognitive loss during normal aging
Background
With advancing age, most aspects of cognitive function suffer some degree of deterioration, ranging from modest declines in healthy animals and humans to devastating losses in people with neurodegenerative diseases associated with aging like Alzheimer and Parkinson disease (Frick, 2008). Ascribing a specific role for estrogens or other parameters to age-related cognitive loss is difficult because all factors are confounded by the aging process itself. Nonetheless, it is notable that in post-menopausal women, gonadal hormone levels plummet to near undetectable levels, and in rats, after a period of persistent diestrus, levels also decline to near undetectable. Thus, estradiol, which tends to promote cognitive function, is extremely low at advanced ages in females.
Animal Studies
Most aging studies have used male rats or mice, but the few studies done in females also show age-related performance declines. Approximately 21–24 month old female rats show impairments in relation to 2–4 month old young females on most spatial memory tasks including Y and T mazes (Aggleton et al., 1989), radial arm maze (Luine et al., 1990; see also Fig. 4), Morris water maze (Markowska et al., 1999; Veng et al., 2003), water radial arm maze (Bimonte et al., 2003), Barnes maze (Barrett et al., 2009) and object placement (Luine et al., 2011). Performance on the non-hippocampal dependent task, object recognition, also show declines with aging (Wallace et al., 2008). As indicated earlier, female rodents show age related declines in spatial memory tasks sooner than males (Markowska et al., 1999; Frick et al., 2000).
Measurement of spine densities in the PFC and hippocampus show that densities in aged female rats are 16% lower, as compared to young rats, in apical dendrites of the PFC and CA1 but not different in basal dendrites or in either dendritic tree of CA3 (Reviewed in Luine and Frankfurt, 2012b). This decline with aging is smaller than the decline in density following ovariectomy (Wallace et al., 2006). Small synapses, as compared to large synapses, appear to be lost with aging (Bloss et al., 2013). Overall, studies indicate that aging in female rats is accompanied by losses in memory abilities, neural spines, reorganization of synapses and lower estradiol levels. Whether there is a causal relationship among the variables still requires further analyses and investigation.
Human studies and the critical period hypothesis
During the 1980’s and 1990’s estradiol administration was shown in several observational studies to promote memory during aging and to possibly delay the onset and/or prevent Alzheimer’s disease. However, several larger and longer trials of HRT in women suffering from Alzheimer’s disease found no cognitive benefits, and this area of work is no longer being actively pursued (reviewed in Luine, 2008). With regard to normal aging and menopause, the eagerly anticipated, large scale, double-blind placebo controlled Women’s Health Initiative Study (WHI) conducted by the National Institutes of Health found that hormone therapy with CEE (not estradiol) (Espeland et al., 2004) or CEE plus MPA (Schumaker et al., 2003) given to women aged 65 years and older and at least 15 years past normal menopause, did not improve cognitive functioning and slightly increased the risk of dementia (reviewed in Luine, 2008; Frick, 2009). Thus, promotion of cognitive function by estradiol during aging was also not supported. However, some previous observational studies of menopausal women had shown that estradiol treatment was associated with better verbal memory, working memory, and visuospatial function, and with a lower risk of dementia, but these results were found particularly among women who had initiated treatment during, or soon after, the menopause (studies reviewed in Frick, 2009). In addition, randomized clinical trials with 17β-estradiol near the onset of menopause also showed beneficial effects of treatment on verbal and working memory (Joffe et al., 2006; Phillips and Sherwin, 1992; Viscoli et al., 2005; Wolf et al., 1999). Since women in the WHI trial were at least fifteen years past menopause when treatments were initiated, several investigators posited that a critical period or window of opportunity may exist for positive estrogen effects (Resnick and Henderson, 2002; Sherwin, 2012; Singh et al., 2013; Maki, 2013). Thus, in order for estrogens to exert positive effects on neural functioning, hormonal replacements must be given close to the initiation of menopause. In addition, some evidence indicated that even a short period of treatment, given around menopause, might confer long term benefits.
Some evidence for the critical period hypothesis in rodents was already extant (Gibbs, 2000), and several groups have provided new cognitive and neural evidence supporting the hypothesis (see Daniel, 2013 for review). In short, if rats receive estrogen replacements immediately after ovariectomy at middle age, even for only a short period, they show superior learning and memory in old age as compared to subjects ovariectomized at the same time but receiving no estrogen and show similar learning and memory abilities as ovariectomized rats that receive continuous estrogen replacement (Rodgers et al., 2010; Bohacek and Daniel, 2010; Kiss et al., 2012). This enduring enhancement of spatial memory in middle-aged rats by estradiol given for a short period after ovariectomy is illustrated in Fig. 9. The mechanism(s) responsible for this apparent change in responsivity to estradiol during the menopausal period is currently unknown. A simple answer is that continuous, unopposed estradiol (no progesterone) has different effects than naturally cycling effects, but no evidence for or against this idea has emerged. Another mechanism for this time limited estrogen effect is changes in ERα expression (Daniel, 2013; Schreihofer and Ma, 2013; Witty et al., 2012). Aged females show general decreases in this receptor with a specific 50% reduction in CA1 synapses which contain ERα immunoreactivity (Adams et al., 2002). Moreover, estrogen increases ERα levels in young but not aged rats (Adams, 2002), and, interestingly, long-term ovarian hormone deprivation in middle-aged rats attenuated the ability of subsequent estradiol treatment to increase levels of ERα (Bohacek and Daniel, 2009). Like the behavioral findings, only ten days of treatment initiated at the time of ovariectomy was sufficient to significantly increase levels of ERα in the hippocampus, but treatment initiated 5 months later was not. Yet, it is still unclear why lowered ERα levels would lead to unresponsivity to estrogens and/or negative effects (WHI trial). ERβ appears to act, in concert with estradiol, as a negative regulator of ERα-mediated transcription which may contribute to unresponsivity (Bean et al., 2014). Gene expression in the hippocampus after estradiol treatment to young as compared to middle-aged mice shows a different pattern of change which may also contribute to mechanisms underlying the critical period (Aenlle and Foster, 2010). Taking these observations together Foster (2012) indicates that an age-related shift in ERα/ERβ expression, combined with declining gonadal estradiol, may impact estrogen’s effects on transcription, cell signaling, neuroprotection, and neuronal growth leading to different effects than those which occur prior to aging. Also related to the concept of aberrant receptor function is the hypothesis that increased levels of ER polymorphisms with aging could contribute to negative effects of estradiol.
Fig. 9.
Estradiol treatment at middle age enhances memory in old age — evidence for the critical period hypothesis. Results show that the enhancing effects on memory of previous midlife estradiol treatment are comparable to ongoing estradiol treatment in aging ovariectomized rats. Middle-aged rats were ovariectomized and received implants resulting in continuous cholesterol control treatment (Cont Ch), continuous estradiol treatment (Cont E), or 40 days of prior exposure to estradiol treatment that was terminated before testing (Prior E). Rats were tested every other month on a working memory task in an eight-arm radial maze during which 1 min and 2.5 h delays were imposed between the 4th and 5th arm choices. Mean number of total retroactive errors (±SEM) averaged across delay trials one (A), three (B), five (C), and seven (D) months after termination of estradiol treatment in the Prior E group. *p < .05 vs. Cont Ch.
Adapted from Rodgers et al. (2010).
In summary, in rats, at least, there appears to be a time defined window where estradiol acts to mitigate age-related cognitive declines and changes in the neural underpinnings of the effects, but exactly how this regulation occurs and how this knowledge might be therapeutically translated (other than by appropriate estradiol treatments) is unclear at this time.
Investigation of the critical period hypothesis is more difficult in women than in rats, but the limited results thus far provide both positive and negative data (Sherwin, 2012; Maki et al., 2011; Maki, 2013; Grodstein, 2013). One older study evaluated women who had undergone bilateral oophorectomy prior to the onset of natural menopause and had either used or never used estrogen replacement therapy (ET) until age 50 (Rocca et al., 2007). The never use group had an increased risk of cognitive impairment or dementia 30 years later as compared with referent women while users had no increased risk of cognitive impairment or dementia which suggests that ET initiated immediately following a surgical menopause for several years has an enduring effect 30 years later. In another study, young (ages 50–62) and older (ages 70–81) postmenopausal women, who received estradiol or placebo for three months, were administered a cholinergic challenge and tested for verbal memory and attention (Dumas et al., 2008). Estradiol pre-treatment significantly attenuated the drug-induced impairments only in the younger group which supports the concept of a critical period. Bagger et al. (2005) also found that treatment with ET for 2 to 3 years around menopause decreased risk of cognitive impairments 5–15 years later by 64% as compared with those who received placebo. Thus, these findings support the idea that 2–3 years of ET, initiated closely in time to the menopause may confer enduring cognitive protection. In contrast, results of other studies have not supported the critical period hypothesis, and it is notable that CEE was utilized in most. Espeland et al. (2013), from the WHIMSY Study Group, gave additional cognitive testing to selected women in the WHI trial who were closer to menopause, aged 50–55, than other WHI participants when they received CEE HRT. The women received HRT for 7 years and were tested approximately 7.2 years after cessation of HRT, at approximately 67 years of age. For the primary outcome, cognitive function and the secondary outcomes of verbal memory, attention, executive function, verbal fluency and working memory, there was no benefit or any increased risk. Hogervorst and Bandelow (2010) performed a meta-analysis of 36 randomized treatment trials (various estrogen formulations) and found that neither age of the women nor duration of time elapsed when treatment was initiated since menopause significantly affected the cognitive outcome. Outcomes in the Kronos Early Estrogen Prevention Study (KEEPS) which randomized postmenopausal women from 42 to 59 years to low dose oral or transdermal estradiol (as compared to CEE in the WHI trials) combined with progesterone or placebo for up to 4 years is awaited (Wharton et al., 2013). It is notable that results for coronary heart disease are consistent with the critical period hypothesis as the WHI provided initial indication of decreased heart disease risk in younger women, and other studies show decreased total myocardial infarction risk and significant reductions in atherosclerosis progression with CEE treatment (Grodstein, 2013). Thus, whether there is a critical period in women for estradiol’s enduring maintenance of cognitive function awaits verification by ongoing and future studies.
The future of research on estrogen treatments for women suffering from memory loss and other symptoms due to premature menopause (oophorectomy), menopause or aging should not necessarily rest on the outcome(s) of experiments testing the critical period hypothesis. If outcomes are positive, then estradiol replacement should be actively given around menopause, but this outcome does not preclude the use of newer and more effective HRTs. It is important to note that Premarin was approved by the FDA for use in postmenopausal women in 1942 (Stefanick, 2005) and that no new estrogenic treatments for menopause have been implemented since that time. New doses of Premarin and transdermal patches containing estradiol have been brought to market but no novel compounds. If ongoing studies do not support the critical period hypothesis, then research to understand why estrogens no longer have positive impacts on neural function after the menopause would be imperative, and the necessity of newer treatment strategies and/or drugs would become paramount. Thus, research must continue and translational strategies should be expanded in order to maximize positive effects of estrogens on neural function.
Novel strategies for estradiol replacement/treatments
Research over the last twenty-five years has established that estradiol influences cognitive function, but a surprising development has been the expansion of possible mechanisms for estrogen’s actions. New receptors have been identified which can mediate genomic (ERβ) and non-genomic changes (membrane receptors). In addition, estradiol has also been identified to be a neurosteroid and most likely a neuromodulator. Thus, understanding which mechanism(s) contribute to cognition has become more challenging. Another complication for research is that it appears that effects of estrogens may change over the lifespan. This is especially critical for aging where it appears that estradiol’s positive effects are lessened as compared to its effects in adulthood, and, in some cases, estrogens may even cause detrimental effects. Thus, more basic research is critical, especially in aged models, in order to understand how to maintain cognitive function during this transition.
In relation to HRT therapies, it was noted above that Premarin has been in use for HRT for approximately 75 years and that no new treatment options are currently available to women. In the 1990’s drug development focused on synthesis of selective estrogen receptor modulator compounds (SERMs) which would act as agonists in bone for treatment of osteoporosis and also mitigate hot flashes but not bind in the uterus or breasts to cause cancer; cognitive issues did not appear to be a focus at that time. First and second generation SERMS, Tamoxifen and Raloxifene, respectively, were relatively successful as agonists in bone and antagonists in mammary tissue (MacLusky, 1997). Thus, they were useful for treating osteoporosis and as an adjuvant therapy for estrogen receptor positive breast cancers, but their mixed agonist/antagonist properties limited their use. Thus, it was a research milestone when ERβ was discovered (Kuiper et al., 1997), and more receptor specific SERMs were synthesized (Minutolo, 2011). Since ERβ is present in the brain but in only minimum levels in the uterus and breasts, ERβ specific SERMS held great promise as HRTs because of expected minimal side effects. Several, DPN, C19 and WAY-200070, enhanced memory in animal models (see the section Human studies for details). Unfortunately, estrogen therapy is currently not approved by the US FDA for the prevention of cardiovascular disease or dementia. These drugs have not yet come to market, but ERβ specific receptor agonists still hold potential for treating certain cancers, as well as endometriosis, inflammatory diseases including rheumatoid arthritis, cardiovascular and CNS conditions (Minutolo et al., 2011). A novel phytoestrogen formulation, referred to as the Phyto-β-SERM formulation, prolonged survival, improved spatial recognition memory, and slowed progression of amyloid pathology in a female mouse model of Alzheimer’s disease and is currently undergoing a trial in women where cognition will be evaluated (Zhao et al., 2013). Finally, Bazedoxifene, a third generation SERM, in combination with conjugated estrogens came to market in January 2014 (www.Duavee.com) for preventing moderate to severe hot flashes and osteoporosis in post-menopausal women. Due to the estrogen agonist/antagonist profile of Bazedoxifene, this drug is expected to have fewer side effects than previous SERMS.
Another more recently described target for drug development is the “Downstream Molecule” concept advanced by Frick (2012). In this model, neuronal molecules and cellular mechanisms, which are downstream from hormone receptors and underlie the memory-enhancing effects of estradiol, would be targeted (see the section Synthesis of estradiol within brain areas). Such drugs would interact with cellular signaling molecules rather than the receptors themselves and thus should mimic the beneficial, but not the deleterious, effects of estrogens because they would be non-steroidal in nature. It is also possible that a “cocktail” of estradiol and such drugs may be required in order to overcome aging effects.
A final approach for drug development is intra-neuronal modulation of estrogen production and release. Increased availability of brain derived estradiol should be beneficial for cognitive functioning. Currently drugs are available to inhibit aromatase and lower estradiol levels but none activate aromatase. Allosteric activation of the enzyme itself or increased synthesis of the enzyme protein could increase local estradiol production and be beneficial for cognition. Possible side effects of increased aromatase are currently not known.
Conclusions
The field of hormonal modulation of learning and memory has advanced enormously since Hormone and Behavior’s landmark volume on Estrogen Effects on Cognition across The Lifespan in 1998. However, it is clear that the breadth and depth of new developments concerning neural mechanisms for estradiol action as well as the dearth of new HRTs indicate that researchers will continue to be engaged in this area of neuroendocrine research in the near and far future.
Acknowledgments
Recent experimental work discussed in this review from my laboratory was supported by The City University of New York, PSC-CUNY, NIH grants GM60654 (VNL), GM60665 (VNL) and RR03037 (HC). Helpful comments by Maya Frankfurt (Hofstra-LIJ Medical School) and Jill Schneider (Leigh University) and provision of Fig. 9 by Jill Daniel are gratefully acknowledged.
Footnotes
References
- Acosta JI, Hiroi R, Camp BW, Talboom JS, Bimonte-Nelson HA. An update on the cognitive impact of clinically-used hormone therapies in the female rats: models, mazes and mechanisms. Brain Res. 2013;1514:18–39. doi: 10.1016/j.brainres.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor-alph in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aenlle KK, Foster TC. Aging alters the expression of genes for neuroprotection and synaptic function following acute estradiol treatment. Hippocampus. 2010;20:1047–1060. doi: 10.1002/hipo.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Blindt HS, Candy JM. Working memory in aged rats. Behav Neurosci. 1989;103:975–983. doi: 10.1037//0735-7044.103.5.975. [DOI] [PubMed] [Google Scholar]
- Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen GC PERF Study Group. Early postmenopausal hormone therapy may prevent cognitive impairment later in life. Menopause. 2005;12:12–17. doi: 10.1097/00042192-200512010-00005. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Ma C, Soma KK, Saldanha CJ. Inhibition of hippocampal aromatization impairs spatial memory performance in a male songbird. Endocrinology. 2013;154:4707–4714. doi: 10.1210/en.2013-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Barrett GL, Bennie A, Trieu J, Ping S, Tsafoulis C. The chronology of age-related spatial learning impairment in two rat strains, as tested by the Barnes maze. 2009;123:533–538. doi: 10.1037/a0015063. [DOI] [PubMed] [Google Scholar]
- Bean LA, Ianov L, Foster TC. Estrogen Receptors, the Hippocampus, and Memory. Neuroscientist 2014. 2014 Feb 7; doi: 10.1177/1073858413519865. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Food deprivation modulates chronic stress effects on object recognition in male rats: Role of monoamines and amino acids. Brain Res. 1999;830:56–71. doi: 10.1016/s0006-8993(99)01380-3. [DOI] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiol Behav. 2002;75:661–673. doi: 10.1016/s0031-9384(02)00670-4. [DOI] [PubMed] [Google Scholar]
- Beltran-Campos V, Pado-Alcala RA, Leon-Jacinto U, Aguilar-Vazquez A, Quirarte GL, Ramirez-Amaya V, Diaz-Cintra S. Increase of mushroom spine density in CA1 apical dendrites produced by water maze training is prevented by ovariectomy. Brain Res. 2011;1369:119–130. doi: 10.1016/j.brainres.2010.10.105. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Beltz AM. Sexual differentiation of human behavior: effects of prenatal and pubertal organizational hormones. Front Neuroendocrinol. 2011;32:183–200. doi: 10.1016/j.yfrne.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Bhavnani BR. Estrogens and menopause: pharmacology of conjugated equine estrogens and their potential role in the prevention of neurodegenerative diseases such as Alzheimer’s. J Steroid Biochem Mol Biol. 2003;85:473–482. doi: 10.1016/s0960-0760(03)00220-6. [DOI] [PubMed] [Google Scholar]
- Biegon A, Kim SW, Alexoff DL, Jayne M, Carter P, Hubbard B, King P, Logan J, Muench L, et al. Unique distribution of aromatase in the human brain: in vivo studies with PET and [N-methyl-11C]vorozole. Synapse. 2010;64:801–807. doi: 10.1002/syn.20791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte HA, Nelson ME, Granholm AE. Age-related deficits as working memory load increases: relationships with growth factors. Neurobiol Aging. 2003;24:37–48. doi: 10.1016/s0197-4580(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bisagno V, Ferguson D, Luine VN. Chronic d-amphetamine induces sexually dimorphic effects on locomotion, recognition memory and brain monoamines. Pharmacol Biochem Behav. 2003;74:859–867. doi: 10.1016/s0091-3057(03)00017-0. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. Cytoplasmic estrogen receptors in rat brain: immunocytochemical evidence using three antibodies with distinct epitopes. Endocrinology. 1992;131:1336–1342. doi: 10.1210/endo.131.3.1380440. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. An estrogen by any other name. Endocrinology. 2008;149:2697–2698. doi: 10.1210/en.2008-0396. [DOI] [PubMed] [Google Scholar]
- Bloss EB, Puri R, Yuk F, Punsoni M, Hara Y, Janssen WG, McEwen BS, Morrison JH. Morphological and molecular changes in aging rat prelimbic prefrontal cortical synapses. Neurobiol Aging. 2013;34:200–210. doi: 10.1016/j.neurobiolaging.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM. The ability of oestradiol administration to regulate protein levels of oestrogen receptor alpha in the hippocampus and prefrontal cortex of midd-aged rats is altered following long-term ovariean hormone deprivation. J Neuroendocrinol. 2009;21:640–647. doi: 10.1111/j.1365-2826.2009.01882.x. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM. The beneficial effects of estradiol on attentional processes are dependent on timing of treatment initiation following ovariectomy in middle-aged rats. Psychoneuroendocrinology. 2010;35:694–705. doi: 10.1016/j.psyneuen.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Boon WC, Chow JD, Simpson ER. The multiple roles of estrogens and the enzyme aromatase. Prog Brain Res. 2010;181:209–232. doi: 10.1016/S0079-6123(08)81012-6. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, Frick KM. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci. 2013;33:15184–15194. doi: 10.1523/JNEUROSCI.1716-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:401–410. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Dun S, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, et al. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- Brown TJ, MacLusky NJ, Shanabrough M, Naftolin F. Comparison of age-and sex-related changes in cell nuclear estrogen-binding capacity and progestin receptor induction in the rat brain. Endocrinology. 1990;126:2965–2972. doi: 10.1210/endo-126-6-2965. [DOI] [PubMed] [Google Scholar]
- Christensen A, Dewing P, Micevych P. Membrane-Initiated Estradiol Signaling Induces Spinogenesis Required for Female Sexual Receptivity. J Neurosci. 2011;30:17583–17589. doi: 10.1523/JNEUROSCI.3030-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM. Estrogens, estrogen receptors, and female cognitive aging: the impact of timing. Horm Behav. 2013;63:231–237. doi: 10.1016/j.yhbeh.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21:6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Lee CD. Estrogen replacement in ovariectomized rats affects strategy selection in the Morris water maze. Neurobiol Learn Mem. 2004;82:142–149. doi: 10.1016/j.nlm.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze task. Horm Behav. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Roberts SL, Dohanich GP. Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiol Behav. 1999;66:11–20. doi: 10.1016/s0031-9384(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Davis DM, Jacobson TK, Aliakbari S, Mizumori SJ. Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiol Learn Mem. 2005;84:132–137. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Dohanich GP. Gonadal steroids, learning and memory. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RI, editors. Hormones, Brain and Behavior. Academic Press; San Diego: 2002. pp. 265–327. [Google Scholar]
- Dohanich G, Fader AF, Javorsky DJ. Estrogen and estrogen-progesterone treatments counteract the effect of scopolamine on reinforced T-maze alternation in female rats. Behav Neurosci. 1994;108:988–992. doi: 10.1037//0735-7044.108.5.988. [DOI] [PubMed] [Google Scholar]
- Dumas J, Hamcur-Bucci C, Naylor M, Sites C, Newhouse P. Estradiol interacts with the cholinergic system to affect verbal memory in postmenopausal women: evidence for the critical period hypothesis. Horm Behav. 2008;53:159–169. doi: 10.1016/j.yhbeh.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA. Estradiol treatment altered anticholinergic-related brain activation during working memory in postmenopausal women. Neuroimage. 2012;60:1394–1403. doi: 10.1016/j.neuroimage.2012.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englemann M, Ebner K, Landgraf R, Wotjak CT. Effects of morris water maze testing on the neuroendocrine stress response and intrahypothalamic release of vasopressin and oxytocin in the rat. Horm Behav. 2006;50:496–501. doi: 10.1016/j.yhbeh.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Engler-Chiurazzi E, Tsang C, Nonnenmacher S, Liang WS, Corneveaux JJ, Prokai L, Huentelman MJ, Bimonte-Nelson HA. Tonic Premarin dose-dependently enhances memory, affects neurotrophin protein levels and alters gene expression in middle-aged rats. Neurobiol Aging. 2011;32:680–697. doi: 10.1016/j.neurobiolaging.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler-Chiurazzi EB, Talboom JS, Braden BB, Tsang CW, Mennenga S, Andrews M, Demers LM, Bimonte-Nelson HA. Continuous estrone treatment impairs spatial memory and does not impact number of basal forebrain cholinergic neurons in the surgically menopausal middle-aged rat. Horm Behav. 2012;62:1–9. doi: 10.1016/j.yhbeh.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MS, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KI, Hogan PE, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women. Women’s Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Shumaker SA, Leng I, et al. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173:1429–1436. doi: 10.1001/jamainternmed.2013.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett JW. Ovulation in rats from preoptic stimulation through platinum electrodes. Important of duration and spread of stimuli. Endocrinology. 1965;76:1195–1201. doi: 10.1210/endo-76-6-1195. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Hendricson AW, Dohanich GP. Estrogen improves performance of reinforced T-maze alternation and prevents the amnestic effects of scopolamine administered systemically or intrahippocampally. Neurobiol Learn Mem. 1998;69:225–240. doi: 10.1006/nlme.1998.3820. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Johnson PE, Dohanich GP. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine of a radial-arm maze. Pharmacol Biochem Behav. 1999;62:711–717. doi: 10.1016/s0091-3057(98)00219-6. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Frick KM. Chronic oral estrogen affects memory and neurochemistry in middle-aged female mice. Behav Neurosci. 2004;118:1340–1351. doi: 10.1037/0735-7044.118.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmar LC, Hemmer M, Denslow ND, Kroll K, Chen J, Cheek A, Richman H, Meredith H, Grau EG. A comparison of the estrogenic potencies of estradiol, ethynylestriol, diethylstilbestrol, nonylphenol and methoxychlor in vivo and in vitro. Aquat Toxicol. 2002;60:101–110. doi: 10.1016/s0166-445x(01)00276-4. [DOI] [PubMed] [Google Scholar]
- Fortress AM, Frick KM. Epigenetic regulation of estrogen-dependent memory. Front Neuroendocrinol. 2014 May 27; doi: 10.1016/j.yfrne.2014.05.001. http://dx.doi.org/10.1016/j.yfrne.2014.05.001. pii: S0091-3022(14)00048-X, [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Foster TC. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus. 2012;22:656–669. doi: 10.1002/hipo.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankfurt M, Gould E, Woolley CS, McEwen BS. Gonadal steroids modify dendritic spine density in ventromedial hypothalamic neurons: a Golgi study in the adult rat. Neuroendocrinology. 1990;51:530–535. doi: 10.1159/000125387. [DOI] [PubMed] [Google Scholar]
- Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm. Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM. Building a better hormone therapy?: how understanding the rapid effects of sex steroid hormones could lead to new therapeutics for age-related memory decline. Behav Neurosci. 2012;126:29–53. doi: 10.1037/a0026660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Berger-Sweeney J. Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57Bl/6 mice. Behav Neurosci. 2001;115:229–237. doi: 10.1037/0735-7044.115.1.229. [DOI] [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Arters JA, Gerger-Sweeney J. Reference memory, anxiety and estrous cyclicity in C57BL/6NJA mice are affected by age and sex. Neuroscience. 2000;95:203–307. doi: 10.1016/s0306-4522(99)00418-2. [DOI] [PubMed] [Google Scholar]
- Frye CA. Estrus-associated decrements in a water maze task are limited to acquisition. Physiol Behav. 1995;57:5–14. doi: 10.1016/0031-9384(94)00197-d. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CA, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behav Brain Res. 2001;126:115–126. doi: 10.1016/s0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Galea LA, Lee T, Kostaras TY, Sidhu JA, Barr AM. High levels of estradiol impair spatial performance in the Morris water maze and increase “depressive-like” behaviors in the female meadow vole. Physiol Behav. 2002;77:217–225. doi: 10.1016/s0031-9384(02)00849-1. [DOI] [PubMed] [Google Scholar]
- Gatewood JD, Morgan MD, Eaton M, McNamara IM, Stevens LF, Macbeth AH, et al. Motherhood mitigates aging-related decrements in learning and memory and psotively affects brain aging in the rat. Brain Res Bull. 2005;66:91–98. doi: 10.1016/j.brainresbull.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr Rev. 2010;31:224–253. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese KP, Mizuno K. The roles of protein kinases in learning and memory. Learn Mem. 2013;20:540–552. doi: 10.1101/lm.028449.112. [DOI] [PubMed] [Google Scholar]
- Gillies GE, McArthue S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. 2010;62:155–198. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski RA. Gonadal hormones and the perinatal development of neuroendocrine function. In: Martini L, Ganong WF, editors. Frontiers in Neuroendocrinology. Oxford Press; New York: 1971. pp. 237–348. [Google Scholar]
- Gresack JE, Frick KM. Environmental enrichment reduces the mnemonic and neural benefits of estrogen. Neuroscience. 2004;128:459–471. doi: 10.1016/j.neuroscience.2004.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodstein F. Hormone therapy in younger women and cognitive health. Invited Commentary. JAMA Intern Med. 2013;173:1437–1438. doi: 10.1001/jamainternmed.2013.6827. [DOI] [PubMed] [Google Scholar]
- Hammond Mauk R, Ninaci D, Nelson D, Gibbs R. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm Behav. 2009;56:309–314. doi: 10.1016/j.yhbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson E. Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology. 1990;15:97–111. doi: 10.1016/0306-4530(90)90018-5. [DOI] [PubMed] [Google Scholar]
- Hampson E. Sex differences in human brain and cognition: the influence of sex steroids in early and adult life. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral Endocrinology. 2. MIT Press; Boston: 2002. [Google Scholar]
- Hampson E, Morley EE. Estradiol concentrations and working memory performance in women of reproductive age. Psychoneuroendocrinology. 2013;38:2897–2904. doi: 10.1016/j.psyneuen.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S. Sex steroids to maintain cognitive function in women after the menopause: a meta-analyses of treatment trials. Maturitas. 2010;66:56–71. doi: 10.1016/j.maturitas.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Kawato S. Adult male rat rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P450 17-alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine V. Acute Estrogen Treatment Facilitates Recognition Memory Consolidation and Alters Monoamine levels in Memory-related Brain Areas. Horm Behav. 2010;58:415–426. doi: 10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Frankfurt M, Luine V. Estrogen-induced memory enhancements are blocked by acute bisphenol A in adult female rats: role of dendritic spines. Endocrinology. 2012;153:3357–3367. doi: 10.1210/en.2012-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. Prenatal gonadal steroid affect adult spatial behavior, CA1 and CA3 pyramidal cell morphology in rats. Horm Behav. 1998;34:183–198. doi: 10.1006/hbeh.1998.1477. [DOI] [PubMed] [Google Scholar]
- Jacome LF, Gautreaux C, Inagaki T, Mohan G, Arellanos A, MacLusky N, Alves S, Lubbers L, Luine VN. ERβ Agonists Enhance Recognition Memory and Alter Monoamines in Ovariectomized Rats. Neurobiol Learn Mem. 2010;94:488–498. doi: 10.1016/j.nlm.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedlicka P, Vlachos A, Schwarzacher SW, Delle T. A role for the spine apparatus in LTP and spatial learning. Behav Brain Res. 2008;192:12–19. doi: 10.1016/j.bbr.2008.02.033. [DOI] [PubMed] [Google Scholar]
- Joffe H, Hall J, Gruber S, Sarmiento I. Estrogen therapy selectively enhances pre-frontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently post-menopausal women. Menopause. 2006;13:411–422. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- Kato A, Hojo Y, Higo S, Komatsuzaki Y, Murakami G, Yoshino H, Uebayashi M, Kawato S. Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Front Neural Circ. 2013 Oct 18;7:149. doi: 10.3389/fncir.2013.00149. http://dx.doi.org/10.3389/fncir.2013.00149. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns KA, Berenbaum SA. Sex differences in spatial ability in children. Behav Genet. 1991;21:383–396. doi: 10.1007/BF01065974. [DOI] [PubMed] [Google Scholar]
- Khan MM, Dhandapani KM, Zhang Q, Brann DW. Estrogen regulation of spine density and excitatory syunapses in rat prefrontal and somatosensory cerebral cortex. Steroids. 2013;78:614–623. doi: 10.1016/j.steroids.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A, Delattre AM, Pereira SI, Carolino RG, Szawka RE, Anselmo-Franci JA, Zanata SM, Ferraz AC. 17β-estradiol replacement in young, adult and middle-aged female ovariectomized rats promotes improvement of spatial reference memory and an antidepresseant effect and alters monoamines and BDNF levels in memory-and depression-related brain areas. Behav Brain Res. 2012;227:100–108. doi: 10.1016/j.bbr.2011.10.047. [DOI] [PubMed] [Google Scholar]
- Korol DI, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav. 2004;45:330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Babayan AH, Gall CM, Lynch G. Estrogen promotes learning-related plasticity by modifying the synaptic cytoskeleton. Neuroscience. 2013;239:3–16. doi: 10.1016/j.neuroscience.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;8(Suppl 1):3–63. doi: 10.1080/13697130500148875. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distributionof estrogen receptors α and β. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Langer G, Bader B, Meoli L, Isensee J, Delbeck M, Noppinger PR, Otto C. A critical review of fundamental controversies in the field of GPR30 research. Steroids. 2010;75:603–610. doi: 10.1016/j.steroids.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Leuner B, Falduta J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MC, Kerr KM, Orr PT, Frick KM. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav Neurosci. 2008;122:716–721. doi: 10.1037/0735-7044.122.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos A, Allen P, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory tasks in female mice. Proc Natl Acad Sci. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Day M, Muñiz L, Bitran D. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Luine VN. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp Neurol. 1985;89:484–490. doi: 10.1016/0014-4886(85)90108-6. [DOI] [PubMed] [Google Scholar]
- Luine V. Neuroendocrinology of memory and cognition. In: Lajtha Abel., editor. Handbook of Neurochemistry and Molecular Neurobiology, 3rd edn, Blaustein Jeff D, Volume Editor: Behavioral Neurochemistry and Neuroendocrinology. Berlin: Springer; 2006. pp. 775–800. [Google Scholar]
- Luine VN. Sex steroids and cognitive function. J Neuroendocrinol. 2008;20:866–872. doi: 10.1111/j.1365-2826.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- Luine V. Recognition memory tasks in neuroendocrine research. Behav Brain Res. 2014 doi: 10.1016/j.bbr.2014.04.032. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Dohanich G. Sex differences in cognitive function in Rodents. In: Becker JB, Berkley KJ, Geary N, Hampson E, Herman JP, Young E, editors. Sex Differences in the Brain: From Genes to Behavior. Oxford University Press; 2008. pp. 227–252. [Google Scholar]
- Luine V, Frankfurt M. Estrogens facilitate memory processing through membrane mediated mechanisms and spine density changes. Front Neuroendocrinol. 2012a;33:388–402. doi: 10.1016/j.yfrne.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Frankfurt M. An Integrative Review of Estradiol Effects on Dendritic Spines and Memory over the Lifespan. In: Kahn Scott M., editor. Sex Steroids. Intech Open Access Publishing; 2012b. ( http://www.intechopen.com/articles/show/title/an-integrative-review-of-estrogen-effects-on-memory-and-dendritic-spines-over-the-lifespan) [Google Scholar]
- Luine V, Frankfurt M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience. 2013;239:34–45. doi: 10.1016/j.neuroscience.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Rodriguez M. Effects of estradiol on radial arm maze performance of young and aged rats. Behav Neural Biol. 1994;62:230–236. doi: 10.1016/s0163-1047(05)80021-4. [DOI] [PubMed] [Google Scholar]
- Luine V, Hearns M, Bowling D. Spatial memory deficits in aged rats: contributions of monoaminergic systems. Brain Res. 1990;537:271–278. doi: 10.1016/0006-8993(90)90368-l. [DOI] [PubMed] [Google Scholar]
- Luine V, Richards ST, Wu VY, Beck K. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, MacLusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luine V, Attalla S, Mohan G, Costa A, Frankfurt M. Dietary phytoestrogens enhance spatial memory and spine density in the hippocampus and prefrontal cortex of ovariectomized rats. Brain Res. 2006;1126:183–187. doi: 10.1016/j.brainres.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, MacLusky NJ. Stress and neural function – Accounting for sex and age. J Neuroendocrinol. 2007;19:743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- Luine VN, Wallace M, Frankfurt M. Age-related deficits in spatial memory and hippocampal spines in virgin, female, Fischer-344 rats. Curr Gerontol Geriatr Res. 2011:Article ID 316386, 7. doi: 10.1155/2011/316386. http://dx.doi.org/10.1155/2011/316386. [DOI] [PMC free article] [PubMed]
- Macbeth AH, Luine VN. Changes in anxiety and cognition due to reproductive experience: a review of data from rodent and human mothers. Neurosci Biobehav Rev. 2010;34:452–467. doi: 10.1016/j.neubiorev.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Macbeth AH, Scharfman HE, MacLusky NJ, Gautreaux C, Luine VN. Effects of multiparity on recognition memory, monoaminergic neurotransmitters, and brain-derived neurotrophic factor (BDNF) Horm Behav. 2008;54:7–17. doi: 10.1016/j.yhbeh.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ. Mechanisms of gonadal steroid action in New concepts in hormone management: selective estrogen receptor modulators (SERMS) J Soc Obstet Gynaecol Can. 1997;(Suppl):4–20. [Google Scholar]
- MacLusky NJ, Luine VN, Hajszan T, Prange-Kiel, Leranth C. The 17 α and βisomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- Maki PM. Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause. 2013;20:695–709. doi: 10.1097/GME.0b013e3182960cf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Dennerstein L, Clark M, Gutherie J, Lamontagne P, Fornelli D, Little D, Henderson VW, Resnick SM. Perimenopausal use of hormone therapy is associated with enhanced memory and hippocampal function later in life. Brain Res. 2011;1379:232–243. doi: 10.1016/j.brainres.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouf MA, Migeon CJ, Carson KA, Petrucci L, Wisniewski AB. Cognitive outcome in adult women affected by congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res. 2006;65:142–150. doi: 10.1159/000091793. [DOI] [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decine in spatial memory: relevance to alterations in the estorus cycle. J Neurosci. 1999;19:8122–8133. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews KA, Kuller LH, Wing RR, Meilahn EN, Plantings P. Prior to use of estrogen replacement therapy, are users healthier than nonusers? Am. J Epidemiol. 1996;143:971–978. doi: 10.1093/oxfordjournals.aje.a008678. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Kanemoto Y, Kasai H. Simultaneous two-photon activation of presynaptic cells and calcium imaging in postsynaptic dendritic spines. Neural Syst Circ. 2011;1:2–11. doi: 10.1186/2042-1001-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K, Cauley J, Yaffe K, Zmuda JM. Estrogen replacement therapy and cognitive decline in older community women. J Am Geriatr Soc. 1999;47:518–523. doi: 10.1111/j.1532-5415.1999.tb02563.x. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Nugent BM. Epigenetic contributions to hormonally-mediated sexual differentiation of the brain. J Neuroendocrinol. 2013;25:1133–1140. doi: 10.1111/jne.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav Neurosci. 2012;126:4–16. doi: 10.1037/a0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Dissociating learning and performance: drug and hormone enhancement of memory storage. Brain Res Bull. 1989;23:339–345. doi: 10.1016/0361-9230(89)90220-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Wright RL, Conrad CD. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: possible involvement of CA1 neurons. Neuroscience. 2005;135:1045–1054. doi: 10.1016/j.neuroscience.2005.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Bimonte-Nelson H, Neisewander JL, Conrad CD. Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: evidence that the duration of hormone deprivation after ovariectomy compromises 17beta-estradiol effectiveness in altering CA1 spines. Horm Behav. 2008;54:386–395. doi: 10.1016/j.yhbeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Mermelstein PG. Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. J Chem Neuroanat. 2011;42:236–241. doi: 10.1016/j.jchemneu.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalj M, Drenjančević I, Včev A, Šumanovac A, Čavka A, Vladetić M, Gmajnić R. Basic cognitive functions across the menstrual cycle in a controlled female cohort. Med Glas (Zenica) 2014;11:177–185. [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal α estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Minutolo F, Macchia M, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor β ligands: recent advances and biomedical applications. Med Res Rev. 2011;31:364–442. doi: 10.1002/med.20186. [DOI] [PubMed] [Google Scholar]
- Morgado-Bernal I. Learning and memory consolidation: linking molecular and behavioral data. Neuroscience. 2011;176:12–19. doi: 10.1016/j.neuroscience.2010.12.056. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Temple V, Oh E, VanRyzin C, Williams A, Cornwell B, Grillon C, Pine DS, Ernst M, Merke DP. Early androgen exposure modulates spatial cognition in congenital adrenal hyperplasia (CAH) Psychoneuroendocrinology. 2008;33:973–980. doi: 10.1016/j.psyneuen.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D, Toni N, Buchs PA. Spine changes associated with long-term potentiation. Hippocampus. 2000;10:596–604. doi: 10.1002/1098-1063(2000)10:5<596::AID-HIPO10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Packard MG. Posttraining estrogen and memory modulation. Horm Behav. 1998;34:126–139. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Pan M, Li Z, Yeung Z, Xu RJ. Dietary supplementation of soy germ phytoestrogens or estradiol improves spatial memory performance and increases gene expression of BDNF, TrkB receptor and synaptic factors in ovariectomized rats. Nutr Metab (Lond) 2010;7:75–83. doi: 10.1186/1743-7075-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parducz A, Hajszan T, Maclusky NJ, Hoyk Z, Csakvari E, Kurunczi A, Prange-Kiel J, Leranth C. Synaptic remodeling induced by gonadal hormones: neuronal plasticity as a mediator of neuroendocrine and behavioral responses to steroids. Neuroscience. 2006;138:977–985. doi: 10.1016/j.neuroscience.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Phan A, Gabor CS, Favaro KJ, Kaschack S, Armstrong JN, MacLusky NJ, Choleris E. Low doses of 17β-estradiol rapidly improve learning and increase hippocampal dendritic spines. Neuropsychopharmacology. 2012;37:2299–2309. doi: 10.1038/npp.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Dong S, Maidment NT, Schlinger BA. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J Neurosci. 2011;31:10034–10038. doi: 10.1523/JNEUROSCI.0566-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Henderson VW. Hormone therapy and risk of Alzheimer disease: a critical time. JAMA. 2002;288:2170–2172. doi: 10.1001/jama.288.17.2170. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Estrogen has mnemonic-enhancing effects in the inhibitory avoidance task. Pharmacol Biochem Behav. 2004;78:551–558. doi: 10.1016/j.pbb.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Bower JH, Maraganore DM, Grossardt BR, deAndrade M, Melton LJ. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- Rodgers SP, Bohacek J, Daniel JM. Transient estradiol exposure during middle age in orvaiectomized rats exerts lasting effects on cognitive function and the hippocampus. Endocrinology. 2010;151:1194–1203. doi: 10.1210/en.2009-1245. [DOI] [PubMed] [Google Scholar]
- Roof RL. Neonatal exogenous testosterone modifies sex difference in radial arm and morris water maze performance in prepubescent and adult rats. Behav Brain Res. 1993;26:1–10. doi: 10.1016/s0166-4328(05)80261-x. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr Rev. 2011;32:532–549. doi: 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Hintz TM, Gomez J, Stormes KA, Barouk S, Malthankar-Phatak DH, McLoskey DP, Luine VN, MacLusky NJ. Changes in hippocampal function of ovariectomized rats after sequential low doses of estradiol to simulate the preovulatory estrogen surge. Eur J Neurosci. 2007;26:2595–2612. doi: 10.1111/j.1460-9568.2007.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreihofer DA, Ma Y. Estrogen receptors and ischemi neuroprotection: who, what, where, and when? Brain Res. 2013;1514:107–122. doi: 10.1016/j.brainres.2013.02.051. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: the organizational–activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, III, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive functioning in women: lessons we have learned. Behav Neurosci. 2012;126:123–127. doi: 10.1037/a0025539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Simpkins JW, Bimonte-Nelson HA, Brinton RD. Window of opportunity for estrogen and progestin intervention in brain aging and Alzheimer’s disease. Brain Res. 2013;1514:1–2. doi: 10.1016/j.brainres.2013.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitruk-Ware R. Hormonal replacement therapy. Rev Endocr Metab Disord. 2002;3:243–256. doi: 10.1023/a:1020028510797. [DOI] [PubMed] [Google Scholar]
- Srivastava DP. Two-step wiring plasticity – a mechanism for estrogen-induced rewiring of cortical circuits. J Steroid Biochem Mol Biol. 2012;131:17–23. doi: 10.1016/j.jsbmb.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Evans PD. Mechanisms underlying the interactions between rapid estrogenic and BDNF control of synaptic connectivity. Neuroscience. 2013a;239:17–33. doi: 10.1016/j.neuroscience.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Penzes P. Insights into rapid modulation of neuroplasticity by brain estrogens. Pharmacol Rev. 2013b;65:1318–1350. doi: 10.1124/pr.111.005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanick ML. Estrogens and progestins: background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. Am J Med. 2005;118:64S–73S. doi: 10.1016/j.amjmed.2005.09.059. [DOI] [PubMed] [Google Scholar]
- Sweat JD. Mechanisms of Memory, The NMDA Receptor. 2. Academic Press; San Diego: 2010. pp. 191–208. [Google Scholar]
- Talboom JS, Engler-Chiurazzi EB, Whiteaker P, Simard AR, Lukas R, Acosta JI, Proka L, Bimonte-Nelson HA. A component of Premarin(®) enhances multiple cognitive functions and influences nicotinic receptor expression. Horm Behav. 2010;58:917–928. doi: 10.1016/j.yhbeh.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Janssen WG, Hoa J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex. 2004;14:215–223. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- Tierney MC, Ryan J, Ancelin ML, Moineddin R, Rankin S, Yao C, MacLusky NJ. Lifelong estrogen exposure and memory in older postmenopausal women. J Alzheimers Dis. 2013;34:601–608. doi: 10.3233/JAD-122062. [DOI] [PubMed] [Google Scholar]
- Towart LA, Alves SE, Znamensky V, Hayashi S, McEwen BS, Milner TA. Sub-cellular relationships between cholinergic terminals and estrogen receptor α in the dorsal hippocampus. J Comp Neurol. 2003;463:390–401. doi: 10.1002/cne.10753. [DOI] [PubMed] [Google Scholar]
- Tropp J, Markus EJ. Sex differences in the dynamic of cue utilization and exp. ortory behavior. Behav Brain Res. 2001;15:143–154. doi: 10.1016/s0166-4328(00)00345-4. [DOI] [PubMed] [Google Scholar]
- Velázquez-Zamora DA, Garcia-Segura LM, González-Burgos I. Effects of selective estrogen receptor modulators on allocentric working memory performance and on dendritic spines in medial prefrontal cortex pyramidal neurons of ovariectomized rats. Horm Behav. 2012;61:512–517. doi: 10.1016/j.yhbeh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Veng LM, Granholm A-C, Rose GM. Age-related sex differences in spatial learning and basal forebrain cholinergic neurons in F344 rats. Physiol Behav. 2003;80:27–36. doi: 10.1016/s0031-9384(03)00219-1. [DOI] [PubMed] [Google Scholar]
- Viscoli C, Brass L, Kernan W, Sarrel P. Estrogen therapy and risk of cognitive decline: results from the Women’s Estrogen for Stroke Trial (WEST) Am J Obstet Gynecol. 2005;192:387–393. doi: 10.1016/j.ajog.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychol Bull. 1995;117:250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf A, Koonce C, Frye C. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem. 2008;89:513–521. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellanos A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in hippocampus and prefrontal cortex. Brain Res. 2006;1126:176–182. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- Wallace M, Frankfurt M, Arellanos A, Inagaki T, Luine V. Impaired recognition memory and decreased frontal cortex spine density in aged rats. Ann N Y Acad Sci. 2008;1097:54–57. doi: 10.1196/annals.1379.026. [DOI] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. Spatial and nonspatial learning across the rat estrous cycle. Behav Neurosci. 1997;111:259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- Watson CS, Alyea RA, Cunningham KA, Jeng YJ. Estrogens of multiple classes and their role in mental health disease mechanisms. Int J Womens Health. 2010;2:153–166. doi: 10.2147/ijwh.s6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MT, Maki PM, McDermott MP. Cognition and mood in perimenopause: a systematic review and meta-analysis. J Steroid Biochem Mol Biol. 2013 Jul;142C:90–98. doi: 10.1016/j.jsbmb.2013.06.001. http://dx.doi.org/10.1016/j.jsbmb.2013.06.001. Epub 2013 Jun 14. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Yuen EY, Liu W, Li X, Zhong P, Karatsoreos IN, McEwen BS, Yan Z. Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Mol Psychiatry. 2014;19:588–598. doi: 10.1038/mp.2013.83. [DOI] [PubMed] [Google Scholar]
- Wharton W, Gleason CE, Miller VM, Asthana S. Rationale and design of the Kronos Early Estrogen Prevention Study (KEEPS) and the KEEPS Cognitive and Affective sub study (KEEPS Cog) Brain Res. 2013;13(1514):12–17. doi: 10.1016/j.brainres.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, editor. Estrogen effects on cognition across the lifespan. Horm Behav. 1998;34:79–210. doi: 10.1006/hbeh.1998.1480. [DOI] [PubMed] [Google Scholar]
- Williams CL, Meck WH. The organizational effects of gonadal steroids on sexually dimorphic spatial ability. Psychoneuroendocrinology. 1991;16:155–176. doi: 10.1016/0306-4530(91)90076-6. [DOI] [PubMed] [Google Scholar]
- Williams CL, Barnett AM, Meck WH. Organizational effects of early gonadal secretions on sexual differentiation in spatial memory. Behav Neurosci. 1990;104:84–97. doi: 10.1037//0735-7044.104.1.84. [DOI] [PubMed] [Google Scholar]
- Witty CF, Foster TC, Semple-Rowland SL, Daniel JM. Increasing hippocampal estrogen receptor alpha levels via viral vectors increases MAP kinase activation and enhances memory in aging rats in the absence of ovarian estrogen. PLoS One. 2012;7:e51385. doi: 10.1371/journal.pone.0051385. http://dx.doi.org/10.1371/journal.pone.0051385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf OT, Kudielka BM, Hellhammer DH, Törber S, McEwen BS, Kirschbaum C. Two weeks of transdermal estradiol treatment in postmenopausal elderly women and its effect on memory and mood: verbal memory changes are associated with the treatment induced estradiol levels. Psychoneuroendocrinology. 1999;24:727–741. doi: 10.1016/s0306-4530(99)00025-6. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Mao Z, Chen S, Schneider LS, Brinton RD. Early intervention with an estrogen receptor β-selective phytoestrogenic formulation prolongs survival, improves spatial recognition memory, and slows progression of amyloid pathology in a female mouse model of Alzheimer’s disease. J Alzheimers Dis. 2013;37:403–419. doi: 10.3233/JAD-122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Serio SJ, Korol DL. Intra-striatal estradiol in female rats impairs response learning within two hours of treatment. Horm Behav. 2011;60:470–477. doi: 10.1016/j.yhbeh.2011.07.014. [DOI] [PubMed] [Google Scholar]