Abstract
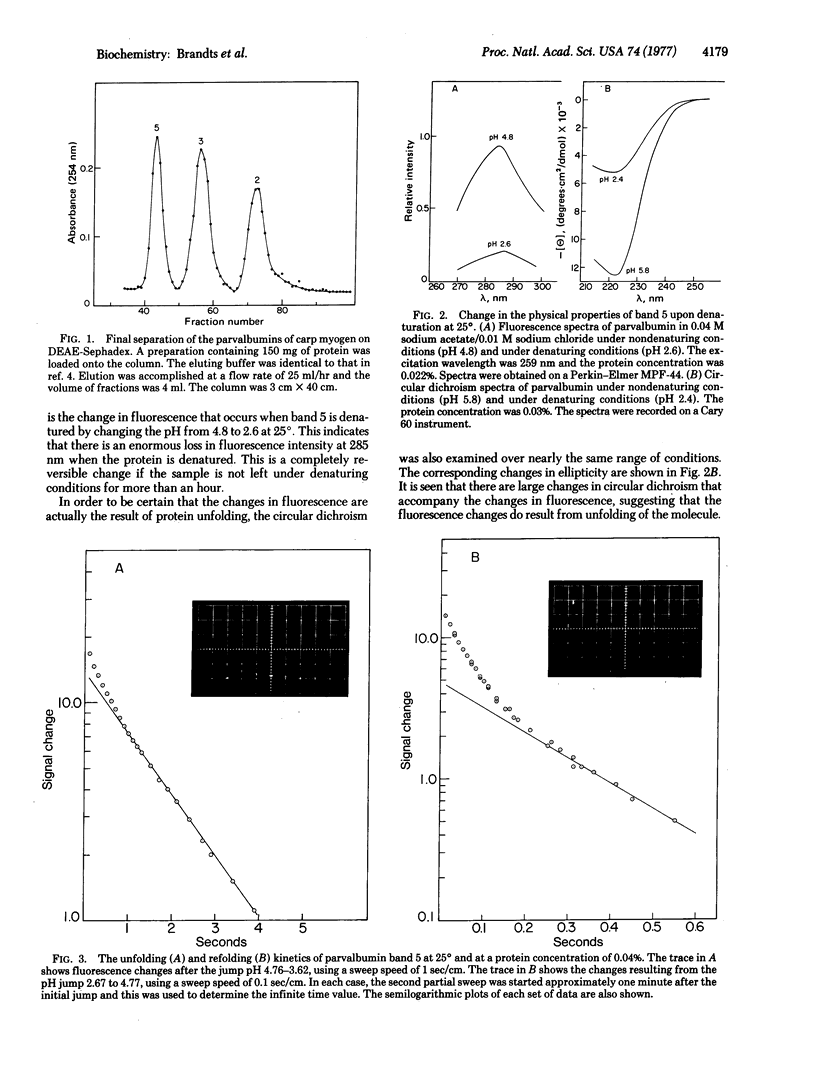
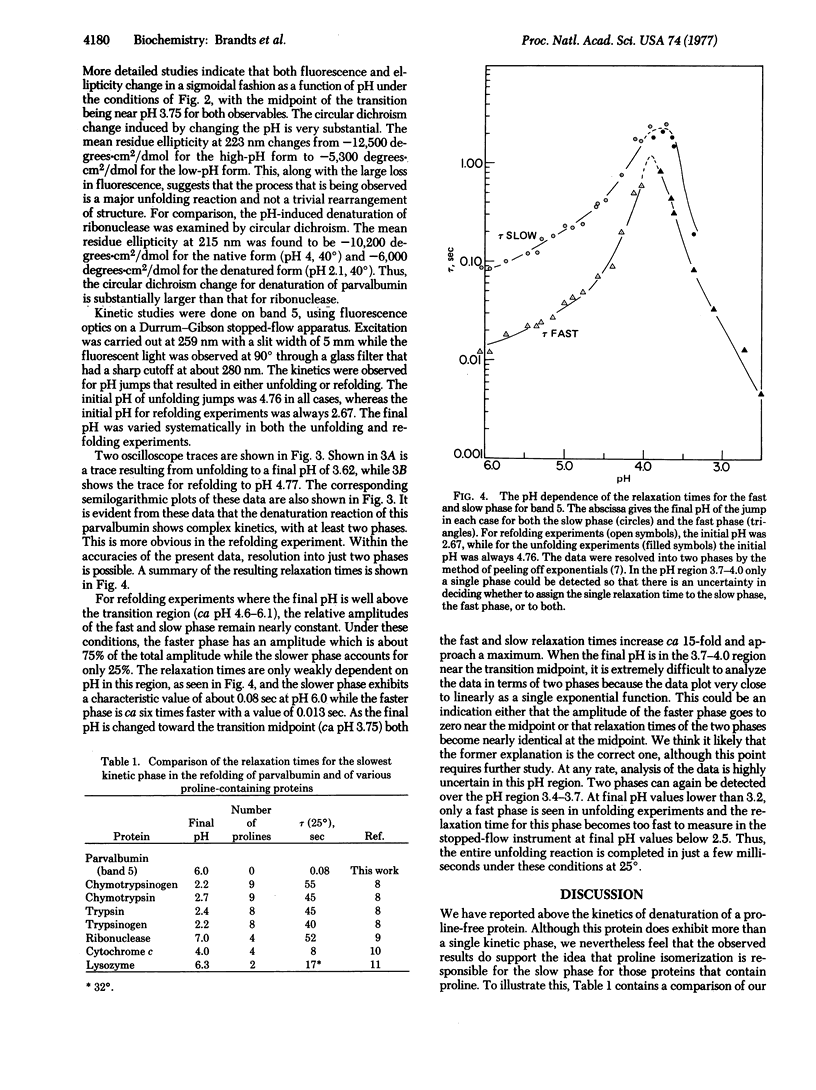
The kinetics for unfolding and refolding of a parvalbumin (band 5) have been examined as a function of pH near the transition region, using stopped-flow techniques. This protein is rather unusual in that it has no proline residues, and therefore serves as a good example to test the hypothesis that the rate-limiting step seen in denaturation reactions is due to the cis-trans isomerization of proline peptide bonds in the denatured state. The kinetics for parvalbumin unfolding and refolding are complex, with the data being resolvable into two fast phases at 25 degrees. The slower of the two phases seen for the parvalbumin is about 100 to 500 times faster than the slow phase seen for proline-containing proteins under the same conditions! These results argue strongly in support of the proline isomerization hypothesis. It is also suggested that the slower phase seen for parvalbumin and the second-slowest phase seen for proline-containing proteins might be due to the cis-trans isomerization of peptide bonds of non-proline residues.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin R. L. Intermediates in protein folding reactions and the mechanism of protein folding. Annu Rev Biochem. 1975;44:453–475. doi: 10.1146/annurev.bi.44.070175.002321. [DOI] [PubMed] [Google Scholar]
- Brandts J. F., Halvorson H. R., Brennan M. Consideration of the Possibility that the slow step in protein denaturation reactions is due to cis-trans isomerism of proline residues. Biochemistry. 1975 Nov 4;14(22):4953–4963. doi: 10.1021/bi00693a026. [DOI] [PubMed] [Google Scholar]
- Donato H., Jr, Martin R. B. Conformations of carp muscle calcium binding parvalbumin. Biochemistry. 1974 Oct 22;13(22):4575–4579. doi: 10.1021/bi00719a016. [DOI] [PubMed] [Google Scholar]
- Epstein H. F., Schechter A. N., Chen R. F., Anfinsen C. B. Folding of staphylococcal nuclease: kinetic studies of two processes in acid renaturation. J Mol Biol. 1971 Sep 28;60(3):499–508. doi: 10.1016/0022-2836(71)90184-7. [DOI] [PubMed] [Google Scholar]
- Garel J. R., Baldwin R. L. The heat-unfolded state of ribonuclease A is an equilibrium mixture of fast and slow refolding species. J Mol Biol. 1975 Jun 5;94(4):611–620. doi: 10.1016/0022-2836(75)90325-3. [DOI] [PubMed] [Google Scholar]
- Hagerman P. J., Baldwin R. L. A quantitative treatment of the kinetics of the folding transition of ribonuclease A. Biochemistry. 1976 Apr 6;15(7):1462–1473. doi: 10.1021/bi00652a017. [DOI] [PubMed] [Google Scholar]
- KONOSU S., HAMOIR G., PECHERE J. F. CARP MYOGENS OF WHITE AND RED MUSCLES. PROPERTIES AND AMINO ACID COMPOSITION OF THE MAIN LOW-MOLECULAR-WEIGHT COMPONENTS OF WHITE MUSCLE. Biochem J. 1965 Jul;96:98–112. doi: 10.1042/bj0960098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechére J. F., Demaille J., Capony J. P. Muscular parvalbumins: preparative and analytical methods of general applicability. Biochim Biophys Acta. 1971 May 25;236(2):391–408. doi: 10.1016/0005-2795(71)90220-0. [DOI] [PubMed] [Google Scholar]
- Segawa S., Husimi Y., Wada A. Kinetics of thermal unfolding of lysozyme. Biopolymers. 1973 Nov;12(11):2521–2537. doi: 10.1002/bip.1973.360121107. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y., Baldwin R. L., Elson E. L. Properties of the refolding and unfolding reactions of ribonuclease A. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1809–1812. doi: 10.1073/pnas.69.7.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsong T. Y. Detection of three kinetic phases in the thermal unfolding of ferricytochrome c. Biochemistry. 1973 Jun 5;12(12):2209–2214. doi: 10.1021/bi00736a005. [DOI] [PubMed] [Google Scholar]