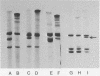
Abstract
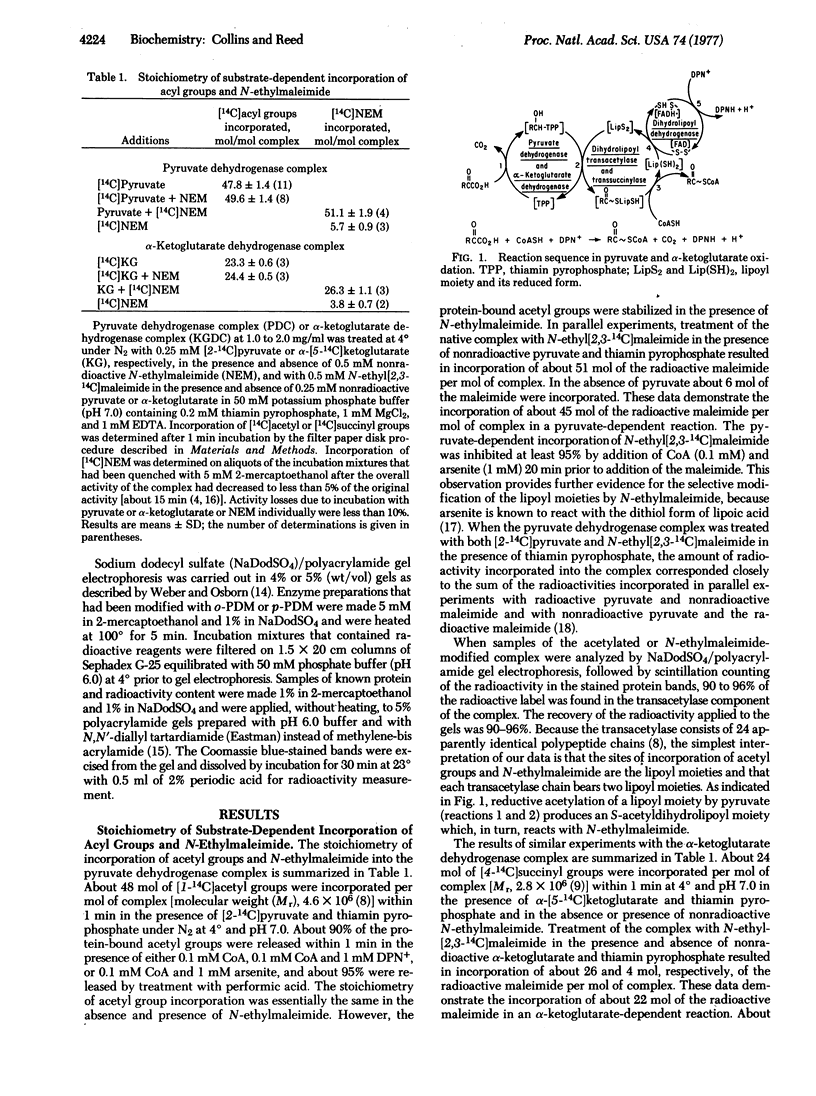
The dihydrolipoyl transacetylase component of the Escherichia coli pyruvate dehydrogenase complex [pyruvate:lipoate oxidoreductase (decarboxylating and acceptor-acetylating), EC 1.2.4.1] bears two sites on each of its 24 polypeptide chains that undergo reductive acetylation by [2-14C]pyruvate and thiamin pyrophosphate, acetylation by [1-14C]acetyl-CoA in the presence of DPNH, and reaction with N-ethyl[2,3-14C]maleimide in the presence of pyruvate and thiamin pyrophosphate. The data strongly imply that these sites are covalently bound lipoyl moieties. The results of similar experiments with the E. coli α-ketoglutarate dehydrogenase complex [2-oxoglutarate:lipoate oxidoreductase (decarboxylating and acceptor-succinylating), EC 1.2.4.2] indicate that its dihydrolipoyl transsuccinylase component bears only one lipoyl moiety on each of its 24 chains. Charging of the 48 acetyl acceptor sites on the transacetylase or the 24 succinyl acceptor sites on the transsuccinylase by pyruvate or α-ketoglutarate, respectively, and thiamin pyrophosphate was observed in the presence of only a few functionally active pyruvate dehydrogenase or α-ketoglutarate dehydrogenase chains. Extensive crosslinking of the transacetylase chains was observed when the pyruvate dehydrogenase complex was treated with pyruvate and thiamin pyrophosphate or with DPNH in the presence of N,N′-o- or N,N′-p-phenylenedimaleimide, respectively. When the α-ketoglutarate dehydrogenase complex was treated with DPNH in the presence of N,N′-p-phenylenedimaleimide, only transsuccinylase monomers and crosslinked transsuccinylase dimers were detected. It appears that the 48 lipoyl moieties in the transacetylase and the 24 lipoyl moieties in the transsuccinylase comprise an interacting network that functions as an acyl group and electron pair relay system through thiol-disulfide and acyl-transfer reactions among all of the lipoyl moieties.
Keywords: multienzyme complexes, thiol-disulfide interchange, crosslinking of subunits
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrose M. C., Perham R. N. Spin-label study of the mobility of enzyme-bound lipoic acid in the pyruvate dehydrogenase multienzyme complex of Escherichia coli. Biochem J. 1976 May 1;155(2):429–432. doi: 10.1042/bj1550429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker H. S. A solubilizable acrylamide gel for electrophoresis. FEBS Lett. 1970 Apr 16;7(3):293–293. doi: 10.1016/0014-5793(70)80185-5. [DOI] [PubMed] [Google Scholar]
- Brown J. P., Perham R. N. Selective inactivation of the transacylase components of the 2-oxo acid dehydrogenase multienzyme complexes of Escherichia coli. Biochem J. 1976 May 1;155(2):419–427. doi: 10.1042/bj1550419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J. R., Pettit R. H., Davis P. F., Reed L. J. Binding of thiamin thiazolone pyrophosphate to mammalian pyruvate dehydrogenase and its effects of kinase and phosphatase activities. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1667–1674. doi: 10.1016/0006-291x(77)90636-2. [DOI] [PubMed] [Google Scholar]
- Chang F. N., Flaks J. G. The specific cross-linking of two proteins from the Escherichia coli 30 s ribosomal subunit. J Mol Biol. 1972 Jul 14;68(1):177–180. doi: 10.1016/0022-2836(72)90272-0. [DOI] [PubMed] [Google Scholar]
- Danson M. J., Perham R. N. Evidence for two lipoic acid residues per lipoate acetyltransferase chain in the pyruvate dehydrogenase multienzyme complex of Escherichia coli. Biochem J. 1976 Dec 1;159(3):677–682. doi: 10.1042/bj1590677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derosier D. J., Oliver R. M., Reed L. J. Crystallization and preliminary structural analysis of dihydrolipoyl transsuccinylase, the core of the 2-oxoglutarate dehydrogenase complex. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1135–1137. doi: 10.1073/pnas.68.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley M. H., Namihira G., Hamilton L., Munk P., Reed L. J. -Keto acid dehydrogenase complexes. 18. Subunit composition of the Escherichia coli pyruvate dehydrogenase complex. Arch Biochem Biophys. 1972 Oct;152(2):655–669. doi: 10.1016/0003-9861(72)90262-7. [DOI] [PubMed] [Google Scholar]
- Grande H. J., Bresters T. W., de Abreu R. A., de Kok A., Veeger C. The pyruvate-dehydrogenase complex from Azotobacter vinelandii. 3. Stoichiometry and function of the individual components. Eur J Biochem. 1975 Nov 15;59(2):355–363. doi: 10.1111/j.1432-1033.1975.tb02462.x. [DOI] [PubMed] [Google Scholar]
- Gutowski J. A., Lienhard G. E. Transition state analogs for thiamin pyrophosphate-dependent enzymes. J Biol Chem. 1976 May 10;251(9):2863–2866. [PubMed] [Google Scholar]
- KOIKE M., REED L. J., CARROLL W. R. alpha-Keto acid dehydrogenation complexes. IV. Resolution and reconstitution of the Escherichia coli pyruvate dehydrogenation complex. J Biol Chem. 1963 Jan;238:30–39. [PubMed] [Google Scholar]
- Moe O. A., Jr, Lerner D. A., Hammes G. G. Fluorescence energy transfer between the thiamine diphosphate and flavine adenine dinucleotide binding sites on the pyruvate dehydrogenase multienzyme complex. Biochemistry. 1974 Jun 4;13(12):2552–2557. doi: 10.1021/bi00709a012. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Hamilton L., Munk P., Namihira G., Eley M. H., Willms C. R., Reed L. J. Alpha-keto acid dehydrogenase complexes. XIX. Subunit structure of the Escherichia coli alpha-ketoglutarate dehydrogenase complex. J Biol Chem. 1973 Aug 10;248(15):5282–5290. [PubMed] [Google Scholar]
- Reed L. J., Oliver R. M. The multienzyme alpha-keto acid dehydrogenase complexes. Brookhaven Symp Biol. 1968 Jun;21(2):397–412. [PubMed] [Google Scholar]
- Speckhard D. C., Frey P. A. Esherichia coli pyruvate dehydrogenase complex: improved purification and the flavin content. Biochem Biophys Res Commun. 1975 Feb 3;62(3):614–620. doi: 10.1016/0006-291x(75)90443-x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]