Abstract
Plants, which are sessile unlike most animals, have evolved a system to reduce growth under stress; however, the molecular mechanisms of this stress response are not well known. During programmed development, a fraction of the leaf epidermal precursor cells become meristemoid mother cells (MMCs), which are stem cells that produce both stomatal guard cells and epidermal pavement cells. Here we report that Arabidopsis plants, in response to osmotic stress, post-transcriptionally decrease the protein level of SPEECHLESS, the transcription factor promoting MMC identity, through the action of a mitogen-activated protein kinase (MAPK) cascade. The growth reduction under osmotic stress was lessened by inhibition of the MAPK cascade or by a mutation that disrupted the MAPK target amino acids in SPEECHLESS, indicating that Arabidopsis reduces growth under stress by integrating the osmotic stress signal into the MAPK–SPEECHLESS core developmental pathway.
Keywords: Arabidopsis thaliana, Mitogen-activated protein kinase, Osmotic stress, Signal integration, SPEECHLESS, Stomata
Introduction
Water deficiency is one of the most serious threats to plant survival in nature. Low (more negative) soil water potential caused by drought (dry soil) or high osmotic pressure (high concentrations of solute) can make it difficult for plants to obtain water. Plants deal with water deficiency by undergoing short- and long-term responses. As a short-term response to drought, plants close their stomata. Stomatal closure decreases water loss, but it also inhibits CO2 intake, therefore this response is a trade-off between water conservation and photosynthesis. As longer term responses, plants produce various materials that make the cytoplasmic osmotic potential more negative, protect the cells or decrease growth. Generally, growth of the shoot is more severely reduced than that of the roots under water-deficient conditions, and a low shoot/root ratio is advantageous for survival under such conditions (Sharp and Davies 1989). It is possible that plants have evolved systems to reduce growth under various stresses to enhance their survival, and that growth reduction under stress is an active process. In the case of salt stress, there is evidence that growth reduction is in part mediated through modulation of growth repressor proteins (Achard et al. 2006).
Molecular mechanisms for perception of osmotic stress are being uncovered. One candidate osmosensor is AtHK1, which is a plasma membrane-localized histidine kinase. AtHK1 can suppress the phenotype of a yeast with a mutation in the gene for the yeast osmosensor, SLN1 (Urao et al. 1999). However, the phenotypes of the loss-of-function mutant of AtHK1 under stresses are moderate (Tran et al. 2007, Kumar et al. 2013, Lau et al. 2014), suggesting that there are other osmosensing mechanisms. Recently, a transmembrane hyperosmolality-activated calcium channel, OSCA1, was identified. Loss-of-function osca1 mutation compromises a range of hyperosmolality responses, indicating that it is a physiologically important osmosensor (Yuan et al. 2014). Many possible secondary messengers, including calcium, reactive oxygen species, calcium-dependent protein kinases and mitogen-activated protein kinases (MAPKs), are implicated in osmotic signaling (Deinlein et al. 2014, Golldack et al. 2014). However, how plants regulate cell proliferation in response to stress is still elusive.
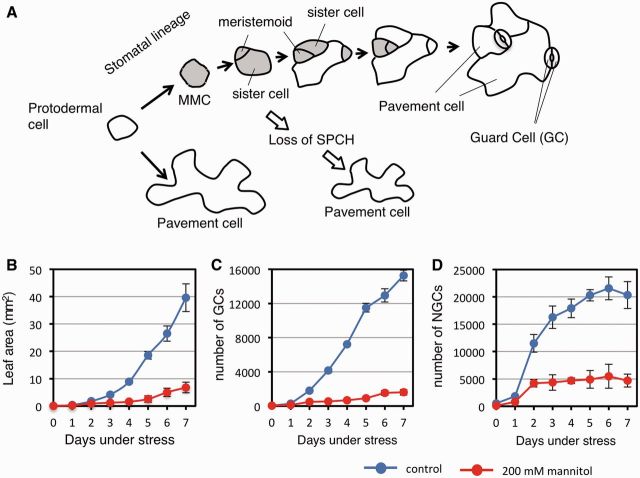
During leaf development, leaf protodermal cells may either terminally differentiate into pavement cells (general epidermal cells) or become meristemoid mother cells (MMCs). MMCs are defined as cells that undergo asymmetric cell division to create a small triangular cell, named a meristemoid, and a larger sister cell. Both cells may undergo additional rounds of asymmetric cell division, but the meristemoid and its sister cell eventually become stomatal guard cells (GCs) and pavement cells, respectively. Thus, the asymmetric divisions impart a stem cell-like character to the stomatal lineage (Nadeau and Sack 2002). Some MMC daughters have MMC identity and, by this stem cell character, the stomatal lineage can produce multiple GCs and pavement cells. Thus the number and activity of MMCs determine the number of epidermal cells, including GCs and non-guard cells (NGCs) (Fig. 1A).
Fig. 1.
Effects of osmotic stress on epidermal development of the first leaf. (A) Genetically programmed pathway of leaf epidermal development. The meristemoid mother cells (MMCs) derived from protodermal cells are precursors of the GC pairs that comprise each stoma and the NGCs. Expression of SPCH is required for cells to be stomatal lineage stem cells (shown in gray). In the spch mutant, all protodermal cells differentiate into pavement cells without first becoming an MMC. (B–D) Effect of 200 mM mannitol on the growth of the first leaves. Col carrying PIP2-GFP, which is useful for visualization of cell margins, was used. Five-day-old plants were transferred onto GM gel plates (blue) or plates containing 200 mM mannitol (red), and the first leaves were excised for measurement of leaf area (B), number of GCs per leaf (abaxial side) (C) and number of NGCs per leaf (abaxial side) (D), every day. Data are shown as the mean ± SD (n ≥ 6).
Genetically programmed molecular pathways that regulate the placement and number of stomata and pavement cells are well understood (Lau and Bergmann 2012, Pillitteri and Torii 2012, Pillitteri and Dong 2013). The master transcriptional regulator for this stem cell identity is SPEECHLESS (SPCH) (MacAlister et al. 2007, Pillitteri et al. 2007, Pillitteri and Torii 2012, Pillitteri and Dong 2013). EPIDERMAL PATTERNING FACTOR 2 (EPF2), which is produced and secreted by MMCs, functions as a negative feedback signal for stem cell number (Hara et al. 2009, Hunt and Gray 2009). EPF1, a secretory peptide resembling EPF2, is produced and secreted by the meristemoid and its early descendants. EPF1 inhibits contact formation of two stomata by regulating the plane of asymmetric cell division in cells contacting an EPF1-producing cell (Hara et al. 2007) and by inhibiting the acquisition of meristemoid identity (Hara et al. 2009). Stomagen (also known as EPFL9), which also resembles EPF1 and EPF2 in amino acid sequence, is secreted by immature mesophyll cells and positively regulates the number of stomata (Kondo et al. 2010, Sugano et al. 2010). EPF1 and EPF2 are perceived and signaled by receptor complexes consisting of the TOO MANY MOUTHS (TMM) receptor-like protein and several ERECTA (ER) and ERECTA LIKE 1 and 2 (ERL1 and ERL2) receptor kinases (Lee et al. 2012, Jewaria et al. 2013). Stomagen also requires TMM to function (Kondo et al. 2010, Sugano et al. 2010). TMM and ER receptors act upstream of a MAPK cascade that phosphorylates and destabilizes the SPCH transcription factor (Lampard et al. 2008). These peptides appear to be differentially recognized by receptor complexes consisting of different combinations of these receptor components (Jewaria et al. 2013). Because genes for these peptides are developmentally regulated, as described above, they can generate signaling loops to ensure the basic stomatal and epidermal cell number and pattern (Hara et al. 2007, Hara et al. 2009, Kondo et al. 2010, Sugano et al. 2010).
These developmental programs ensure robust development of basic plant form. However, plants reduce growth of aerial parts when they face drought or osmotic stresses (Sharp and Davies 1989). Although some of the signaling intermediates in hormone-driven environmental responses are known (Achard et al. 2007), the developmental effectors of such responses are not. Here, we aimed to clarify how plants reduce growth under osmotic stress. For this purpose, we focused on leaf epidermal growth, because leaf size and stomatal number are important factors during the adaptation to osmotic and drought stresses, and the core regulatory pathways for cell fate and proliferation are known (Pillitteri and Torii 2012).
Results
Time course changes in leaf cell numbers under osmotic stress.
To examine the time course of leaf growth under osmotic stress, 5-day-old Arabidopsis seedlings were transferred onto a gel medium containing an osmoticum, 200 mM mannitol, and the growth of the first true leaves was monitored. Plant growth was repressed by mannitol, and the repression was more in the shoot than in the root (Supplementary Fig. S1A). After 7 d of mannitol treatment (12 d after seed sowing), the leaf size (measured as area) of mannitol-treated plants was 17.1% of that of unstressed (control) plants, and the numbers of GCs and NGCs on the abaxial sides of the first leaves were 10.5% and 27.7%, respectively, of the control values (Fig. 1B–D). Stomatal index [number of stomata/(number of epidermal cells + number of stomata)] and stomatal density (number of stomata/total leaf area) were also decreased by osmotic stress (Supplementary Fig. S1B, C).
Numbers of MMCs are quickly decreased by osmotic stress.
In this study, we focused on changes in cell identity and cell numbers during short-term (24 h) osmotic stress treatment. Leaf size, and the numbers of GCs and NGCs were significantly repressed after 24 h treatment with mannitol (columns for wild-type plants in Fig. 2A–E) or another osmoticum, polyethylene glycol (PEG) 6000 (Fig. 2F–H). Consistently, the number of cells expressing CycB1::GUS [a DNA region encompassing the CYCB1 promoter and that coding for the destruction box of CYCB1 fused in-frame to the β-glucosidase gene (GUS)], a marker of the mitotic phase, decreased after the 24 h period of osmotic stress (Supplementary Fig. S2A, B). Also the number of cells in mitotic phase was decreased by the osmotic stress (Supplementary Fig. S2C). The degree of reduction in leaf size was similar to that of cell number, in the case of both mannitol and PEG treatment, indicating that the reduction of leaf size was mostly mediated by a decrease in cell number. To learn more about patterns of cell changes after stress treatment, we examined the distribution of cell sizes after the 24 h mannitol treatment. The fraction of small NGCs, many of which are probably MMCs, was lower in the mannitol-treated plants than in the control plants (Supplementary Fig. S3). We hypothesized that the decrease in cell numbers observed under osmotic stress was caused by a decrease in the number of MMCs. To examine this hypothesis, we examined plants carrying the gene encoding green fluorescent protein (GFP) under the control of the EPF2 promoter (EPF2pro::GFP); in these plants, GFP is primarily expressed in MMCs (Hara et al. 2009), the source of stomata and stomatal lineage pavement cells (Geisler et al. 2000). The number of GFP-positive cells decreased greatly during the 24 h period of osmotic stress (Fig. 3A, B). In contrast, when we examined plants carrying the same reporter gene under the control of the EPF1 promoter (EPF1pro::GFP), a marker which is first expressed in meristemoids (i.e. not in MMCs (Hara et al. 2007), GFP expression was not detectably affected by 24 h osmotic stress, but was decreased only at later time points (Supplementary Fig. S4). These results indicate that the MMC population is rapidly decreased by osmotic stress.
Fig. 2.
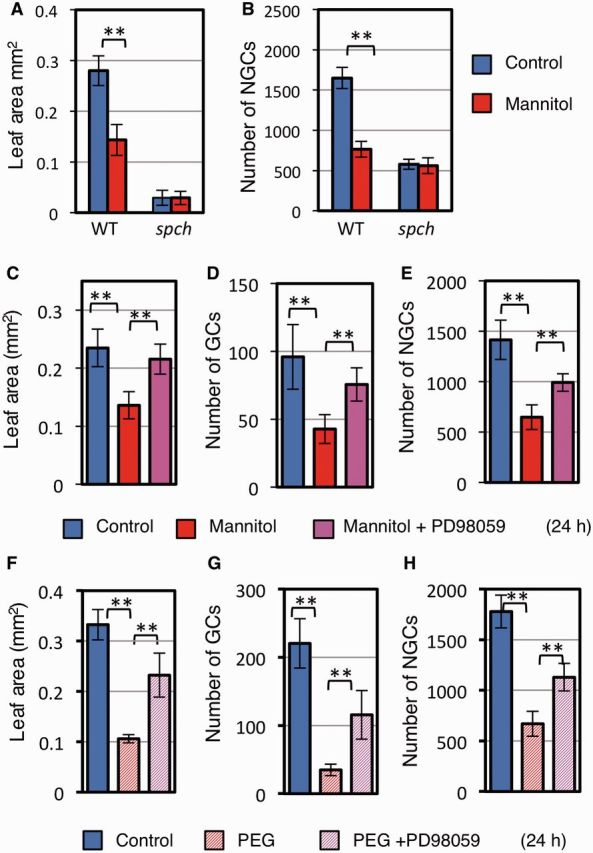
The MAPK cascade mediates osmotic stress-induced cell proliferation stop. (A, B) Effect of the spch mutation on osmotic stress-induced growth reduction. Mannitol treatment was for 24 h. (A) Leaf area. (B) Number of NGCs. Note that spch plants lack GCs. (C–H). The role of the MAPK cascade for stopping osmotic stress-induced cell proliferation. Col carrying PIP2–GFP was used. Leaf area (C, F), number of GCs (D, G) and number of NGCs (E, H) of the first leaves after 24 h treatment with mannitol (C–E) or PEG (F–H), in the presence or absence of 25 µM PD98059. WT, wild-type. Data are shown as the mean ± SD (n ≥ 6). **P < 0.01.
Fig. 3.
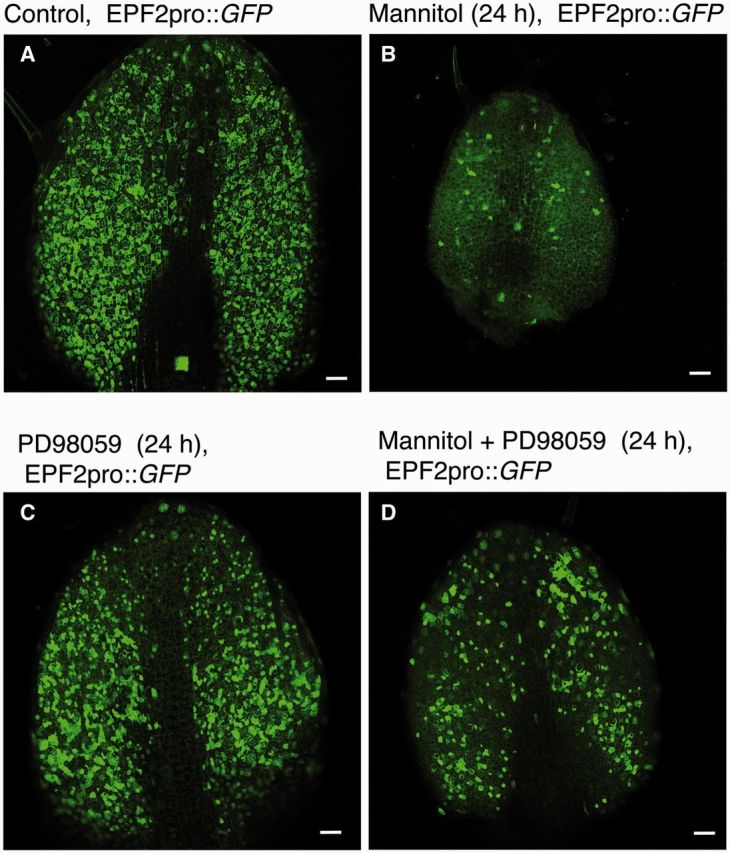
The number of MMCs following osmotic stress is decreased via an MAPK cascade. Five-day-old plants carrying an MMC marker EPF2pro::GFP were grown for 24 h in (A) control liquid medium, (B) medium containing 200 mM mannitol, (C) medium containing 25 µM PD98059 (a MAPK kinase inhibitor) or (D) medium containing 200 mM mannitol plus 25 µM PD98059. Scale bars = 50 µm.
Decrease in the number of MMCs is solely responsible for the decrease in epidermal cell numbers
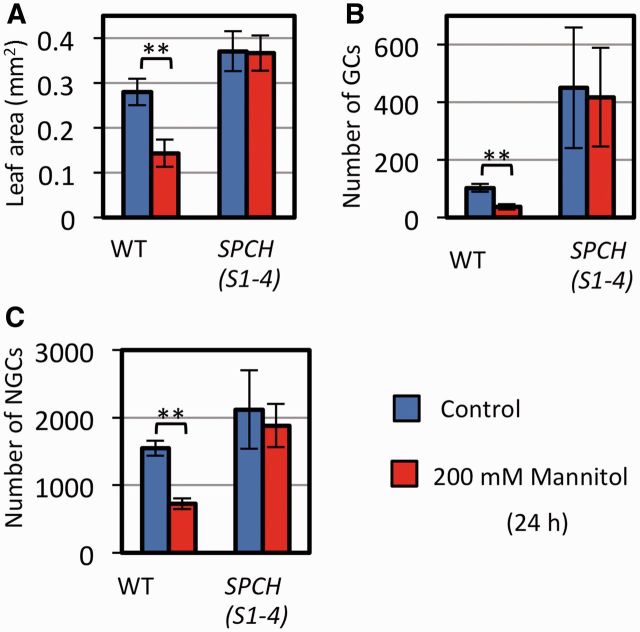
We next asked whether the decrease in the number of MMCs is responsible for the stress-induced decrease in cell proliferation. To answer this question, we used the spch mutant, which lacks MMCs and, consequently, lacks the entire stomatal lineage (MacAlister et al. 2007, Pillitteri et al. 2007). The number of epidermal cells and the leaf size of spch plants were unaffected by osmotic stress (Fig. 2A, B). These results indicate that the decrease in cell number under the osmotic stress is caused by a decrease in the number of MMCs.
Osmotic stress decreased leaf growth independent of ER, ERL1, ERL2, TMM and their peptide ligands
A key question is whether the stress signal regulates the well-known developmental signaling pathway, which constitutes the secretory peptides, ER-containing receptor complexes, the MAPK cascade and SPCH (Lau and Bergmann 2012, Pillitteri and Torii 2012, Pillitteri and Dong 2013). First, we tested the involvement of ER-family receptor kinases, which are absolutely required for the functions of EPF1 and EPF2 (Hara et al. 2007, Hara et al. 2009). We found that the er erl1 erl2 triple mutant was able to respond to osmotic stress by decreasing the numbers of both GCs and NGCs, and by decreasing leaf size (Fig. 4A–C), indicating that ER-family receptors are not required for the reduction in epidermal growth induced by osmotic stress. Consistent with this, experiments with plants carrying a mutation in the gene encoding TMM, the co-receptor of ER-family receptors (Lee et al. 2012), showed that functional TMM was not required for the osmotic stress response (Fig. 4D–F). The EPF-family peptides were also not required for osmotic stress-induced growth changes: upon stress induction, the expression level of the EPF1 gene was unchanged and that of the EPF2 gene decreased (Supplementary Fig. S5A). Because EPF2 negatively regulates epidermal cell proliferation (Hara et al. 2009), the decrease in EPF2 mRNA was not responsible for the stress-responsive decrease in cell number. Expression of the gene encoding stomagen, a positive regulator of the number of stomata, was decreased by mannitol (Supplementary Fig. S5B). Therefore, we examined whether the decrease in STOMAGEN gene expression is necessary for the growth decrease, by using plants constitutively expressing STOMAGEN under the Cauliflower mosaic virus 35S promoter. Overexpression of STOMAGEN did not greatly affect leaf size (Fig. 4G), but it increased the number of GC pairs under normal conditions (Fig. 4H), as has been reported previously (Hunt et al. 2010, Kondo et al. 2010, Sugano et al. 2010). The GC and NGC production was greatly decreased by osmotic stress in plants constitutively expressing STOMAGEN (Fig. 4H, I), indicating that the observed decrease in STOMAGEN expression with mannitol treatment was not necessary to mediate the response.
Fig. 4.
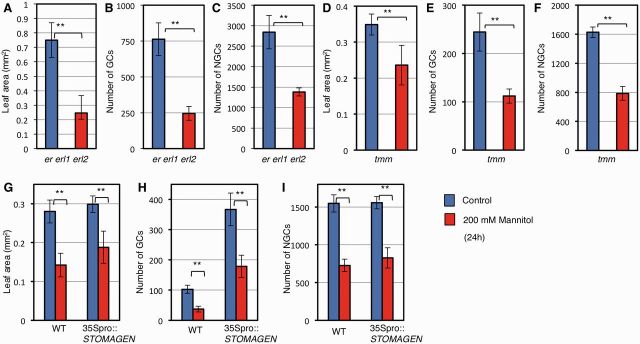
ERECTA–TMM receptor complexes and the decrease in STOMAGEN are not responsible for the mannitol-induced decrease in cell proliferation. Five-day-old plants were transferred to liquid nutrient medium without (blue) or with (red) 200 mM mannitol, and cultured for 24 h. Results for plants carrying (A–C) the er erl1 erl2 triple mutant, (D–F) tmm or (G–I) 35Spro::STOMAGEN are shown. Leaf area (A, D), number of GCs (B, E) and number of NGCs (C, F) in the abaxial leaf surface of the first leaf are shown. Data are shown as the mean ± SD (n ≥ 6). **P < 0.01.
The MAPK cascade integrates stress signaling and the basal developmental program
The MAPKs MPK3 and MPK6, which function downstream of the ER-family receptors to regulate epidermal development (Pillitteri and Torii 2012), are also implicated in stress signaling (Rodriguez et al. 2010). One hypothesis is that these kinases actually serve as the integration point between programmed development and stress responses. To examine the role of a MAPK cascade in modulating epidermal development in response to osmotic stress, we first used a MAPK kinase inhibitor, PD98059 (Dudley et al. 1995). PD98059 counteracted the mannitol-induced decrease in GFP-positive cells in plants carrying EPF2pro::GFP (Fig. 3A–D), the decrease in the number of cells in mitotic phase (Supplementary Fig. S2C), the decrease in leaf size (Fig. 2C) and the decrease in epidermal cell number (Fig. 2D, E) in wild-type plants. These results indicate that a MAPK cascade mediates the stress-induced inhibition of cell proliferation. Although mannitol has been widely used for studies of osmotic stress in plants, a recent study has indicated that mannitol also has an osmotic stress-independent effect on Arabidopsis growth (Trontin et al. 2014). Therefore, we tested whether PD98059 inhibits the growth reduction caused by another osmoticum, PEG. Reduction in leaf sizes and of GC and NGC numbers caused by PEG were all alleviated by PD98059 (Fig. 2F–H).
Decrease in SPCH protein is necessary and sufficient for a large part of the growth repression
We next examined the behaviour of SPCH, which is the master regulator of MMC identity. In plants that carried the SPCH–GFP fusion gene under the control of the SPCH promoter (SPCHpro::SPCH-GFP), the signal from the SPCH–GFP product was decreased after 24 h treatment with mannitol (Fig. 5A, B) or PEG (Fig. 5J, K). To test whether the observed decrease in SPCH–GFP was due to regulation of SPCH gene expression or of the SPCH protein, we examined the same fusion gene expressed under the constitutive 35S promoter (35Spro::SPCH-GFP). Again, the signal from the SPCH–GFP product was decreased after 24 h of mannitol-induced stress (Fig. 5D, E), suggesting that the signal decrease was caused by post-transcriptional regulation. Previously, SPCH was shown to be destabilized by MAPK-mediated phosphorylation (Lampard et al. 2008). Consistent with this, we found that the MAPK kinase inhibitor PD98059 reversed the effect of mannitol (Fig. 5C, F) or PEG (Fig. 5L) on the SPCH–GFP signal, suggesting that a MAPK cascade mediates the decrease in SPCH under osmotic stress. The results of Western blot analysis confirmed the effect of PD98059 on the SPCH–GFP level (Fig. 5M).
Fig. 5.
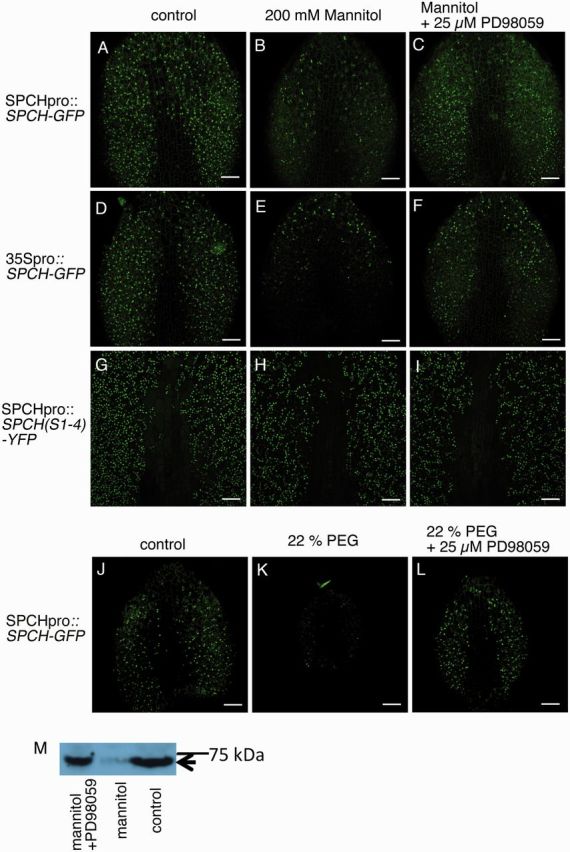
SPCH–GFP protein was decreased by osmotic stress in a MAPK-dependent manner. (A–I) Effects of 200 mM mannitol and a MAPK kinase inhibitor (PD98059) on the levels of SPCH–GFP and SPCH(S1-4)–GFP. Scale bars = 50 µm. Plants carrying (A–C) SPCHpro::SPCH-GFP, (D–F) 35Spro::SPCH-GFP or (G–I) SPCHpro::SPCH(S1-4)-YFP were analyzed. Seedlings were grown in (A, D, G) nutrient liquid medium, (B, E, H) nutrient liquid medium containing 200 mM mannitol or (C, F, I) liquid medium containing 200 mM mannitol plus 25 µM PD98059 for 24 h. The SPCH–GFP level was decreased by osmotic stress, and the decrease was dependent on a MAPK pathway. (J–K) Effects of 22% PEG and a MAPK kinase inhibitor (PD98059) on SPCH–GFP. (J) Control, (K) 22% PEG, (L) 22% PEG plus 25 µM PD98059. (M) Western blot analysis showing the protein levels of SPCH–GFP. The position corresponding to SPCH–GFP is indicated by an arrow.
We next asked whether the decrease in the level or activity of SPCH was responsible for the decrease in cell proliferation caused by the osmotic stress. For this purpose, we used a SPCH variant, SPCH(S1-4), in which the MAPK target serine and threonine residues are mutated to alanine (Lampard et al. 2008), and examined plants carrying a fusion gene encoding SPCH(S1-4)–yellow fluorescent protein (YFP) under the control of the SPCH promoter [SPCHpro::SPCH(S1-4)-YFPI]. The signal for the fusion protein SPCH(S1-4)–YFP was much less sensitive to mannitol (Fig. 5G, H) than the SPCH–GFP signal controlled by an equivalent promoter in the plants described above (Fig. 5A, B). Furthermore, in plants carrying SPCHpro::SPCH(S1-4)-YFP, the leaf size and numbers of GCs and NGCs were not decreased by mannitol treatment (Fig. 6A–C). These results indicate that the decreased growth under osmotic stress is mediated by a MAPK-mediated decrease in SPCH.
Fig. 6.
The MAPK target sites of SPCH are necessary for stress-induced growth reduction and drought tolerance. (A–C) Epidermal growth of Arabidopsis carrying stabilized SPCH after 24 h of mannitol treatment. (A) Leaf size, (B) number of GCs per leaf and (C) number of NGCs per leaf. Blue, control; red, 200 mM mannitol. Data are shown as the mean ± SD (n ≥ 6). **P < 0.01.
Discussion
Growth reduction under drought and osmotic stresses has been thought to be an adaptive plant response, but it has been difficult to determine whether the growth reduction is only a consequence of the inability to grow under harsh conditions or whether it is an active plant response. High concentrations of salts, which can create both osmotic stress and an ionic imbalance, also negatively affect plant growth. Sodium chloride increases the stability of a DELLA protein, REPRESSOR OF GA1-3 (RGA), and Arabidopsis carrying mutations in genes encoding DELLA proteins exhibit decreased growth reduction under NaCl compared with wild-type plants (Achard et al. 2006). These results suggest that plants evolved a specific system to reduce growth under salt stress. Here we showed that Arabidopsis quickly reduces leaf growth under osmotic stress by modulating a core developmental regulatory loop that controls leaf epidermal growth.
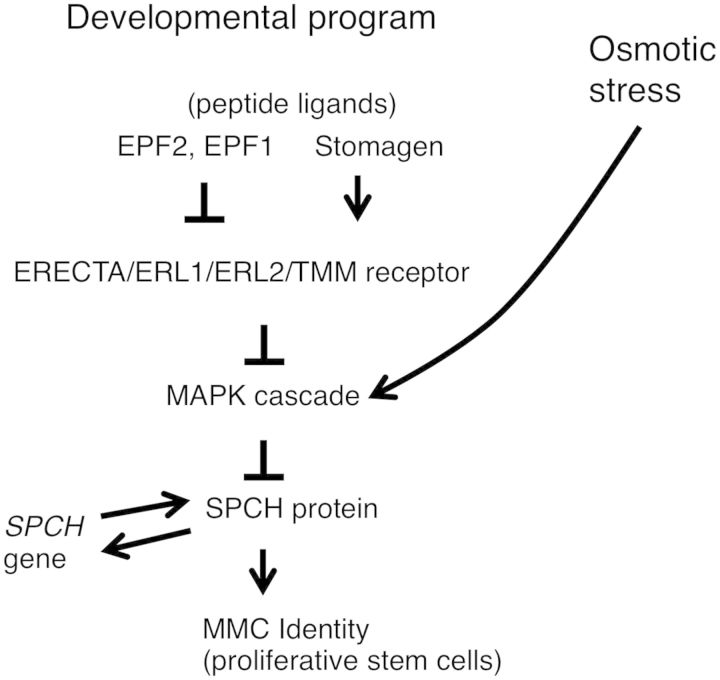
In this study, we showed that osmotic stress caused a reduction in the number of MMCs (Fig. 3B) and a decrease in leaf growth and stomatal number. The spch mutant, which lacks MMCs, did not exhibit these osmotic stress-related effects (Fig. 2A, B), indicating that the reduction in the MMC population was responsible for the reduction in leaf growth and stomatal number in wild-type plants. In wild-type plants, a MAPK kinase inhibitor inhibited the osmotic stress induced-decrease in SPCH levels (Fig. 5), the reduction of the number of MMCs (Fig. 3) and the reduction of the number of stomatal and non-stomatal cells and leaf size (Fig. 2C–H). Furthermore, mutations in the MAPK target sites of SPCH also inhibited the osmotic stress-induced reduction of leaf growth (Fig. 4K–M). Taken together, our results indicate that a MAPK-mediated decrease in SPCH protein levels under stress conditions is responsible for the decrease in the number of MMCs and hence the reduction in the number of epidermal cells. YDA (MKKK4, a MAPK kinase kinase)–MKK4 and MKK5 (MAPK kinases)–MPK3 and MPK6 (MAPKs) mediate a developmental pathway regulating the stomatal lineage (Bergmann et al. 2004, Wang et al. 2007). On the other hand, MKKK20 (a MAPK kinase kinase), MKK4 and MAP3/4/6 are suggested to mediate osmotic stress signaling (Ichimura et al. 2000, Kim et al. 2011, Kim et al. 2012). Although the role of YDA in osmotic stress signaling has not been examined due to the seedling-lethal phenotype of the yda loss-of-function mutant and plants carrying a gain-of function YDA gene (Lukowitz et al. 2004), it is possible that MKK4 might function as an integration point of developmental and osmotic stress signaling. Thus, although specific components of the MAPK cascade mediating this pathway are yet to be defined, our findings demonstrate that Arabidopsis plants control their growth, in response to osmotic stress, by modulating the core signaling pathway involving the MAPK–SPCH signaling module that normally ensures the basic developmental program (Fig. 7).
Fig. 7.
A proposed model for the molecular mechanisms responsible for decreases in cell proliferation in response to osmotic stress. In response to osmotic stress, plants activate a mitogen-activated protein kinase (MAPK) module, which in turn decreases the level of SPEECHLESS (SPCH) and forces stem cells to differentiate terminally into pavement cells. This results in a decreased number of stomata and reduced leaf growth. EPF, EPIDERMAL PATTERNING FACTOR; ER, ERECTA; ERL, ERECTA-LIKE; MMC, meristemoid mother cell; TMM, TOO MANY MOUTHS.
Osmotic stress reduces proliferation of not only leaf epidermal cells, but also mesophyll cells. It is likely that growth of the different cell layers is co-ordinated. Perhaps the proliferation of inner cells (mesophyll cell precursors) depends on epidermal cell proliferation. However, it is also possible that inner cells may have an independent system to regulate cell proliferation in response to osmotic stress. Another interesting question is whether analogous systems that integrate environmental stress and developmental programs operate in different organs. The stress-induced active growth reduction system appears to have evolved to optimize the survival rate at the expense of rapid growth under harsh environmental conditions. The knowledge of this mechanism opens up the possibility to optimize the balance of biomass and stress adaptation in field crops.
Materials and Methods
Growth conditions, and stress and chemical treatments
Arabidopsis thaliana Columbia (Col) ecotype was used. Plants were grown on a GM gel medium [Murashige and Skoog’s salts (Murashige and Skoog 1962), 1% (w/v) sucrose, 100 mg l–1 inositol, 10 mg l–1 thiamine-HCl, 1 mg l–1 pyridoxine-HCl, 1 mg l–1 nicotinic acid, 0.05% MES-KOH (pH 5.7), 0.4% Phytagel™ (Sigma-Aldrich)] for 5 d at 22°C For all 24 h osmotic stress experiments, except those involving reverse transcription–PCR (RT–PCR) or CYCB1-GUS staining, 5-day-old plants were moved to liquid nutrient medium [Gamborg’s B5 salts (Gamborg et al. 1968), 1% sucrose, 0.05% MES-KOH, 100 mg l–1 inositol, 10 mg l–1 thiamine-HCl, 1 mg l–1 pyridoxine-HCl, 1 mg l–1 nicotinic acid] containing no osmoticum (control), 200 mM mannitol, 22% PEG-600 or one of the above osmotica plus 25 µM PD98059.
For the RT–PCR experiments, CYCB1-GUS staining experiment and 1 week time course experiments, 5-day-old plants were moved onto GM gel medium with or without 200 mM mannitol.
Image analysis for epidermal cell number and size quantification
The plasma membrane of the first leaves was stained with 10 µM FM4-64 dye (Invitrogen), and images were captured with a confocal microscope, LSM710 (Carl Zeiss) or FV300 (Olympus). For all 24 h stress treatment experiments, all epidermal cells of the abaxial side of the first true leaf were manually counted by using the ImageJ software (http://imagej.nhi.gov/ij). Col ecotype plants carrying a fusion gene encoding a plasma membrane marker, PIP2–GFP (Cutler et al. 2000), were used as the wild type for experiments including only the wild type, and cell margins were visualized with the GFP signal. For measurements of NGC cell area, we used Packing Analyzer V2 software (Aigouy et al. 2010).
In the time course experiment (Fig. 1B–D, 2–7 d treatment), we counted cell numbers in 0.11 mm2 areas of the distal and proximal regions of the abaxial parts (excluding the midvein) of the first leaf, and we used the average of the two values to estimate the total cell numbers. This was because it was not realistic to count all cells in large leaves. The time course experiment was performed twice, and data from a representative experiment are shown.
Plant materials
The genetic background of er;erl1;erl2 (Shpak et al. 2005), tmm (Salk 011959) (Hara et al. 2007) and all transformants used in this study is Col. Arabidopsis carrying 35Spro::SPCH-GFP or 35Spro::STOMAGEN-GFP was produced as follows. The EGFP gene was PCR-amplified with primers EGFPSmaIFw (5′-tcccccgggaaaATGGTGAGCAAGGGCGAGGAGCTGTTCAC-3′) and EGFPPacIRv (5′-ccttaattaaGTTACTTGTACAGCTCGTCC-3′), where lower case letters indicate sequences added to create restriction enzyme sites, and the amplified DNA was digested with SmaI and PacI restriction enzymes, and cloned between the SmaI and PacI sites of a binary vector pHM3 to create pHM3-EGFP. The SPCH gene was PCR-amplified from genomic DNA of Arabidopsis ecotype Col, by using primers SPCHXbaIFw (5′-tgctctagaATGCAGGAGATAATACCGGATT-3′) and SPCHAsc1Rv (5′-tttggcgcgcctcctcctccGCAGAATGTTTGCTG-3′), and the amplified DNA was digested with XbaI and SmaI restriction enzymes, and then cloned between XbaI and SmaI sites of pHM3-EGFP, creating the plasmid pHM3-SPCH-EGFP (carrying 35Spro::SPCH-GFP). The STOMAGEN gene was PCR-amplified from genomic DNA of Arabidopsis ecotype Col, by using primers STOMAGENXho1Fw (5′-ccgctcgagaaaATGAAGCATGAAATGATGAAC-3′) and STOMAGENSma1Rv (5′-tcccccgggTCTATGACAAACACATCTATAATGATAAG-3′), and the amplified DNA was digested with XhoI and SmaI restriction enzymes, and then cloned between XhoI and SmaI sites of pHM3-EGFP, creating pHM3-STOMAGEN-EGFP (carrying 35Spro::STOMAGEN-EGFP). pHM3-SPCH-EGFP and pHM3-STOMAGEN-EGFP were transformed into Arabidopsis ecotype Col. Arabidopsis carrying CER6pro::H2B-GFP was produced as follows. A 1.2 kb promoter region of CER6 was PCR-amplified by using primers pCER6Fw (5′-TCCCAAGCTTTACTCTTCGATATCGGTTGTTG-3′) and pCER6Rv (5′-CGTCGGAGAGTTTTAATG-3′), and the coding region of the HISTONE H2B gene (AT5G22880) was amplified by using primers H2BFw (5′-AAAATGGCGAAGGCAGATAAGAAACCAG-3′) and H2BRv (5′-CCGCTCGAGAACTCGTAAACTTCGTAAC-3′); and the amplified fragments were cloned in vector pMM1 carrying sGFP. CER6pro::H2B-GFP was transformed into Arabidopsis (Col).
Immunoblot analysis
Five-day-old plants carrying SPCHpro::SPCH-GFP were cultured in liquid nutrient medium, or liquid nutrient medium containing 200 mM mannitol or 200 mM mannitol plus 25 µM PD98059 for 24 h. Then the first and second leaves were collected in liquid nitrogen, and frozen samples were homogenized in a buffer containing 100 mM Tris–HCl (pH 8.0), 200 mM NaCl, 10% glycerol, 1% NP-40, 5 mM EDTA, 10 mM dithiothreitol (DTT) and 1 : 100 (v/v) protease inhibitor cocktail (Nacalai Tesque). The homogenates were centrifuged and the supernatant was used for Western blot analysis. Anti-GFP (1181446000, Roche) and horseradish peroxidase-linked anti-mouse IgG (NA931VS, GE Healthcare) were used for SPCH–GFP detection.
Semi-quantitative RT–PCR
Five-day-old plants were transferred to GM gel medium with or without 200 mM mannitol for 12 or 24 h, and first and second leaves were used for RNA extraction. Total RNA was isolated by using the RNeasy micro kit (Qiagen) according to the manufacturer’s instructions. Total RNA (1 µg) was treated with RNase-free DNase, and reverse-transcribed with a 10 µl reaction mixture by using Superscript II (Invitrogen). After the reaction, 40 µl of water was added and 1 µl of the solution was used as template for the PCR. Primers used were as follows: for STOMAGEN, STOMAGENXho1Fw and STOMAGENSma1Rv; for EPF1, EPF1F (5′-CCGCTCGAGAAAATGAAGTCTCTTCTTCTCCTTGCCT-3′) and EPF1R (5′-TCCCCCGGGAGGGACAGGGTAGGACTTATTGTTG-3′); for EPF2, EPF2F (5′-ccgctcgagaaaATGACGAAGTTTGTACGCAAGTATATG-3′) and EPF2R (5′-tcccccgggAGCTCTAGATGGCACGTGATAGTAT-3′); for AtIPT9, AtIPT9F (5′-GGATTGTATCTGCGATGGTTTATGTATG-3′) and AtIPT9R (5′-CTCCATTGGGTCCTGAAAGCA-3′). AtIPT9 was used as a control gene.
Supplementary data
Supplementary data are available at PCP online.
Funding
This study was supported by the Ministry Education, Culture, Sports, Science, and Technology (MEXT) [KAKENHI grant Nos, 19060005 and 25113006]; the Japan Society for the Promotion of Science (JSPS) [grant No. 25091060 to T.K.]; the Indian Council of Agricultural Research (ICAR), New Delhi, India [support for A.K. under the ICAR-IF (International Fellowship) Scheme].
Supplementary Material
Acknowledgments
Seeds carrying SPCHpro::SPCH-GFP or CYCB1-GUS were provided by Keiko Torii (University of Washington) and Peter Doener (The University of Edinburgh), respectively. Packing analyzer V2 was provided by Benoit Aigouy (IBDM). We thank Chinatsu Egusa (Osaka University) for making the CER6pro::HistoneH2B-GFP construct and plants carrying it.
Glossary
Abbreviations
- Col
Columbia
- EPF
EPIDERMAL PATTERNING FACTOR
- ER
ERECTA
- ERL
ERECTA LIKE
- GC
guard cell
- GFP
green fluorescent protein
- GUS
β-glucuronidase
- MAPK
mitogen-activated protein kinase
- MMC
meristemoid mother cell
- NGC
non-guard cell
- PEG
polyethylene glycol
- RT–PCR
reverse transcription–PCR
- SPCH
SPEECHLESS
- TMM
TOO MANY MOUTHS
- YFP
yellow fluorescent protein
Disclosures
The authors have no conflicts of interest to declare.
References
- Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, et al. Integration of plant responses to environmentally activated phytohormonal signals. Science. 2006;311:91–94. doi: 10.1126/science.1118642. [DOI] [PubMed] [Google Scholar]
- Achard P, Liao L, Jiang C, Desnos T, Bartlett J, Fu X, et al. DELLAs contribute to plant photomorphogenesis. Plant Physiol. 2007;143:1163–1172. doi: 10.1104/pp.106.092254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigouy B, Farhadifar R, Staple DB, Sagner A, Roper JC, Julicher F, et al. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 2010;142:773–786. doi: 10.1016/j.cell.2010.07.042. [DOI] [PubMed] [Google Scholar]
- Bergmann DC, Lukowitz W, Somerville CR. Stomatal development and pattern controlled by a MAPKK kinase. Science. 2004;304:1494–1497. doi: 10.1126/science.1096014. [DOI] [PubMed] [Google Scholar]
- Cutler SR, Ehrhardt DW, Griffitts JS, Somerville CR. Random GFP::cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc. Natl Acad. Sci. USA. 2000;97:3718–3723. doi: 10.1073/pnas.97.7.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinlein U, Stephan AB, Horie T, Luo W, Xu G, Schroeder JI. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014;19:371–379. doi: 10.1016/j.tplants.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl Acad. Sci. USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg OL, Miller RA, Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968;50:151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Geisler M, Nadeau J, Sack FD. Oriented asymmetric divisions that generate the stomatal spacing pattern in arabidopsis are disrupted by the too many mouths mutation. Plant Cell. 2000;12:2075–2086. doi: 10.1105/tpc.12.11.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golldack D, Li C, Mohan H, Probst N. Tolerance to drought and salt stress in plants: unraveling the signaling networks. Front. Plant Sci. 2014;5:151. doi: 10.3389/fpls.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Kajita R, Torii KU, Bergmann DC, Kakimoto T. The secretory peptide gene EPF1 enforces the stomatal one-cell-spacing rule. Genes Dev. 2007;21:1720–1725. doi: 10.1101/gad.1550707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Yokoo T, Kajita R, Onishi T, Yahata S, Peterson KM, et al. Epidermal cell density is autoregulated via a secretory peptide, EPIDERMAL PATTERNING FACTOR 2 in Arabidopsis leaves. Plant Cell Physiol. 2009;50:1019–1031. doi: 10.1093/pcp/pcp068. [DOI] [PubMed] [Google Scholar]
- Hunt L, Bailey KJ, Gray JE. The signalling peptide EPFL9 is a positive regulator of stomatal development. New Phytol. 2010;186:609–614. doi: 10.1111/j.1469-8137.2010.03200.x. [DOI] [PubMed] [Google Scholar]
- Hunt L, Gray JE. The signaling peptide EPF2 controls asymmetric cell divisions during stomatal development. Curr. Biol. 2009;19:864–869. doi: 10.1016/j.cub.2009.03.069. [DOI] [PubMed] [Google Scholar]
- Ichimura K, Mizoguchi T, Yoshida R, Yuasa T, Shinozaki K. Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J. 2000;24:655–665. doi: 10.1046/j.1365-313x.2000.00913.x. [DOI] [PubMed] [Google Scholar]
- Jewaria PK, Hara T, Tanaka H, Kondo T, Betsuyaku S, Sawa S, et al. Differential effects of the peptides stomagen, EPF1 and EPF2 on activation of MAP kinase MPK6 and the SPCH protein level. Plant Cell Physiol. 2013;54:1253–1262. doi: 10.1093/pcp/pct076. [DOI] [PubMed] [Google Scholar]
- Kim SH, Woo DH, Kim JM, Lee SY, Chung WS, Moon YH. Arabidopsis MKK4 mediates osmotic-stress response via its regulation of MPK3 activity. Biochem. Biophys. Res. Commun. 2011;412:150–154. doi: 10.1016/j.bbrc.2011.07.064. [DOI] [PubMed] [Google Scholar]
- Kim JM, Woo DH, Kim SH, Lee SY, Park HY, Seok HY, et al. Arabidopsis MKKK20 is involved in osmotic stress response via regulation of MPK6 activity. Plant Cell Rep. 2012;31:217–224. doi: 10.1007/s00299-011-1157-0. [DOI] [PubMed] [Google Scholar]
- Kondo T, Kajita R, Miyazaki A, Hokoyama M, Nakamura-Miura T, Mizuno S, et al. Stomatal density is controlled by a mesophyll-derived signaling molecule. Plant Cell Physiol. 2010;51:1–8. doi: 10.1093/pcp/pcp180. [DOI] [PubMed] [Google Scholar]
- Kumar MN, Jane WN, Verslues PE. Role of the putative osmosensor Arabidopsis histidine kinase1 in dehydration avoidance and low-water-potential response. Plant Physiol. 2013;161:942–953. doi: 10.1104/pp.112.209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampard GR, Macalister CA, Bergmann DC. Arabidopsis stomatal initiation is controlled by MAPK-mediated regulation of the bHLH SPEECHLESS. Science. 2008;322:1113–1116. doi: 10.1126/science.1162263. [DOI] [PubMed] [Google Scholar]
- Lau OS, Bergmann DC. Stomatal development: a plant’s perspective on cell polarity, cell fate transitions and intercellular communication. Development. 2012;139:3683–3692. doi: 10.1242/dev.080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau OS, Davies KA, Chang J, Adrian J, Rowe MH, Ballenger CE, et al. Direct roles of SPEECHLESS in the specification of stomatal self-renewing cells. Science. 2014;345:1605–1609. doi: 10.1126/science.1256888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Kuroha T, Hnilova M, Khatayevich D, Kanaoka MM, McAbee JM, et al. Direct interaction of ligand–receptor pairs specifying stomatal patterning. Genes Dev. 2012;26:126–136. doi: 10.1101/gad.179895.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowitz W, Roeder A, Parmenter D, Somerville C. A MAPKK kinase gene regulates extra-embryonic cell fate in Arabidopsis. Cell. 2004;116:109–119. doi: 10.1016/s0092-8674(03)01067-5. [DOI] [PubMed] [Google Scholar]
- MacAlister CA, Ohashi-Ito K, Bergmann DC. Transcription factor control of asymmetric cell divisions that establish the stomatal lineage. Nature. 2007;445:537–540. doi: 10.1038/nature05491. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. [Google Scholar]
- Nadeau JA, Sack FD. Stomatal development in Arabidopsis. Arabidopsis Book. 2002;1:e0066. doi: 10.1199/tab.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillitteri LJ, Dong J. Stomatal development in Arabidopsis. Arabidopsis Book. 2013;11:e0162. doi: 10.1199/tab.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillitteri LJ, Sloan DB, Bogenschutz NL, Torii KU. Termination of asymmetric cell division and differentiation of stomata. Nature. 2007;445:501–505. doi: 10.1038/nature05467. [DOI] [PubMed] [Google Scholar]
- Pillitteri LJ, Torii KU. Mechanisms of stomatal development. Annu. Rev. Plant Biol. 2012;63:591–614. doi: 10.1146/annurev-arplant-042811-105451. [DOI] [PubMed] [Google Scholar]
- Rodriguez MC, Petersen M, Mundy J. Mitogen-activated protein kinase signaling in plants. Annu. Rev. Plant Biol. 2010;61:621–649. doi: 10.1146/annurev-arplant-042809-112252. [DOI] [PubMed] [Google Scholar]
- Sharp RE, Davies J. Regulation of growth and development of plants growing with a restricted supply of water. In: Jones HG, Flowers TJ, Jones MB, editors. Plants Under Stress: Biochemistry, Physiology and Ecology and Their Application to plant improvement. Cambridge University Press, New York; 1989. pp. 71–93. [Google Scholar]
- Shpak ED, McAbee JM, Pillitteri LJ, Torii KU. Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science. 2005;309:290–293. doi: 10.1126/science.1109710. [DOI] [PubMed] [Google Scholar]
- Sugano SS, Shimada T, Imai Y, Okawa K, Tamai A, Mori M, et al. Stomagen positively regulates stomatal density in Arabidopsis. Nature. 2010;463:241–244. doi: 10.1038/nature08682. [DOI] [PubMed] [Google Scholar]
- Tran LS, Urao T, Qin F, Maruyama K, Kakimoto T, Shinozaki K, et al. Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc. Natl Acad. Sci. USA. 2007;104:20623–20628. doi: 10.1073/pnas.0706547105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trontin C, Kiani S, Corwin JA, Hematy K, Yansouni J, Kliebenstein DJ, et al. A pair of receptor-like kinases is responsible for natural variation in shoot growth response to mannitol treatment in Arabidopsis thaliana. Plant J. 2014;78:121–133. doi: 10.1111/tpj.12454. [DOI] [PubMed] [Google Scholar]
- Urao T, Yakubov B, Satoh R, Yamaguchi-Shinozaki K, Seki M, Hirayama T, et al. A transmembrane hybrid-type histidine kinase in Arabidopsis functions as an osmosensor. Plant Cell. 1999;11:1743–1754. doi: 10.1105/tpc.11.9.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell. 2007;19:63–73. doi: 10.1105/tpc.106.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F, Yang H, Xue Y, Kong D, Ye R, Li C, et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature. 2014;514:367–371. doi: 10.1038/nature13593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.