Abstract
Purpose
Azithromycin and tetracyclines are commonly prescribed in the United States for the treatment of meibomian gland dysfunction (MGD). The efficacy of these antibiotics has been thought to be their anti-inflammatory and anti-bacterial actions, which suppress MGD-associated posterior blepharitis and growth of lid bacteria. However, we recently discovered that azithromycin can act directly on human meibomian gland epithelial cells (HMGECs) to stimulate their function. In this study we sought to determine whether tetracycline antibiotics can duplicate this azithromycin effect.
Methods
Immortalized HMGEC were cultured in the presence of vehicle, azithromycin, doxycycline, minocycline or tetracycline for 5 days. Cells were evaluated for cholesterol and neutral lipid staining and the lipid composition of cellular lysates was analyzed by high performance thin-layer chromatography.
Results
Our results demonstrate that azithromycin’s ability to stimulate the differentiation of human meibomian gland cells is unique, and is not duplicated by doxycycline, minocycline or tetracycline. Azithromycin, but not the other antibiotics, significantly increased the cellular accumulation of cholesterol, cholesterol esters, phospholipids and lysosomes. These differentiative actions of azithromycin were paralleled by an increased expression of sterol regulatory element-binding protein 1.
Conclusions
Our findings show that the stimulatory effects of azithromycin on human meibomian gland epithelial cell function are unique, and not duplicated by the antibiotics doxycycline, minocycline or tetracycline. Our results further suggest that this stimulatory influence of azithromycin may contribute to its beneficial effect in treating MGD and its associated evaporative dry eye disease.
Keywords: meibomian gland, azithromycin, doxycycline, minocycline, tetracycline
Introduction
Meibomian gland dysfunction (MGD), the leading cause of dry eye disease throughout the world, afflicts hundreds of millions of people and has no cure.1,2 In the United States antibiotics such as azithromycin and tetracyclines, especially doxycycline, minocycline and tetracycline are widely used off-label in the treatment of MGD.1 The therapeutic effects of these antibiotics were believed to be indirect, suppressing MGD-associated posterior blepharitis and bacterial lipase activity on the lid.2
However, we recently discovered that azithromycin can act directly on human meibomian gland epithelial cells (HMGECs) to induce cholesterol, phospholipid and lysosome accumulation, and ultimately a holocrine-like secretion.3,4 We hypothesize that this intracellular effect of azithromycin is due in large part to its cationic amphiphilic structure.5 If our hypothesis is correct, then azithromycin’s action on HMGECs is unique, and will not be duplicated by tetracyclines. To test this hypothesis, we compared the effects of azithromycin and tetracyclines on HMGEC differentiation and proliferation in defined conditions.6,7 We also determined the relative ability of these antibiotics to induce sterol regulatory element-binding protein 1 (SREBP-1) and cyclin B1, which are key regulators of lipogenesis and the cell cycle, respectively.8,9
Materials and Methods
Immortalized human meibomian gland epithelial cells (HMGECs) were cultured in media that promote either proliferation (serum-free) or differentiation (serum-containing), according to published techniques.6 Cells were treated with ethanol vehicle, azithromycin (10 μg/ml; Santa Cruz Biotechnology, Dallas, TX), doxycycline (0.1 to 10 μg/ml; Sigma-Aldrich, St. Louis, MO), minocycline (0.1 to 10 μg/ml; Sigma-Aldrich) or tetracycline (0.1 to 10 μg/ml; Sigma-Aldrich) for 5 days. These doses were selected based upon our preliminary experiments with HMGECs and on their established safety in human corneal cells.10,11
Cell numbers were counted with a hemocytometer. Cellular neutral lipid and lysosome accumulation were evaluated with LipidTOX green neutral lipid stain (Life Technologies, Grand Island, NY) and LysoTracker Red DND-99 (Life Technologies). All experiments were repeated at least three times. Each experiment was performed in duplicate under the same conditions. Data from one representative experiment were used for quantitation. Eight random pictures were taken of each group on the same day with the same exposure time, and the whole cell area in each picture was selected for the measurement of fluorescent intensities. The data show the mean fluorescence intensity of all the cells in each picture, and cell number was similar in all groups. Total cellular lipid composition was analyzed by high performance thin-layer chromatography (HPTLC).12 Each sample contained the same amount of cellular extract, and cell lysates were analyzed by immunoblot. Membranes were incubated with antibodies specific for SREBP-1 (Santa Cruz, H-160, 1:500), cyclin B1 (Cell Signaling Technology, Danvers, MA; 1:500) or β-actin (Cell Signaling Technology, 1:10,000), followed by HRP-conjugated secondary antibodies (1:5,000, Sigma-Aldrich). The fluorescence intensities of LipidTOX and LysoTracker, as well as immunoblot band intensities, were quantified by using ImageJ.
For statistical evaluation, ANOVA and Newman-Keuls multiple comparisons tests were performed with Prism 5 software (GraphPad Software, Inc., La Jolla, CA).
Results
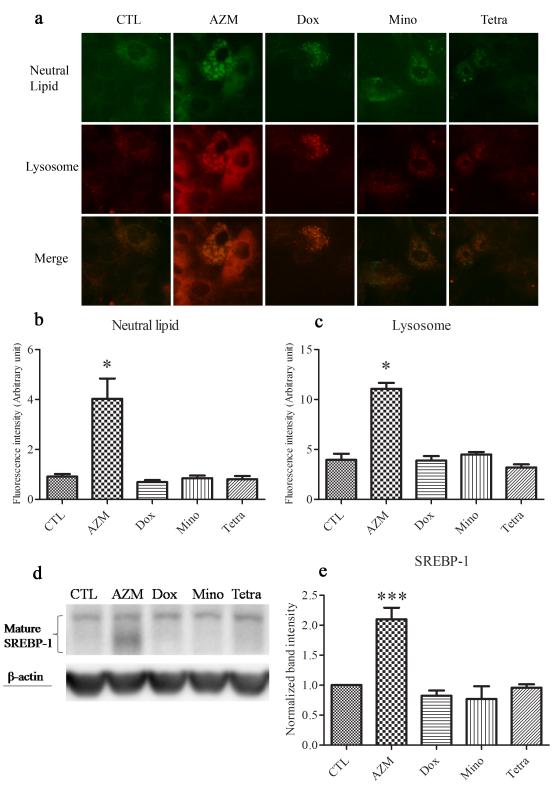
Our results demonstrate that azithromycin’s ability to stimulate the differentiation of HMGECs is unique, and is not duplicated by doxycycline, minocycline or tetracycline. Azithromycin, but not the other antibiotics, significantly enhanced the cellular accumulation of neutral lipid-containing lysosomes in these cells (Figure 1). In contrast, minocycline and tetracycline promoted the formation of small, non-lysosomal neutral lipid droplets. The differentiative actions of azithromycin were paralleled by an increased expression of SREBP-1, which appeared as a cluster of protein bands between 59 and 68 kDa13 (Figure 1).
Figure 1. Effect of antibiotics on lipid and lysosome accumulation in HMGECs.
Cells were treated with vehicle, 10μg/ml azithromycin (AZM), 5 μg/ml doxycycline (Dox), 5 μg/ml minocycline (Mino) or 5 μg/ml tetracycline (Tetra) in differentiation-promoting medium for 5 days. a. Neutral lipid was stained with LipidTOX (green), lysosomes with LysoTracker (red). b, c. The fluorescence intensities of LipidTOX (b) and LysoTracker (c) were quantified using ImageJ. d, e. SREBP-1 was evaluated by immunoblotting. Band intensities were normalized to β-actin and quantified using ImageJ. *p<0.05, ***p<0.001. The experiments were repeated at least 3 times with similar results. Data from a single experiment are shown. Error bars represent the mean ± standard error.
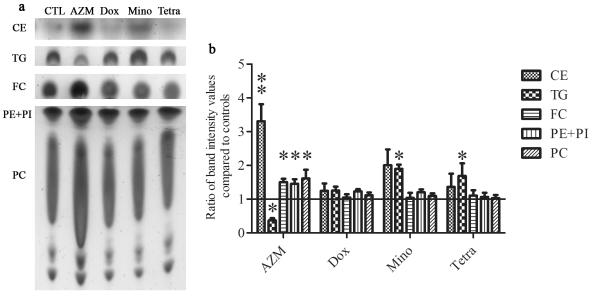
We also found that azithromycin, but not the tetracycline antibiotics, significantly augmented the cellular levels of free cholesterol, cholesterol esters, phosphatidylethanolamine, phosphatidylinositol and phosphatidylcholine, and decreased the content of triglycerides (Figure 2). By contrast, minocycline and tetracycline significantly increase the cellular triglyceride amounts.
Figure 2. The effect of antibiotics on lipid composition in HMGECs.
a. Cells were treated as described in Fig. 1. Extracted lipid samples were normalized to cell numbers. b. Lipid band intensity was quantified using ImageJ. Control band instensity was set to 1 and data are reported as fold-change compared to control. **p<0.01, *p<0.05. Unlabeled bands are unidentified lipids. Band intensity analysis includes data from five independent experiments. Error bars represent the mean ± standard error. Abbreviations include free cholesterol (FC), cholesterol ester (CE), triglyceride (TG), phosphatidylethanolamine (PE), phosphatidylcholine (PC) and phosphatidylinositol (PI).
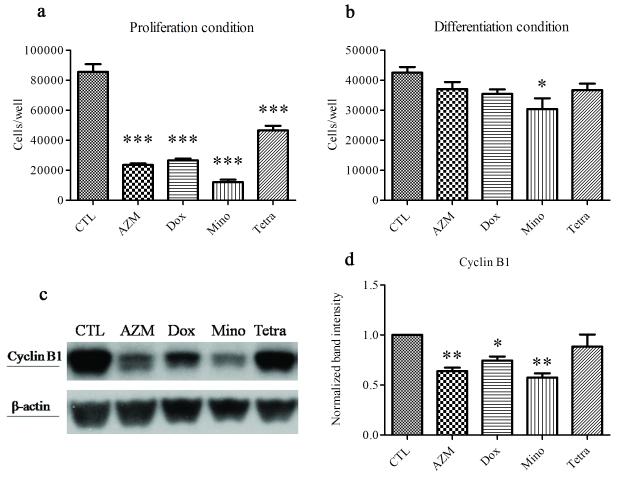
All the antibiotics reduced cell proliferation when cultured in serum-free conditions, whereas only minocycline reduced cell numbers in serum-containing cultures (Figure 3). The anti-proliferative effects of azithromycin, doxycycline and minocycline were associated with a significant decrease in cyclin B1 levels.
Figure 3. The influence of antibiotics on the proliferation of HMGECs.
Cells were seeded in 12-well plates (10,000 cells/well in proliferation-promoting (i.e. serum-free) medium, 50,000 cells/well in differentiation-promoting (i.e. serum-containing) medium, n = 3 wells/group) and treated as described in Fig. 1 prior to counting or lysis. a. Under proliferation conditions, all four drugs significantly reduced cell proliferation (***p<0.001). b. Under differentiation conditions, only minocycline significantly reduced cell proliferation (*p<0.05). c, d. Cells in differentiation-promoting conditions were evaluated by immunoblot for cyclin B1. Band intensities were normalized to β-actin and quantified using ImageJ. **p<0.01, *p<0.05. Data from one experiment are shown as a representative of three independent experiments. Error bars represent the mean ± standard error.
For these studies we used azithromycin at a 10 μg/ml dose and doxycycline, minocycline and tetracycline at concentrations of 5 μg/ml. Our reasons were 3-fold. First, we had previously shown this azithromycin dosage to be optimal and clinically relevant.3,14,15 Second, our preliminary studies demonstrated that the 10 μg/ml concentrations of doxycycline, minocycline and tetracycline were very cytotoxic when cells were cultured in serum-free medium. And third, our dose-response experiments (0.1, 0.5, 1.0, 3.0 and 5.0 μg/ml) showed that the 5 μg/ml levels were tolerable in both serum-free and serum-containing cultures.
Discussion
Our study supports our hypothesis that the ability of azithromycin to promote the differentiation of human meibomian gland epithelial cells is unique, and not duplicated by doxycycline, minocycline or tetracycline. Azithromycin, but not the other antibiotics, significantly increased the cellular content of free cholesterol, cholesterol esters, phospholipids, lysosomes and SREBP-1, a key transcription factor for lipogenesis. This azithromycin-induced accumulation of neutral lipids occurred primarily within lysosomes. In contrast, minocycline and tetracycline augmented triglyceride levels and the formation of non-lysosomal lipid droplets. These results suggest that azithromycin, but not doxycycline, minocycline or tetracycline, may be beneficial as a clinical treatment for stimulating human meibomian gland epithelial cell function.
The maturation of HMGECs involves the generation of lysosomes, the accumulation of lipids within these vesicles, and ultimately the release of lipid-laden contents by holocrine secretion.7 Our previous research suggests that the capacity of azithromycin to stimulate this differentiation process is due to its cationic amphiphilic nature.4 Cationic amphiphilic compounds are known to promote the propagation of lipid-containing lysosomes, and especially the accumulation of phospholipids.4,16 Doxycycline, minocycline and tetracycline do not possess this amphiphilic structure.
The tetracycline derivatives, though, were able to act directly on the HMGEC’s. These antibiotics, as well as azithromycin, all suppressed cellular proliferation in serum-free cultures. This “nonantibiotic” effect has also been observed in other cells,17 and for azithromycin, doxycycline and minocycline was accompanied by a decrease in cyclin B1, a regulator of G2/M transition and mitosis.18 We also found that minocycline and tetracycline increased, whereas azithromycin decreased, intracellular levels of triglycerides. This stimulatory influence was associated with the formation of small, non-lysosomal neutral lipid droplets. These actions of minocycline and/or tetracycline are analogous to those they induce in hepatic cells.19,20
Given our experimental findings, it is of interest that tetracycline derivatives reportedly relieve the symptoms of patients with MGD.21,22 This response may be due to the anti-inflammatory and anti-bacterial actions of these antibiotics, which may suppress the MGD-associated posterior blepharitis and growth of lid bacteria.1 However, the additional lipid-promoting activity of azithromycin may account for its significantly greater efficacy, as compared to doxycycline, in alleviating the signs (i.e. ocular surface staining and conjunctival redness) of MGD-associated evaporative dry disease.22
Acknowledgments
This research was supported by NIH grant EY05612 and the Margaret S. Sinon Scholar in Ocular Surface Research Fund, and the Guoxing Yao Research Fund. Yang Liu had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses. A provisional patent has been filed around this technology. The intellectual property for this application is owned by the Schepens Eye Research Institute/Massachusetts Eye and Ear.
Disclosure of funding: This research was supported by NIH grant EY05612 and the Margaret S. Sinon Scholar in Ocular Surface Research Fund, and the Guoxing Yao Research Fund.
Footnotes
Conflict of interest disclosures: A provisional patent has been filed around this technology. The intellectual property for this application is owned by the Schepens Eye Research Institute/Massachusetts Eye and Ear.
References
- 1.Geerling G, Tauber J, Baudouin C, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Investigative ophthalmology & visual science. 2011;52(4):2050–2064. doi: 10.1167/iovs.10-6997g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knop E, Knop N, Millar T, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investigative ophthalmology & visual science. 2011;52(4):1938–1978. doi: 10.1167/iovs.10-6997c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Kam WR, Ding J, et al. Effect of azithromycin on lipid accumulation in immortalized human meibomian gland epithelial cells. JAMA ophthalmology. 2014;132(2):226–228. doi: 10.1001/jamaophthalmol.2013.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Kam WR, Ding J, et al. One man’s poison is another man’s meat: using azithromycin-induced phospholipidosis to promote ocular surface health. Toxicology. 2014;320:1–5. doi: 10.1016/j.tox.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Bambeke F, Montenez JP, Piret J, et al. Interaction of the macrolide azithromycin with phospholipids. I. Inhibition of lysosomal phospholipase A1 activity. European journal of pharmacology. 1996;314(1-2):203–214. doi: 10.1016/s0014-2999(96)00552-3. [DOI] [PubMed] [Google Scholar]
- 6.Liu S, Hatton MP, Khandelwal P, et al. Culture, immortalization, and characterization of human meibomian gland epithelial cells. Investigative ophthalmology & visual science. 2010;51(8):3993–4005. doi: 10.1167/iovs.09-5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan DA, Liu Y, Kam WR, et al. Serum-induced differentiation of human meibomian gland epithelial cells. Investigative ophthalmology & visual science. 2014 doi: 10.1167/iovs.13-13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galderisi U, Jori FP, Giordano A. Cell cycle regulation and neural differentiation. Oncogene. 2003;22(33):5208–5219. doi: 10.1038/sj.onc.1206558. [DOI] [PubMed] [Google Scholar]
- 9.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. The Journal of clinical investigation. 2002;109(9):1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kernt M, Hirneiss C, Neubauer AS, et al. Minocycline is cytoprotective in human corneal endothelial cells and induces anti-apoptotic B-cell CLL/lymphoma 2 (Bcl-2) and X-linked inhibitor of apoptosis (XIAP) The British journal of ophthalmology. 2010;94(7):940–946. doi: 10.1136/bjo.2009.165092. [DOI] [PubMed] [Google Scholar]
- 11.Kim HS, Luo L, Pflugfelder SC, et al. Doxycycline inhibits TGF-beta1-induced MMP-9 via Smad and MAPK pathways in human corneal epithelial cells. Investigative ophthalmology & visual science. 2005;46(3):840–848. doi: 10.1167/iovs.04-0929. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Ding J. The combined effect of azithromycin and insulin-like growth factor-1 on cultured human meibomian gland epithelial cells. Investigative ophthalmology & visual science. 2014 doi: 10.1167/iovs.14-14782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Briggs MR, Hua X, et al. Nuclear protein that binds sterol regulatory element of low density lipoprotein receptor promoter. II. Purification and characterization. The Journal of biological chemistry. 1993;268(19):14497–14504. [PubMed] [Google Scholar]
- 14.Akpek EK, Vittitow J, Verhoeven RS, et al. Ocular surface distribution and pharmacokinetics of a novel ophthalmic 1% azithromycin formulation. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2009;25(5):433–439. doi: 10.1089/jop.2009.0026. [DOI] [PubMed] [Google Scholar]
- 15.Chiambaretta F, Garraffo R, Elena PP, et al. Tear concentrations of azithromycin following topical administration of a single dose of azithromycin 0.5%, 1.0%, and 1.5% eyedrops (T1225) in healthy volunteers. European journal of ophthalmology. 2008;18(1):13–20. doi: 10.1177/112067210801800103. [DOI] [PubMed] [Google Scholar]
- 16.Halliwell WH. Cationic amphiphilic drug-induced phospholipidosis. Toxicologic pathology. 1997;25(1):53–60. doi: 10.1177/019262339702500111. [DOI] [PubMed] [Google Scholar]
- 17.Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. Journal of the American Academy of Dermatology. 2006;54(2):258–265. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Buolamwini JK. Cell cycle molecular targets in novel anticancer drug discovery. Current pharmaceutical design. 2000;6(4):379–392. doi: 10.2174/1381612003400948. [DOI] [PubMed] [Google Scholar]
- 19.Antherieu S, Rogue A, Fromenty B, et al. Induction of vesicular steatosis by amiodarone and tetracycline is associated with up-regulation of lipogenic genes in HepaRG cells. Hepatology. 2011;53(6):1895–1905. doi: 10.1002/hep.24290. [DOI] [PubMed] [Google Scholar]
- 20.Labbe G, Fromenty B, Freneaux E, et al. Effects of various tetracycline derivatives on in vitro and in vivo beta-oxidation of fatty acids, egress of triglycerides from the liver, accumulation of hepatic triglycerides, and mortality in mice. Biochemical pharmacology. 1991;41(4):638–641. doi: 10.1016/0006-2952(91)90640-q. [DOI] [PubMed] [Google Scholar]
- 21.Foulks GN, Borchman D, Yappert M, et al. Topical azithromycin and oral doxycycline therapy of meibomian gland dysfunction: a comparative clinical and spectroscopic pilot study. Cornea. 2013;32(1):44–53. doi: 10.1097/ICO.0b013e318254205f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kashkouli MB, Fazel AJ, Kiavash V, et al. Oral azithromycin versus doxycycline in meibomian gland dysfunction: a randomised double masked open label clinical trial. The British journal of ophthalmology. 2014 doi: 10.1136/bjophthalmol-2014-305410. [DOI] [PubMed] [Google Scholar]