Abstract
We have determined the nucleotide sequence of a Hpa II restriction fragment of the phage T7 DNA containing a promoter for the phage-specified RNA polymerase. (Hpa II is a restriction endonuclease from Haemophilus parainfluenzae.) Mapping of the Hpa II restriction fragments on the T7 genome shows this promoter to be the second of tandem promoters separated by approximately 170 base pairs that begin transcription by the T7 RNA polymerase at approximately 15% of the genome. Features of the sequence involved in recognition by the T7 RNA polymerase are discussed and include the following region of hyphenated 2-fold symmetry (boxed regions are related through a 2-fold axis of symmetry at the center of the sequence shown). (See article). This sequence includes the initiation site, since the message transcribed from this fragment begins pppG-G-G-A. Combination of our results with work of others has permitted this fragment to be mapped at the junction of T7 genes 1 and 1.1. The RNA transcribed from this fragment begins within gene 1 and contains the RNase III cleavage site that lies between genes 1 and 1.1. This sequence is compared to other processing sites in T7 early message.
Full text
PDF
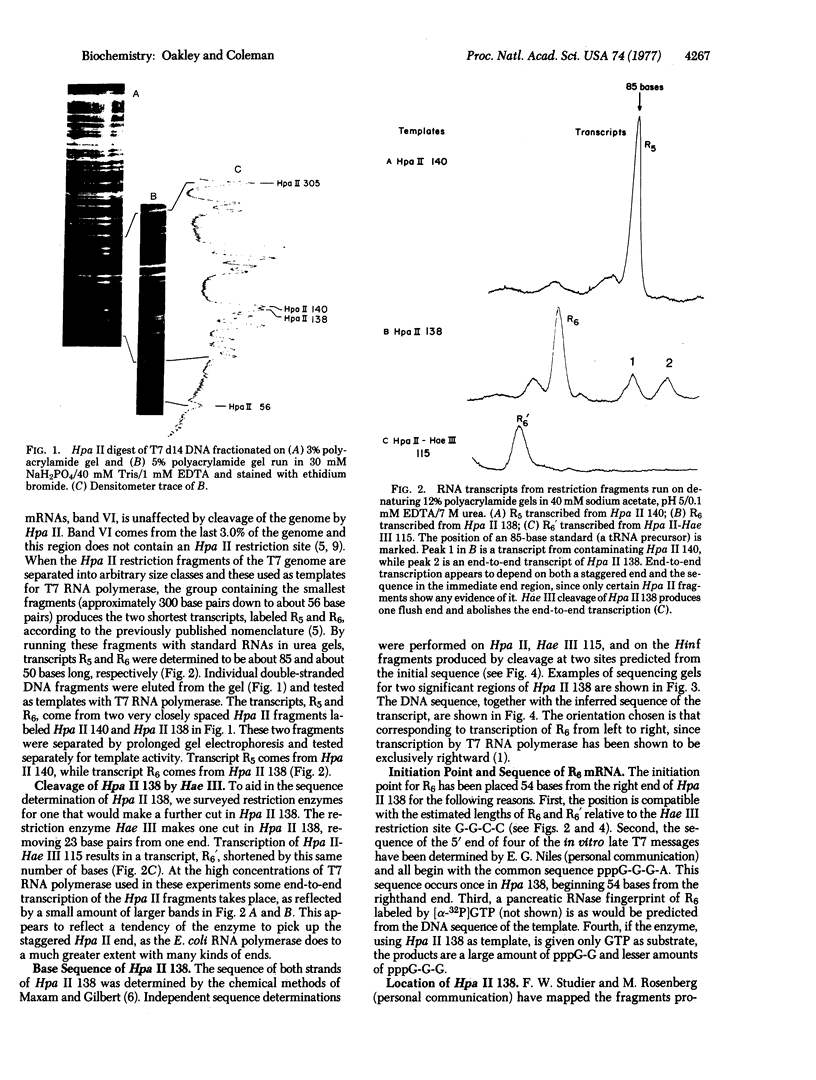

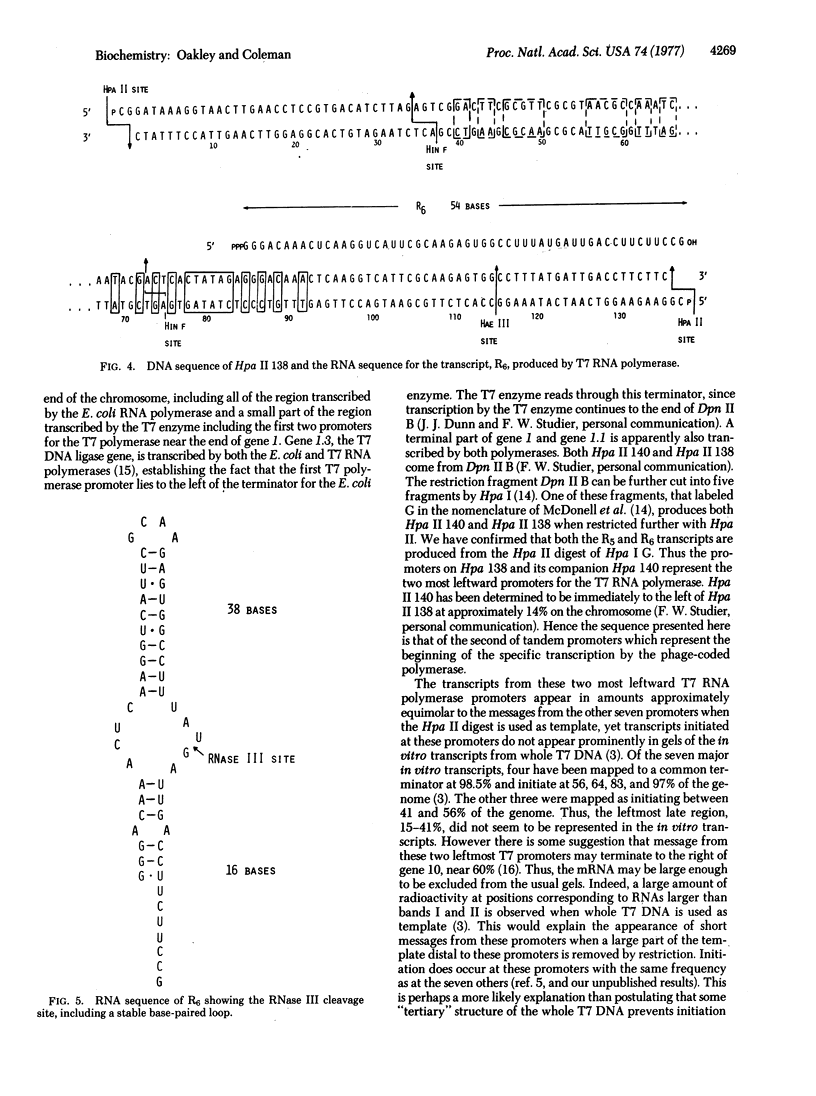

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chamberlin M., McGrath J., Waskell L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature. 1970 Oct 17;228(5268):227–231. doi: 10.1038/228227a0. [DOI] [PubMed] [Google Scholar]
- Dunn J. J. RNase III cleavage of single-stranded RNA. Effect of ionic strength on the fideltiy of cleavage. J Biol Chem. 1976 Jun 25;251(12):3807–3814. [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Effect of RNAase III, cleavage on translation of bacteriophage T7 messenger RNAs. J Mol Biol. 1975 Dec 15;99(3):487–499. doi: 10.1016/s0022-2836(75)80140-9. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs are generated by site-specific cleavages. Proc Natl Acad Sci U S A. 1973 May;70(5):1559–1563. doi: 10.1073/pnas.70.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb M., Chamberlin M. Characterization of T7-specific ribonucleic acid polymerase. IV. Resolution of the major in vitro transcripts by gel electrophoresis. J Biol Chem. 1974 May 10;249(9):2858–2863. [PubMed] [Google Scholar]
- Hercules K., Schweiger M., Sauerbier W. Cleavage by RNase 3 converts T3 and T7 early precursor RNA into translatable message. Proc Natl Acad Sci U S A. 1974 Mar;71(3):840–844. doi: 10.1073/pnas.71.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Oakley J. L., Pascale J. A., Coleman J. E. T7 RNA polymerase: conformation, functional groups, and promotor binding. Biochemistry. 1975 Oct 21;14(21):4684–4691. doi: 10.1021/bi00692a019. [DOI] [PubMed] [Google Scholar]
- Pribnow D. Bacteriophage T7 early promoters: nucleotide sequences of two RNA polymerase binding sites. J Mol Biol. 1975 Dec 15;99(3):419–443. doi: 10.1016/s0022-2836(75)80136-7. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E., Dunn J. J. A nucleotide sequence from a ribonuclease III processing site in bacteriophage T7 RNA. Proc Natl Acad Sci U S A. 1977 Mar;74(3):822–826. doi: 10.1073/pnas.74.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Kramer R. A. Nucleotide sequence surrounding a ribonuclease III processing site in bacteriophage T7 RNA. Proc Natl Acad Sci U S A. 1977 Mar;74(3):984–988. doi: 10.1073/pnas.74.3.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Kramer R. A., Steitz J. A. The specificity of RNase III cleavage of bacteriophage T7 early messenger RNAs. Brookhaven Symp Biol. 1975 Jul;(26):277–285. [PubMed] [Google Scholar]
- Sekiya T., Khorana H. G. Nucleotide sequence in the promoter region of the Escherichia coli tyrosine tRNA gene. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2978–2982. doi: 10.1073/pnas.71.8.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skare J., Niles E. G., Summers W. C. Localization of the leftmost initiation site for T7 late transcription, in vivo and in vitro. Biochemistry. 1974 Sep 10;13(19):3912–3916. doi: 10.1021/bi00716a015. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Summers W. C., Siegel R. B. Transcription of late phage RNA by T7 RNA polymerase. Nature. 1970 Dec 19;228(5277):1160–1162. doi: 10.1038/2281160a0. [DOI] [PubMed] [Google Scholar]