Abstract
Endophytic actinomycetes encompass bacterial groups that are well known for the production of a diverse range of secondary metabolites. Vochysia divergens is a medicinal plant, common in the “Pantanal” region (Brazil) and was focus of many investigations, but never regarding its community of endophytic symbionts. During a screening program, an endophytic strain isolated from the V. divergens, was investigated for its potential to show biological activity. The strain was characterized as Microbispora sp. LGMB259 by spore morphology and molecular analyze using nucleotide sequence of the 16S rRNA gene. Strain LGMB259 was cultivated in R5A medium producing metabolites with significant antibacterial activity. The strain produced 4 chemically related β-carbolines, and 3 Indoles. Compound 1-Vinyl-β-carboline-3-carboxylic acid displayed potent activity against the Gram-positive bacterial strains Micrococcus luteus NRRL B-2618 and Kocuria rosea B-1106, and was highly active against two human cancer cell lines, namely the prostate cancer cell line PC3 and the non-small-cell lung carcinoma cell line A549, with IC50 values of 9.45 and 24.67 µM, respectively. 1-Vinyl-β-carboline-3-carboxylic acid also showed moderate activity against the yeast Saccharomyces cerevisiae ATCC204508, as well as the phytopathogenic fungiPhyllosticta citricarpa LGMB06 and Colletotrichum gloeosporioides FDC83.
Keywords: Pantanal, β-carboline, Indoles, antibiotics, anticancer agents, Endophytic Microbispora
Introduction
It has been well established that microorganisms are a virtually unlimited source of natural products, many of which have potential therapeutic applications. Without such discoveries “there would be a significant therapeutic deficit in several important clinical areas, such as infectious and cardiovascular disease, most solid tumors, and immune-inflammatory diseases” [24]. Therefore, there is an urgent need to search for effective new antibacterial or antifungal agents in treatment of infectious diseases at present. While terrestrial microorganisms were largely explored over the past 50 years, endophytes isolated from medicinal plants became of significant importance for the production of new compounds [4, 16]. Endophytic actinomycetes are Gram-positive bacteria reside in the internal tissues of plants via symbiotic, parasitic, or mutualistic means, without causing immediately overt negative effects for the plant [25]. The genus Microbispora was originally described for actinomycetes that produce characteristic paired spores on the aerial mycelium. Members of the genera Microbispora were isolated from various hosts, and are known for production of bioactive secondary metabolites, with antibacterial [10], antifungal [22] and antitumor [15] activities. We were particularly interested in endophytes from medicinal plants found in the Pantanal, a unique tropical wetland region of Brazil that stretches also into Bolivia and Paraguay. Due to the dynamic character of Pantanal, few trees are able to tolerate long periods of flooding, that begins in November and in adjacent areas can last until mid-June. Among the plant species that have tolerance to high levels of flooding is the Vochysia divergens [1]. Vochysia divergens is a medical plant, common in South America, and it is largely used because of its bactericide activity against Staphylococcus aureus and its antinociceptive activity [5].
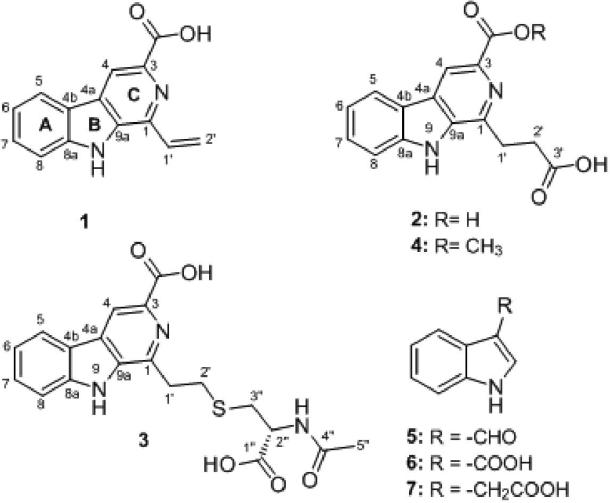
In the present work, we describe the isolation of an endophytic actinomycete strain from V. divergens (Pantanal, Brazil), which generates compounds with biological activity, and the identification of this strain based on spore characteristics and phylogenetic analyze using 16S rRNA. Fermentation of this strain on R5A-medium, followed by extraction and purification yield 4 β-carbolines and 3 indoles (Fig. 1). Compound 1-vinyl-β-carboline-3-carboxylic acid showed large biological activity, in contrast the compounds JBIR-133, kitasetaline and methyl 1-(propionic acid)-β-carboline-3-carboxylic acid neither revealed activities. The bioactivity tests indicate that the vinyl side chain attached at 1-position in compound 1 is essential for both the antitumor and the antibiotic activity of this natural product, and the studies presented here provide further insights into the structure activity relationships (SAR) of β-carbolines.
Fig. 1.
Chemical structure of compounds 1-7
Material and Methods
Taxonomy
Strain LgMB259 was isolated by V. divergens leaves collected from the Pantanal, in the region of Nhecolândia (S18°10.07’, W57°23.03’) in Brazil. To the endophytic isolation, the preference was given to leaves with no marks, scratches or wounds. To eliminate epiphytic microorganisms, a purification protocol of six steps was used [23]. The leaves were fragmented and inoculated in Petri dishes with medium PDA (Potato Dextrose Agar). The plates were incubated at 28 °C for 30 days, and the growth was daily verified. The living cultures were deposited in the LabGeM collection, Federal University of Paraná, Curitiba, Paraná, Brazil (http://www.labgem.ufpr.br/).
To the Scanning Electron Microscope strain LGMB259 was grow up in plates ISP medium 3 [12] at 37°C for 15 days, and was fixed in Karnovsky solution (glutaraldehyde 2.5%, paraformaldehyde 2.5% in sodium cacodylate buffer 0.05 M, CaCl2 0.001 M, pH 7.2) for 24 hours. Sample was dehydrated in ascending series of ethanol, 30, 50, 70, 90 and 100% for 10 minutes at each step, the last step 100%, repeated three times. The acrylic resin infiltration was started with a pre-infiltration of PA resin and ethanol in the ratio 1:1 for approximately 5 hours, followed by infiltration with pure resin for one night. Finally, the sample was placed at room temperature for polymerizing. The analysis of the strain was performed under light microscope ‘Zeiss Axioskop 2’, by acquiring photographs in digital camera.
Genomic DNA extraction was carried out using the UltraClean™ Microbial DNA Kit (MO Bio, Carlsbad, CA, USA) according to manufacturer's protocol. The primers 9f (5’ – GAGTTTGATCCTGGCTCAG) and 1541r (5’- AAGGAGGTGATCCAGCC) were used to amplify the gene 16S rRNA [20]. The PCR product was purified using ethanol precipitation. The product of PCR was sequenced using BigDye Terminator Cycle Sequencing Kit v3.1 (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions, and sequences were analyzed on an ABI3100 DNA Sequencer (Applied Biosystems, Foster City, CA, USA). The sequence was compared with available sequences in the Genbank database of NCBI (http://www.ncbi.nlm.nih.gov/), and was aligned using the CLUSTAL_W v.1.81 program [27]. Alignment was manually verified and adjusted prior to the construction of a phylogenetic tree. The phylogenetic tree was constructed using the Maximum likelihood method in the Garli version 2.0 [28]. The confidence values for branches of the phylogenetic tree were determined using bootstrap analyses based on 1000 resampling.
Fermentation, Extraction and Isolation
The Microbispora sp. LGMB259 was cultivated on ISP3-agar plates at 37 °C for 7 days. Chunks of agar with the fully-grown strains were used to inoculate five (250 mL) Erlenmeyer flasks, each containing 50 mL of R5A medium [11]. Individual cultures were grown at 37 °C for 3 days and subsequently used as seed cultures for the scale-up fermentation. The seed cultures were used to inoculate 80 Erlenmeyer flasks (250 mL) each containing 100 mL of R5A medium. Fermentation (8 L) was continued at 37 °C with, shaking (250 rpm) for 10 days. The obtained orange culture broth was centrifuged and filtered over celite. The biomass (mycelium) was extracted with MeOH (5 X 500 mL) and then the recovered organics were evaporated in vacuo at 40 °C to yield 5.4 g of crude extract. The supernatant was mixed with 5% (w/v) XAD-16 resin and stirred overnight, followed by filtration. The water fraction was extracted with EtOAC (5 X 500 mL) and then recovered organics were evaporated in vacuo at 40 °C to yield 170 mg of water extract. The resin was washed with water (3 X 600 mL) and then extracted with MeOH until the eluent was colorless. The MeOH was subsequently mixed with water and extract with EtOAC (5 X 500 mL), and then recovered organics were evaporated in vacuo at 40 °C to yield 480 mg of XAD extract. The crude extract was then subjected to a Reverse Phase C18 column chromatography (20 X 8cm, 250g) eluted with a gradient of H2O-MeOH (100:0-0:100), followed of HPLC purification to yield the compounds 2 (mg) and 3 (mg). The water extract was subjected to semipreparative HPLC and resulted in compounds 5 (10.8 mg) and 6 (12.3 mg) in pure form. The XAD extract was subject to HPLC, Sephadex LH-20 (MeOH; 1 × 20 cm), and further purified by HPLC and offered compounds 1, 3, 4, 7 (11 mg, 40 mg, 11 mg, 2.3 mg , 9.6 mg) (Supplementary file, Fig. S2).
For preparative scale separation, Phenomenex (Torrance, CA 90501-1430 ) C18 column (10 × 250 mm, 5 μm) was used on a Varian (Varian, Palo Alto, CA, USA) ProStar Model 210 equipped with a photodiode diode array detector and a gradient elution profile (solvent A: H2O, solvent B: acetonitrile; flow rate: 5.0 mL min-1; 0-2 min 85% A and 15% B, 2-23 min, 85-20% A, 23-24 min 20% A and 80 % B, 24-25 min 20-85% A, 25-26 min 85% A and 15% B). UV spectra were recorded on an Ultrospec 8000 spectrometer (GE, Pittsburgh, USA). HRESI mass spectra were recorded on AB SCIEX Triple TOF® 5600 System. HPLC-MS analyses were carried out in Waters 2695 LC module (Waters corp. Milford, MA, USA) using a Symmetry Anal C18 5 μm column (4.6 × 250 mm, Waters corp. Milford, MA 01757) and a gradient elution profile (solvent A: H2O, solvent B: acetonitrile; flow rate: 0.5 mL min-1; 0-4 min 90% A and 10% B, 4-22 min, 90-0% A, 22-27 min 0% A and 100% B, 27-29 min 0-90% A, 29-35 min 90% A and 10 % B). NMR spectra were measured on a Varian VnmrJ 500 (1H, 500 MHz; 13C, 125.7 MHz) spectrometer; the δ-values were referenced to the respective solvent signals. All solvents used were of ACS grade and purchased from the Pharmco-AAPER (Brookfield, CT). Rf values were measured on Polygram SIL G/UV254 (Macherey-Nagel & Co.). Size exclusion chromatography was performed on Sephadex LH-20 (GE Healthcare).
Antimicrobial and Antifungal Activity
The Gram-negative bacterium Escherichia coli DH5α (Invitrogen) and the Gram-positive bacteria Micrococcus luteus NRRL B-2618 and Kocuria rosea B-1106 were maintained in lysogeny broth (LB) liquid media and Mueller-Hinton agar. A sterile loopful of each organism was inoculated into a 7 mL culture of LB broth and incubated in a 37 °C orbital shaker at 200 RPM for 10 hours. Each test organism was streaked on a sterile Mueller-Hinton agar plate with a sterile cotton swab. Compounds 1-3 were dissolved in methanol and were aliquoted in 100 μg amounts per each 6 mm sterile filter disc and were allowed to dry in a laminar flow hood. The discs were placed on the plates, which were then incubated for 24 hours at 37 °C. The resulting of inhibition zone was measured in millimeters.
The fungal strains Saccharomyces cerevisiae ATCC 204508, Phyllosticta citricarpa LGMB06 and Colletotrichum gloeosporioides FDC83 were used in disc diffusion assays. Solutions of amphotericin B and test compounds were made in MeOH. Each sterile paper disc was loaded with 20 mL solution and was allowed to dry in the biosafety cabinet for 4 h. The dried discs were then placed on the V8 agar plate following the homogeneous distribution of fungus. MeOH was used as a negative control. The plates were then incubated at 24 °C for 3, 7, 10 and 14 days. Inhibition zone were then measured.
Cell Viability Assay
Conversion of resazurin (7-hydroxy-10-oxido-phenoxazin-10-ium-3-one) to its fluorescent product resorufin was monitored to assess viability of human lung non-small cell carcinoma 549 and prostate adenocarcinoma PC3 cell lines. DMEM/F-12 Kaighn's modification media (Life Technologies, NY, USA) was used to grow A549 and PC3 cells (ATCC, Manassas, VA, USA) with 10% heat-inactivated fetal bovine serum (FBS), 100 U mL−1 penicillin, 100 μg mL−1, streptomycin, 2 mM L-glutamine. Cells were seeded at a density of 3 × 103 cells per well in 96-well clear bottom culture plates (Corning, NY, USA), incubated 24 hours at 37 °C in a humidified atmosphere containing 5% CO2 and were subsequently exposed to known toxin (1.5 mM hydrogen peroxide, 10 μg mL−1 actinomycin D) and test compounds for two days. To assess cell viability, 150 μM of resazurin (Sigma, St. Louis, MO, USA) was added to each well, plates were shaken briefly for 10 seconds and incubated for another 3 hours at 37 °C to allow viable cells to convert resazurin into resorufin. The fluorescence intensity for resorufin was detected on a scanning microplate spectrofluorometer FLUOstar Omega (BMG Labtech, Cary, NC, USA) using an excitation wavelength of 560 nm and an emission wavelength of 590 nm.
Results
Strain Isolation and Cultivation
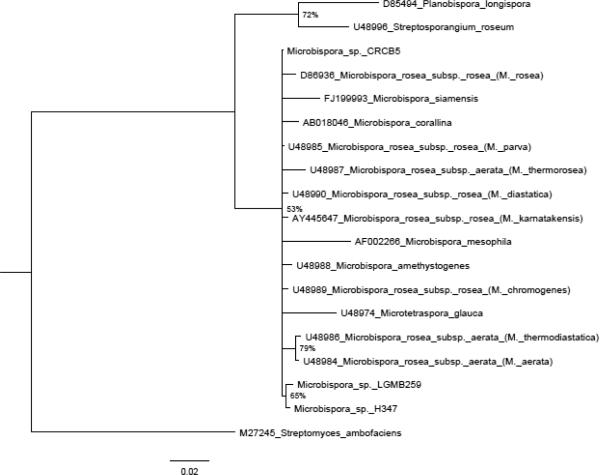
In our search about biodiversity and bioactive compounds, the strain LGMB259 was isolated from leaf tissues of V. divergens. Strain LGMB259 produced branched and non-fragmented substrate mycelia. Non-motile spores in characteristic longitudinal pairs were borne on short sporophores branching from aerial hyphae. Each spore was oval and its surface was smooth (Fig. 2). Neither sporangium-like bodies nor any other special structures were observed, characteristic of the genus Microbispora. Strain LGMB259 was confirmed to belong to the Microbispora genus by 16S rRNA analysis. The strain showed the highest level similarity with sequences deposited in the Genbank (99%); strain Microbispora sp. H347, Microbispora sp. CRCB5 and type strain Microbispora rosea subsp. rosea JCM8971. However, in the phylogenetic analysis (Fig. 3) was not possible to assign this isolate to a single species, due the low Bootstrap support.
Fig. 2.
Scanning electron micrograph of spherical paired spores with smooth surfaces of strain Microbispora sp. LGMB259 grown on ISP medium 3 for 15 days at 37 °C. Bar, 10 μm.
Fig. 3.
Maximum Likelihood Phylogenetic tree for Microbispora genus using 16S rRNA gene. Only clades with more than 50% of are shown. The tree was rooted with Streptosporangium roseum (U48996).
Structure Elucidation
A large-scale fermentation of the strain in R5A-medium afforded 5.4 g of crude extract, 170 mg of water extract and 480 mg of XAD extract. Isolation and purification of the obtained extracts using various chromatographic techniques afforded compounds 1~7 in pure forms (Supplementary file, Fig. S2).
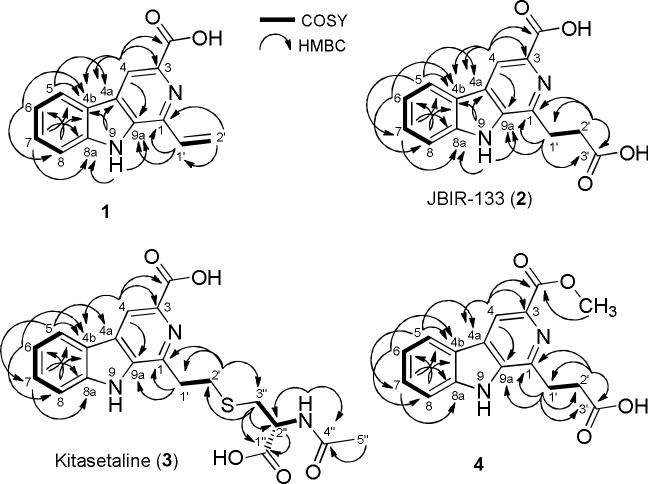
The physicochemical properties of compounds 1~4 are summarized in Table 1. Compound 1 was isolated as pale yellow solid (4.1 mg L−1), the molecular formula of compound 1 was deduced as C14H10N2O2 on the basis of HR-ESI-MS (Table 1). The proton NMR spectrum of 1 in DMSO-d6 (Table 2) displayed one broad H/D exchangeable signal at δ 12.21 of an OH or NH group. In addition, the 1H NMR spectrum displayed four aromatic proton signals at δ 8.39 (d, J = 8.0 Hz), 7.67 (dd, J = 7.5, 1.0 Hz), 7.63 (ddd, J = 8.0, 7.5, 1.5 Hz), and 7.33 (ddd, J = 8.0, 8.0, 1.0 Hz), representing a tetra-substituted benzene along with one aromatic proton singlet at δ 8.82. Three additional olefinic proton signals were observed at δ 7.46 (dd, J = 17.0, 10.5 Hz), 6.68 (dd, J = 17.0, 1.5 Hz) and 5.77 (dd, J = 11.0, 1.5 Hz). The 13C NMR/HSQC spectra (Table 2) along with the UV spectrum confirmed the structure of compound 1 to be a β-carboline bearing a vinyl-side chain. The 1H-1H COSY and HMBC correlations (Fig. 4) of compound 1 finalized the structure, showing 3JC-H and JC-H HMBC correlations from the CH2-2′ and CH-1′ to C-1, respectively, confirming the attachment of the vinyl group at the C-1 position (Supplementary file, Fig. S3-13).
Table 1.
Physico-chemical properties of compounds 1-4
| 1a) | 2a) | 3a) | 4a) | |
|---|---|---|---|---|
| Molecular Formula | C14H10N2O2 | C15H12N2O4 | C19H19N3O5S | C16H14N2O4 |
| Appearance | Pale-yellow solid, UV-absorbing (254 nm) | Pale-yellow solid, UV-absorbing (254 nm) | Pale-yellow solid, UV-absorbing (254 nm) | Pale-yellow solid, UV-absorbing (254 nm) |
| HPLC-R t | 12.86 (min) | 12.48 (min) | 12.37 (min) | 20.06 (min) |
| R f | 0.28 (DCM/10% MeOH) | 0.10 (DCM/20% MeOH) | 0.34 (DCM/20% MeOH) | 0.13 (DCM/20% MeOH) |
| Anisaldehyde/H2SO4 reagent | Yellow | Yellow | Yellow | Yellow |
| (+)-APCI-MS: m/z | 239 [M + H]+, 477 [2M + H]+, 193 [(M-COOH) + H]+ | 285 [M + H]+, 267 [M – H2O]+, 239 [(M-COOH) + H]+ | 402 [M + H]+, 273 [(M-Cys) + H]+ | 299 [M + H]+, 267 [M – H2O]+, 239 [(M-COOH) + H]+ |
| (–)-APCI-MS: m/z | 237 [M – H]–, 475 [2M – H]–, 191 [(M-COOH) – H]– | - | 400 [M – H]–, 801 [2M – H]–, 271 [(M-Cys) – H]– | - |
| (+)-HRESI-MS: m/z | 239.0814 [M + H]+ | - | - | - |
| Calcd. | 239.0815 for C14H11N2O2 [M + H]+ | - | - | - |
| (–)-HRESI-MS: m/z | 237.0662 [M – H]– | - | - | - |
| Calcd. | 237.0669 for C14H9N2O2 [M – H]– | - | - | - |
| UV/VIS (MeOH): λmax (log ε) | 394 sh (3.08), 366 sh (3.42), 282 (4.30), 213 (4.17) nm | 374, 278, 240, 213 nm | 375, 278, 241, 214 nm | 374, 272, 240, 218 nm |
For HPLC, UV and Mass spectrometry data, see supporting information Fig. S4-7, S13, S19 and S25.
Table 2.
13C and 1H NMR data of compounds 1-4 in DMSO-d6 (mult., J in [Hz])
| No. | 1a) | 2a) | 3a) | 4a) | ||||
|---|---|---|---|---|---|---|---|---|
| δ C b) | δ H c) | δ C b) | δ H c) | δ C b) | δ H c) | δ C b) | δ H c) | |
| 1 | 138.3 Cq | - | 143.8 Cq | - | 142.9 Cq | - | 144.6 Cq | - |
| 3 | 136.5 Cq | - | 134.0 Cq | - | 134.2 Cq | - | 135.8 Cq | - |
| 3-CO | 166.4 Cq | - | 165.3 Cq | - | 165.2 Cq | - | 166.1 Cq | - |
| 3COCH3 | - | - | - | - | - | - | 51.9 CH3 | 3.90 (s) |
| 4 | 116.4 CH | 8.85 (s) | 116.5 CH | 8.93 (s) | 116.9 CH | 9.02 (s) | 116.2 CH | 8.79 (s) |
| 4a | 129.3 Cq | - | 128.7 Cq | - | 129.0 Cq | - | 128.4 Cq | - |
| 4b | 121.0 Cq | - | 121.1 Cq | - | 121.1 Cq | - | 121.3 Cq | - |
| 5 | 122.2 CH | 8.39 (d, 8.0) | 122.7 CH | 8.44 (d, 8.0) | 122.9 CH | 8.49 (d, 8.0) | 122.1 CH | 8.37 (d, 7.5) |
| 6 | 120.4 CH | 7.33 (ddd, 8.0, 8.0, 1.0) | 120.9 CH | 7.36 (t, 8.0) | 121.0 CH | 7.39 (t, 8.0) | 120.2 CH | 7.30 (t, 8.0) |
| 7 | 128.9 CH | 7.63 (ddd, 8.0, 7.5, 1.5) | 129.6 CH | 7.66 (t, 8.5) | 130.1 CH | 7.70 (t, 8.0) | 128.4 CH | 7.59 (t, 7.0) |
| 8 | 112.4 CH | 7.67 (dd, 7.5, 1.0) | 112.7 CH | 7.71 (d, 8.5) | 112.8 CH | 7.74 (d, 8.5) | 112.4 CH | 7.66 (d, 8.5) |
| 8a | 141.3 Cq | - | 141.8 Cq | - | 142.1 Cq | - | 140.8 Cq | - |
| 9 | - | 12.22 (s) | - | 12.54 (brs) | - | 12.67 (brs) | - | 12.20 (brs) |
| 9a | 134.9 Cq | - | 135.6 Cq | - | 135.6 Cq | - | 135.8 Cq | - |
| 1′ | 131.1 CH | 7.46 (dd, 17.0, 10.5) | 27.1 CH2 | 3.50 (t, 7.5) | 32.0 CH2 | 3.57 (t, 7.5) | 28.5 CH2 | 3.38 (t, 7.5) |
| 2′ | 120.7 | 6.68 (dd, 17.0, 1.5, Ha) 5.77 (dd, 11.0, 1.5, Hb) |
31.8 CH2 | 2.91 (t, 7.5) | 30.3 CH2 | 3.09 (t, 7.5) | 32.0 CH2 | 2.87 (t, 8.0) |
| 3′ | - | - | 173.9 Cq | - | - | - | 174.2 Cq | - |
| N-Acetyl-l-Cys | ||||||||
| 1″ | - | - | - | - | 172.3 Cq | - | - | - |
| 2″ | - | - | - | - | 52.1 CH | 4.44 (m) | - | - |
| 2″-NH | - | - | - | - | - | 8.30 (d, 8.5) | - | - |
| 3″ | - | - | - | - | 33.1 CH2 | 3.03 (dd, 13.5, 5.0) 2.86 (dd, 13.5, 8.5) |
- | - |
| 4″ | - | - | - | - | 169.5 Cq | - | - | - |
| 5″ | - | - | - | - | 22.4 CH3 | 1.84 (s) | - | - |
Supporting information Fig. S8-12, S14-18, S20-24 and S26-30 for the NMR spectra
125 MHz
500 MHz
Fig. 4.
Selected 1H,1H-COSY (▬) and HMBC (→) correlations of β-carbolines 1-4
The molecular weights of compounds 2, 3 e 4 were determined as 284, 401 and 299 Daltons, respectively, based on the (+) and (−)-APCI-MS (Table 1). The proton NMR spectrum along with the 13C NMR/HSQC spectra (Table 2) of compounds 2, 3 and 4 showed that they contains the same β-carboline core as compound 1 with Δm/z = 46, 163 and 60 amu higher than 1, respectively. Furthermore, the signals of the vinyl group characteristic for compound 1 were missing in the spectra of compounds 2, 3 and 4, and instead two triplet methylene signals were observed (Table 2). Thorough analyses of the 1D and 2D NMR spectra (Table 2, Fig. 4) of compounds 2, 3 and 4, followed by a substructure search in AntiBase [18] revealed the identity of 2, 3 and 4 with JBIR-133, kitasetaline and methyl 1-(propionic acid)- β-carboline -3-carboxylic acid, respectively (Supplementary file, Fig. S14-31).
Compounds 5~7 were characterized by mass spectra and NMR data as indole-3-carbaldehyde (5), indole-3-carboxylic acid (6) and indole-3-acetic acid (7), according to the data in the literature [18] (Supplementary file, Fig. S32-40).
Antibacterial and Antifungal Activities
The antibacterial activity of the crude extracts and β-carboline compounds (1~3) were determined against the Gram-negative and Gram-positive bacteria (Table 3). 1-vinyl-β-carboline-3-carboxylic acid (1) displayed potent antibacterial activity against the Gram-positive bacterial strains K. rosea and M. luteus. Compound 1 also showed moderate antifungal activity against C. gloeosporioides, P. citricarpa and S. cerevisiae (Table 3), while the other congeners (compounds 2~3) neither revealed antibacterial nor antifungal activities against the above mentioned bacterial and antifungal strains at 100 μg/disc.
Table 3.
Inhibition zone (in millimeters) of LGMB259 crude extract and compounds 1-3 tested antibacterial and antifungal activities at 100 μg/disc
| Extracts /Isolated compounds |
Kocuria rosea |
Micrococcus luteus |
Escherichia coli |
Phyllosticta citricarpa |
Colletotrichum gloeosporioides |
Saccharomyces cerevisiae |
|---|---|---|---|---|---|---|
| R5A-medium | 33 | 30 | --- | NE | NE | NE |
| Compound 1 | 31 | 30 | --- | 9 | 17 | 16 |
| Compound 2 | --- | --- | --- | --- | --- | --- |
| Compound 3 | --- | --- | --- | --- | --- | --- |
| Control | 30 | 31 | 35 | 27 | 25 | 50 |
--- denotes no measurable halo, NE not evaluated antibacterial control: Ampicillin (1 mg/disc), Antifungal control: amphotericin B (1 mg/disc)
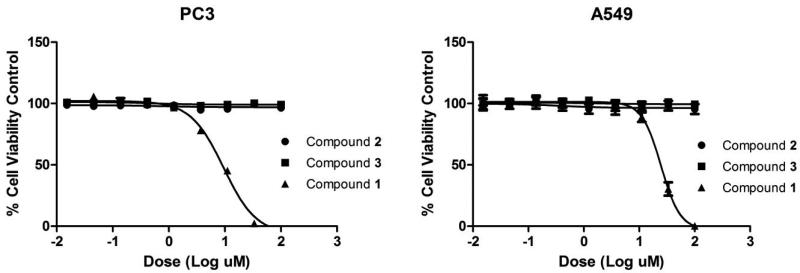
Cytotoxicity Assays
The cytotoxic activities of 1-vinyl-β-carboline-3-carboxylic acid, JBIR-133 and kitasetaline were determined, using PC3 (prostate) and A549 (non-small cell lung) human cancer cell lines (Fig. 5A and 5B). Cell viability assays showed that 1-vinyl-β-carboline-3-carboxylic acid was highly active against both PC3 (IC50 = 9.45 μM) and A549 (IC50 = 24.67 μM) cell lines. In contrast, JBIR-133 and kitasetaline were not active up to 100 μM.
Fig. 5.
Dose response curve of β-carbolines 1-3, in PC3 and A549 cell lines at 48h in viability assay.
Discussion
This research represents the very first attempt to isolate endophytic actinomycetes from V. divergens, which is a typical herbal medicine, which produces compounds with antibacterial activitiy like sericic acid [14] and anti-allodynic properties like tormentic acid [5]. This strain LGMB259 showed morphology characteristic of Microbispora genus. Highs values (99%) of similarity with 3 strains in the database GenBank was observed. The first strain Microbispora sp. H347 was isolated from sample of Korean soil, and was utilized in a phylogenetic study of the Genus Microbispora, however this strain formed a clade with the type stains M. rose and M. parva and have no support to classification as a unique species [19]. The others stains correlated are Microbispora sp. CRCB5, strain utilized in cellulose- decomposing, but was not characterized on species level [8], and the type strain Microbispora rosea subsp. rosea JCM8971 [21]. Strain LGMB259 formed a clade with Microbispora sp. H347 in the 16S rRNA phylogenetic analysis, this analysis showed conflicting topologies and no support to classification in species level. So we assumed that the 16S rRNA analysis is more appropriate for discrimination on the genus level, and a multigene study is necessary to phylogenetic characterization of this genus.
1-Vinyl-β-carboline-3-carboxylic acid (1), was responsible for the antibacterial activity of the extract from R5A-medium, this compound showed high cytotoxic activity and moderate activity against the yeast S. cerevisiae ATCC204508, and the phytopathogens P. citricarpa LGMB06 and C. gloeosporioides FDC83. In contrast, JBIR-133 (2) and kitasetaline (3) neither revealed biological activity, as well the compound methyl 1-(propionic acid)- β-carboline -3-carboxylic acid (4) [17], indicating that the vinyl side chain attached at 1-position in compound 1 is crucial for the biological activities of this natural product. β-Carbolines are nitrogen-containing heterocyclic compounds that consists of a pyridine ring fused to an indole skeleton [6]. Only ~30 β-carboline derivatives have been reported from bacteria so far [18]. They show an interesting biological activity spectrum, ranging from antibacterial over fungicidal to herbicidal. Some of them were reported as having affinity to the benzodiazepine-receptor [7, 26]. 1-Vinyl-β-carboline-3-carboxylic acid (1) was reported for the first time as natural product from Nocardiopsis dassonvillei (Ichihara, T., Japanese patent application JP 57-169481 A. 1982). However NMR assignments of this natural product based on extensive 1D and 2D NMR analyses and antifungal activity are reported here for the first time. Compounds JBIR-133 (2) [3] and kitasetaline (3) [2] were recently reported as metabolites of genetically modified Kitasatospora setae NBRC 14216 strains. Both compounds are reported here for the first time from a wild type bacterial strain.
Indoles are a group of compounds produced by a big group of organisms, and are ubiquitously present in higher plants and a wide range of microbes particularly those in association with plant [9]. We have isolated indole-3-carbaldehyde (5), indole-3-acetic acid (6) and indole-3-carboxylic acid (7) from strain Microbispora LGMB259. Recent findings about indole-3-acetic acid suggest that this compound serves as a signaling molecule in certain plant-associated microbes and it might exert an indispensable impact in microbe-plant interaction and was correlated with plant growth promotion [20]. Furthermore the compound indole-3-carboxylic acid was related to an active role in the induced resistance upon infection by fungi in plants [13]. These data suggest the possibility of strain LGMB259 be utilized in biological control, since this microorganism produces compounds with antimicrobial activity (including phytopathogens), compounds that improve defense systems in plants and was isolated as an endophytic microbe.
In summary, the strain was characterized as Microbispora sp. LGMB259 by morphologic and phylogenetic analysis. This strain produced as activity metabolite 1-vinyl-β-carboline-3-carboxylic acid (1), a compound that displayed high antibacterial activity against selected Gram-positive bacterial, moderate antifungal activity and considerable cytotoxic activity against PC3 and A549 human cancer cell lines. Overall, these bioactivity studies provide new insights into the structure-activity-relationships (SAR) of β-carbolines, and show that the vinyl side chain is essential for the observed biological activities. This research also opens an interesting possibility about the utilization of strain LGMB259 as biological control, due the capacity of survive in the plants tissues and produce compounds with biological interest in the plant defense.
Supplementary Material
Acknowledgements
We thank Dr. Jack Goodman (University of Kentucky) for the HRESIMS measurements and Dr Francisco André Ossamu Tanaka (NAP/MEPA) for the Scanning Electron Microscope analysis. D. C. S. thanks the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for a short term scholarship. This work was supported in part by the University of Kentucky College of Pharmacy, the University of Kentucky Markey Cancer Center and the National Center for Advancing Translational Sciences (UL1TR000117). This work was also supported by NIH grant CA 91091 to J.R, and Fundação Araucária – Apoio ao desenvolvimento científico e tecnológico do Paraná.
References
- 1.Arieira J, Nunes Da Cunha C. Fitossociologia de uma floresta inundável monodominante de Vochysia divergens Pohl (Vochysiaceae), no Pantanal Norte, MT, Brasil. Acta Bot Bras. 2006;20:569–580. [Google Scholar]
- 2.Aroonsri A, Kitani S, Hashimoto J, Kosone I, Izumikawa M, Komatsu M, Fujita N, Takahashi Y, Shin-Ya K, Ikeda H, Nihira T. Pleiotropic control of secondary metabolism and morphological development by KsbC, a butyrolactone autoregulator receptor homologue in Kitasatospora setae. Appl Environ Microbiol. 2012;78:8015–24. doi: 10.1128/AEM.02355-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aroonsri A, Kitani S, Ikeda H, Nihira T. Kitasetaline, a novel beta-carboline alkaloid from Kitasatospora setae NBRC 14216T. J Biosci Bioeng. 2012;114:56–8. doi: 10.1016/j.jbiosc.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 4.Berdy J. Bioactive microbial metabolites. J Antibiot. 2005;58:1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- 5.Bortalanza LB, Ferreira J, Hess SC, Delle Monache F, Yunes RA, Calixto J B. Anti-allodynic action of the tormentic acid, a triterpene isolated from plant, against neuropathic and inflammatory persistent pain in mice. Eur J Pharmacol. 2002;453:203–8. doi: 10.1016/s0014-2999(02)02428-7. [DOI] [PubMed] [Google Scholar]
- 6.Bracher F, Hildebrand D, Haberlein H. 1-Substituted β-carboline-3-carboxylates with highaffinities to the benzodiazepine recognition site. Nat Prod Res. 2004;18:391–6. doi: 10.1080/14786410310001630483. [DOI] [PubMed] [Google Scholar]
- 7.De Sarro G, Carotti A, Campagna F, McKernan R, Rizzo M, Falconi U, Palluotto F, Giusti P, Rettore C, De Sarro A. Benzodiazepine receptor affinities, behavioral, and anticonvulsant activity of 2aryl-2,5-dihydropyridazino[4,3-b]indol- 3(3H)-ones in mice. Pharmacol Biochem Behav. 2000;65:47587. doi: 10.1016/s0091-3057(99)00230-0. [DOI] [PubMed] [Google Scholar]
- 8.Eida MF, Nagaoka T, Wasaki J, Kouno K. Isolation and Characterization of Cellulose-decomposing Bacteria Inhabiting Sawdust and Coffe Residue Composts. Microbes Environ. 2012;27:226–233. doi: 10.1264/jsme2.ME11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Deby B, Bazaid S, Gherbawy Y, Elhariry H. Characterization of endophytic bacteria associated with rose plant (Rosa damascena trigintipeta) during flowering stage and their plant growth promoting traits. J Plant Interact. 2012;3:248–253. [Google Scholar]
- 10.Esumi Y, Suzuki Y, Itoh Y, Uramoto M, Kimura K, Goto M, Yoshihama M, Ichikawa T. Propeptin, a new inhibitor of prolyl endopeptidase produced by Microbispora II. Determination of chemical structure. J Antibiot. 2002;55:296–300. doi: 10.7164/antibiotics.55.296. [DOI] [PubMed] [Google Scholar]
- 11.Fernández E, Weissbach U, Sánchez Reillo C, Braña AF, Méndez C, Rohr J, Salas JA. Identification of two genes from Streptomyces argillaceus encoding glycosyltransferases involved in transfer of a disaccharide during biosynthesis of the antitumor drug mithramycin. J Bacteriol. 1998;180:4929–37. doi: 10.1128/jb.180.18.4929-4937.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fogle MR, Douglas DR, Jumper CA, Straus DC. Growth and mycotoxin production by Chaetomium globosum. Mycopathologia. 2007;164:49–56. doi: 10.1007/s11046-007-9023-x. [DOI] [PubMed] [Google Scholar]
- 13.Gamir J, Pastor V, Cerezo M, Flors V. Identification of indole-3-carbolylic acid as mediator of priming against Plectosphaerella cucumerina. Plant Physiol Bioch. 2012;61:169–179. doi: 10.1016/j.plaphy.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Hess SC, Brum RL, Honda NK, Cruz AB, Moretto E, Cruz RB, Messana I, Ferrari F, Cechinel-Filho V, Yunes RA. Antibacterial activity and phytochemical analysis of Vochysia divergens (Vochysiaceae). J Ethnopharmacol. 1995;74:97–100. doi: 10.1016/0378-8741(95)01260-k. [DOI] [PubMed] [Google Scholar]
- 15.Ivanova V, Laatsch H, Kolarova M, Aleksieva K. Structure elucidation of a new natural diketopiperazine from a Microbispora aerata strain isolated from Livingston Island, Antarctica. Nat Prod Res. 2013;27:164–70. doi: 10.1080/14786419.2012.665911. [DOI] [PubMed] [Google Scholar]
- 16.Kim TU, Cho SH, Han JH, Shin YM, Lee HB, Kim SB. Diversity and physiological properties of root endophytic actinobacteria in native herbaceous plants of Korea. J Microbiol. 2012;50:50–7. doi: 10.1007/s12275-012-1417-x. [DOI] [PubMed] [Google Scholar]
- 17.Kornsakulkarn J, Saepua S, Boonruangprapa T, Suphothina S, Thongpanchang C. New β-carboline and indole alkaloids from Actinomycete Actinomadura sp. BCC 24717. Phytochem Lett. 2013;6:491–494. [Google Scholar]
- 18.Laatsch H. AntiBase. Wiley-VCH; Weinheim, Germany: 2012. [Google Scholar]
- 19.Lee SO, Choi GJ, Choi YH, Jang KS, Park DJ, Kim CJ, Kim JC. Isolation and Characterization of Endophytic Actinomycetes from Chinese Cabbage Roots as Antagonists to Plasmodiophora brassicae. J Microbiol Biotechn. 2008;18:1741–1746. doi: 10.4014/jmb.0800.108. [DOI] [PubMed] [Google Scholar]
- 20.Lin L, Xu X. Indole-3-Acetic Acid Production by Endophytic Streptomyces sp. En-1 Isolated from medicinal Plants. Curr Microbiol. 2013;67:209–217. doi: 10.1007/s00284-013-0348-z. [DOI] [PubMed] [Google Scholar]
- 21.Meyers PR. Gyrase subunit B amino acid signatures for the actinobacterial family Streptosporangiaceae. Syst App Microb. 2014;4:252–260. doi: 10.1016/j.syapm.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Patel M, Conover M, Horan A, Loebenberg D, Marquez J, Mierzwa R, Puar MS, Yarborough R, Waitz JA. Sch 31828, a novel antibiotic from a Microbispora sp.: taxonomy, fermentation, isolation and biological properties. J Antibiot. 1988;41:794–7. doi: 10.7164/antibiotics.41.794. [DOI] [PubMed] [Google Scholar]
- 23.Petrini O. Taxonomy of endophytic fungi of aerial plant tissues. Microbiol. 1986;2:175–187. [Google Scholar]
- 24.Price AC, Choi RJ, Health Z, Li SW, White CO. Inhibition of β-ketoacyl-[acyl carrier protein] synthases by thiolactomycin and cerulenin: structure and mechanism. J Biol Chem. 2001;276:6551–6559. doi: 10.1074/jbc.M007101200. [DOI] [PubMed] [Google Scholar]
- 25.Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN. Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett. 2008;278:1–9. doi: 10.1111/j.1574-6968.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- 26.Shaaban M, Schroder D, Shaaban KA, Helmke E, Grün-Wollny I, Wagner-Döbler I, Laatsch H. Flazin, perlolyrin, and other β-carbolines from marine-derived Bacteria. Rev Latinoamer Quím. 2007;35:58–67. [Google Scholar]
- 27.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zwickl DL. Ph.D. thesis. The University of Texas; Austin, US: 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.