Abstract
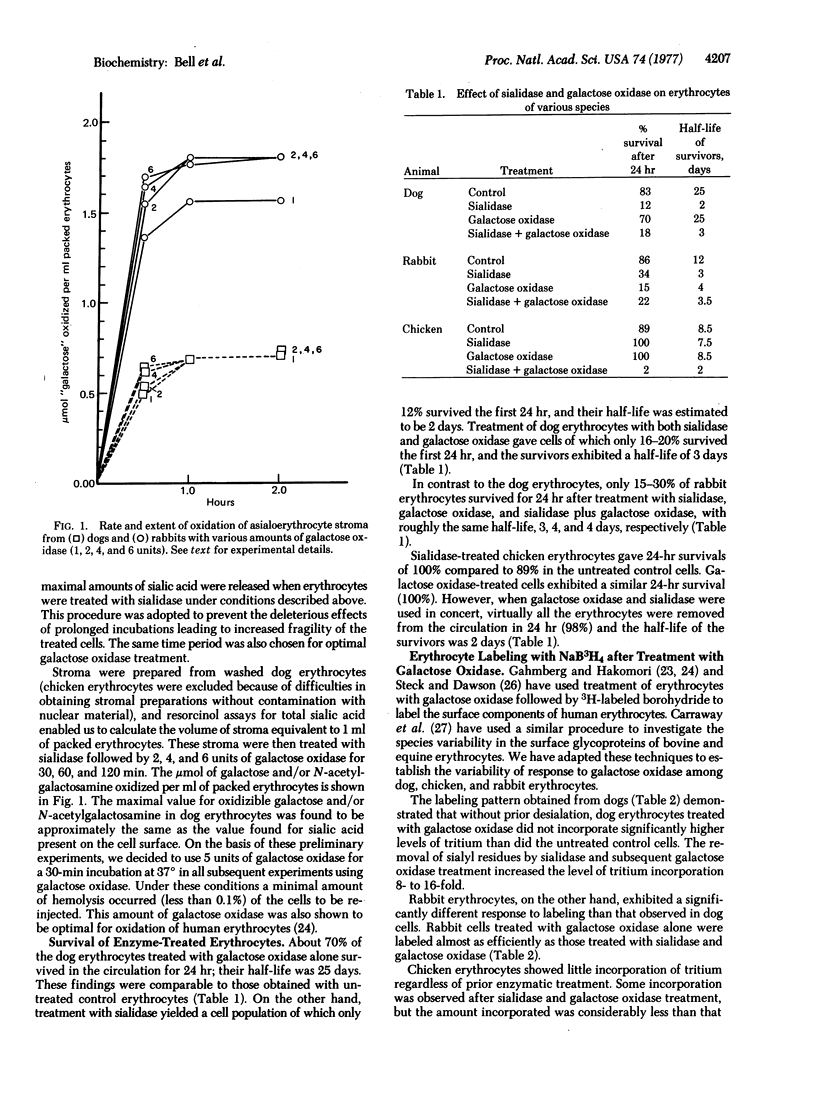
Previous studies have shown that sialidase-treated mammalian erythrocytes were rapidly eliminated from circulation. In contrast, chicken asialoerythrocytes remained fully viable. This investigation was undertaken to ascertain the reason for this difference in behavior as well as to determine the extent of the similarity of the physiological mechanism for the elimination from circulation of asialoglycoproteins and mammalian asialoerythrocytes. To that end, erythrocytes from dogs, rabbits, and chickens were each subjected to the action of galactose oxidase (D-galactose:oxygen 6-oxidoreductase; EC 1.1.3.9) both before and after sialidase (acylneuraminyl hydrolase; EC 3.2.1.18) treatment. The viability of the autologously transfused erythrocytes in circulation was monitored by Na2-51CrO4 labeling. Galactose oxidase had no deleterious effect on the viability of dog or chicken erythrocytes, nor did it restore the viability of dog or rabbit asialoerythrocytes. On the other hand, desialated chicken erythrocytes, which were fully viable, were rendered nonviable upon treatment with galactose oxidase. It may be concluded therefore that (a) the physiological mechanism of elimination of mammalian asialoerythrocytes from circulation is not the same as that for plasma asialoglycoproteins and (b) the treatment of chicken asialoerythrocytes with galactose oxidase results in the oxidation at carbons 6 of the galactosyl- or N-acetylgalactosaminyl residues, thereby rendering the erythrocytes nonviable.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AVIGAD G., AMARAL D., ASENSIO C., HORECKER B. L. The D-galactose oxidase of Polyporus circinatus. J Biol Chem. 1962 Sep;237:2736–2743. [PubMed] [Google Scholar]
- Aminoff D., Bell W. C., Fulton I., Ibgebrigtsen N. Effect of sialidase on the viability of erythrocytes in circulation. Am J Hematol. 1976;1(4):419–432. doi: 10.1002/ajh.2830010407. [DOI] [PubMed] [Google Scholar]
- Aminoff D., Bruegge W. F., Bell W. C., Sarpolis K., Williams R. Role of sialic acid in survival of erythrocytes in the circulation: interaction of neuraminidase-treated and untreated erythrocytes with spleen and liver at the cellular level. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1521–1524. doi: 10.1073/pnas.74.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Carraway K. L., Colton D. G., Shin B. C., Triplett R. B. Species variability in the modification of erythrocyte surface proteins by enzymatic probes. Biochim Biophys Acta. 1975 Mar 13;382(2):181–192. doi: 10.1016/0005-2736(75)90176-5. [DOI] [PubMed] [Google Scholar]
- Cassidy J. T., Jourdian G. W., Roseman S. The sialic acids. VI. Purification and properties of sialidase from Clostridium perfringens. J Biol Chem. 1965 Sep;240(9):3501–3506. [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Durocher J. R., Payne R. C., Conrad M. E. Role of sialic acid in erythrocyte survival. Blood. 1975 Jan;45(1):11–20. [PubMed] [Google Scholar]
- Gahmberg C. G. External labeling of human erythrocyte glycoproteins. Studies with galactose oxidase and fluorography. J Biol Chem. 1976 Jan 25;251(2):510–515. [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973 Jun 25;248(12):4311–4317. [PubMed] [Google Scholar]
- Gattegno L., Bladier D., Cornillot P. Ageing in vivo and neuraminidase treatment of rabbit erythrocytes: influence on half-life as assessed by 51Cr labelling. Hoppe Seylers Z Physiol Chem. 1975 Apr;356(4):391–397. doi: 10.1515/bchm2.1975.356.1.391. [DOI] [PubMed] [Google Scholar]
- Gattegno L., Bladier D., Cornillot P. The role of sialic acid in the determination of survival of rabbit erythrocytes in the circulation. Carbohydr Res. 1974 Jun;34(2):361–369. doi: 10.1016/s0008-6215(00)82911-0. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Putman D., Louis L., Neerunjun D. Comparative effect and fate of non-entrapped and liposome-entrapped neuraminidase injected into rats. Biochem J. 1974 May;140(2):323–330. doi: 10.1042/bj1400323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancik J., Schauer R. Sialic acid--a determinant of the life-time of rabbit erythrocytes. Hoppe Seylers Z Physiol Chem. 1974 Apr;355(4):395–400. doi: 10.1515/bchm2.1974.355.1.395. [DOI] [PubMed] [Google Scholar]
- Jancik J., Schauer R., Streicher H. J. Influence of membrane-bound N-acetylneuraminic acid on the survival of erythrocytes in man. Hoppe Seylers Z Physiol Chem. 1975 Aug;356(8):1329–1331. [PubMed] [Google Scholar]
- Kean E. L. Nuclear cytidine 5'-monophosphosialic acid synthetase. J Biol Chem. 1970 May 10;245(9):2301–2308. [PubMed] [Google Scholar]
- Kean E. L., Roseman S. The sialic acids. X. Purification and properties of cytidine 5'-monophosphosialic acid synthetase. J Biol Chem. 1966 Dec 10;241(23):5643–5650. [PubMed] [Google Scholar]
- Lunney J., Ashwell G. A hepatic receptor of avian origin capable of binding specifically modified glycoproteins. Proc Natl Acad Sci U S A. 1976 Feb;73(2):341–343. doi: 10.1073/pnas.73.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radola B. J. Analytical and preparative isoelectric focusing in gel-stabilized layers. Ann N Y Acad Sci. 1973 Jun 15;209:127–143. doi: 10.1111/j.1749-6632.1973.tb47523.x. [DOI] [PubMed] [Google Scholar]
- Radola B. J. Isoelectric focusing in layers of granulated gels. II. Preparative isoelectric focusing. Biochim Biophys Acta. 1975 Mar 28;386(1):181–195. doi: 10.1016/0005-2795(75)90258-5. [DOI] [PubMed] [Google Scholar]
- Regoeczi E., Hatton M. W., Charlwood P. A. Carbohydrate-mediated elimination of avian plasma glycoprotein in mammals. Nature. 1975 Apr 24;254(5502):699–701. doi: 10.1038/254699a0. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Sempere J. M., Gancedo C., Asensio C. Determination of galactosamine and N-acetylgalactosamine in the presence of other hexosamines with galactose oxidase. Anal Biochem. 1965 Sep;12(3):509–515. doi: 10.1016/0003-2697(65)90217-4. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Dawson G. Topographical distribution of complex carbohydrates in the erythrocyte membrane. J Biol Chem. 1974 Apr 10;249(7):2135–2142. [PubMed] [Google Scholar]
- Zahler P. Blood group antigens in relation to chemical and structural properties of the red cell membrane. Vox Sang. 1968;15(2):81–101. doi: 10.1111/j.1423-0410.1968.tb03414.x. [DOI] [PubMed] [Google Scholar]
- van Dijk W., Ferwerda W., van den Eijnden D. H. Subcellular and regional distribution of CMP-N-acetylneuraminic acid synthetase in the calf kidney. Biochim Biophys Acta. 1973 Jul 5;315(1):162–175. doi: 10.1016/0005-2744(73)90139-3. [DOI] [PubMed] [Google Scholar]
- van den Eijnden D. H. The subcellular localization of cytidine 5'-monophospho-N-acetylneuraminic acid synthetase in calf brain. J Neurochem. 1973 Oct;21(4):949–958. doi: 10.1111/j.1471-4159.1973.tb07539.x. [DOI] [PubMed] [Google Scholar]


