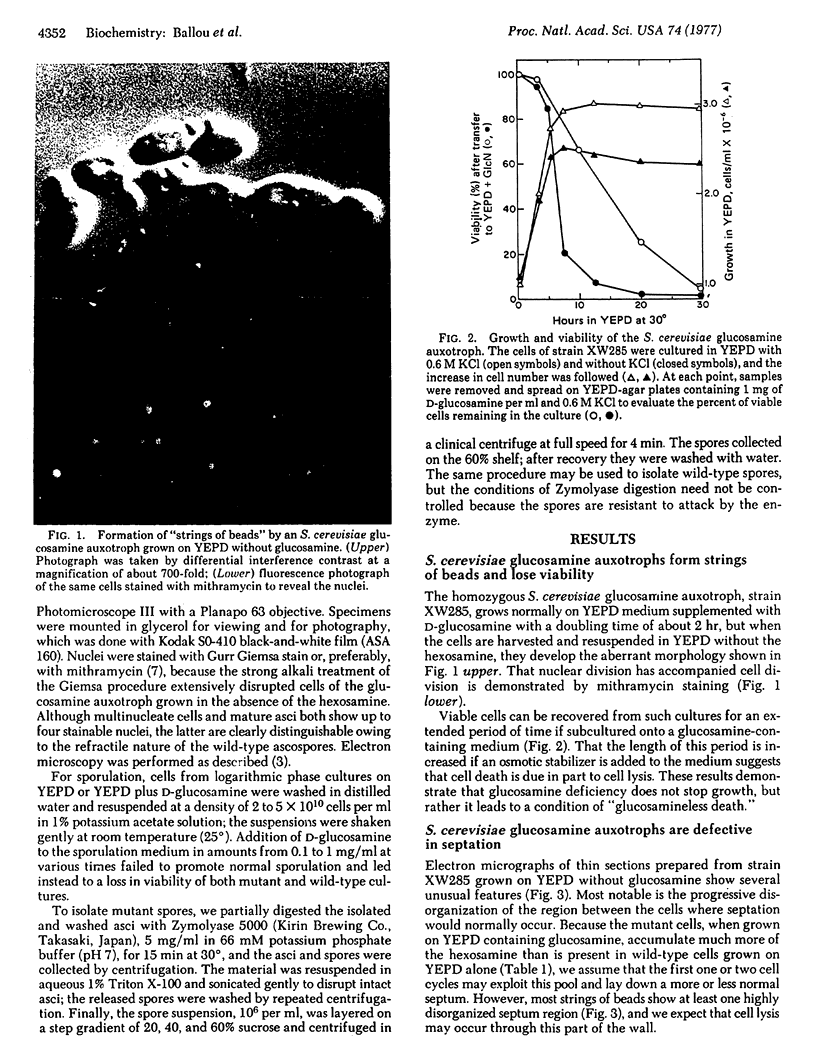
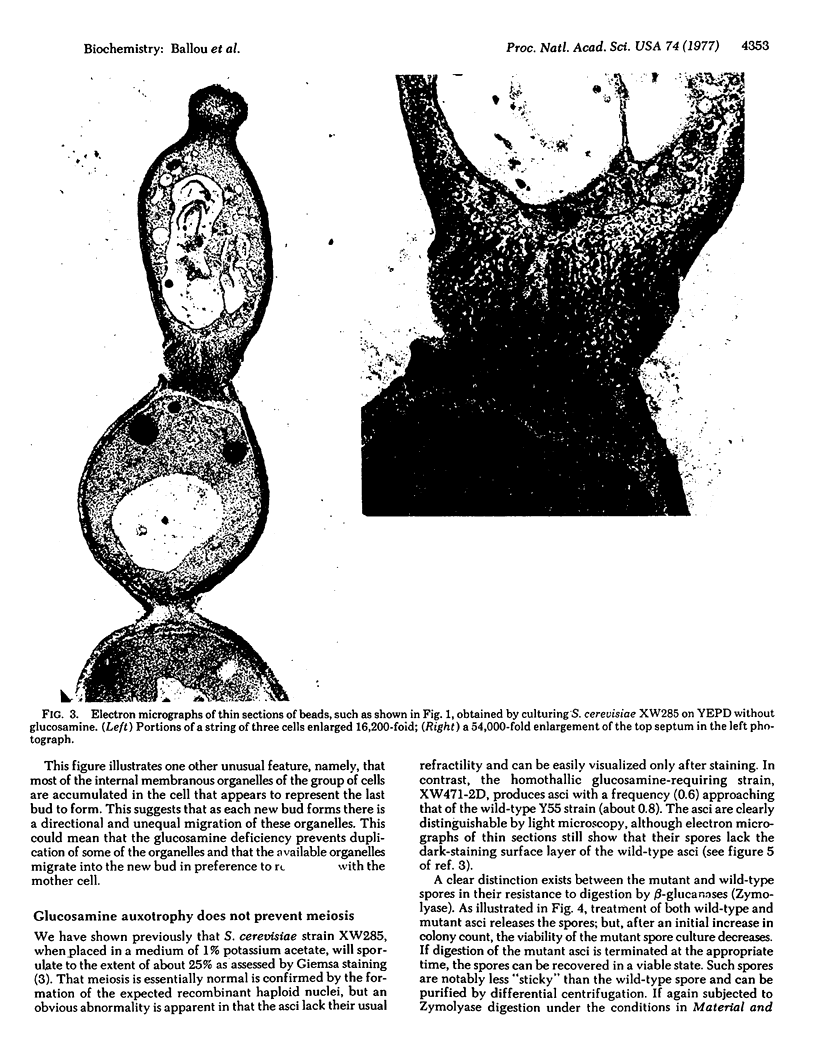
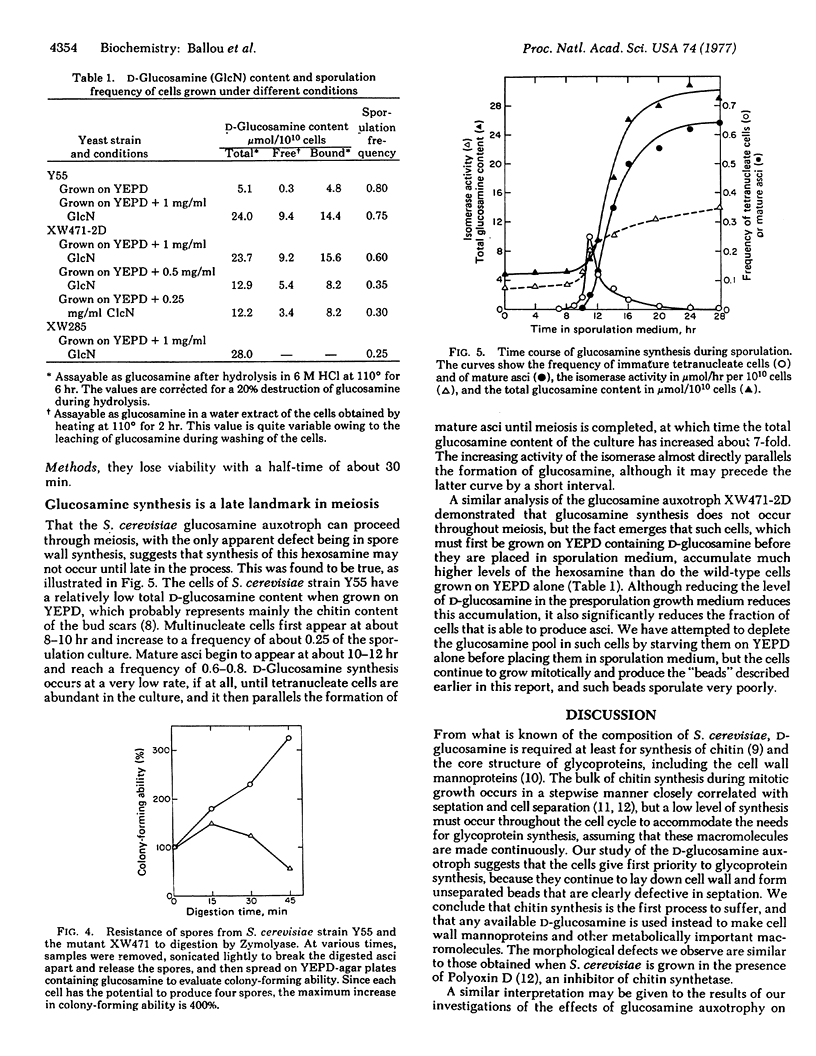
Abstract
Saccharomyces cerevisiae mutants, unable to make D-glucosamine owing to a defect in the enzyme 2-amino-2-deoxy-D-glucose-6-phosphate ketol-isomerase (amino-transferring) (EC 5.3.1.19), show aberrations both in sporulation and in vegetative growth. They grow normally on a medium of yeast extract, peptone, and dextrose (YEPD) containing D-glucosamine (1 mg/ml), and such cells accumulate 4 to 5 times the amount of D-glucosamine present in wild-type cells cultured on YEPD alone. When such mutant cells are shifted to YEPD alone, they continue to increase in cell mass for about 10 hr (three to four cell cycles) and produce strings of beads in which the cells fail to separate. Although each of the “cells” contains a nucleus, electron micrographs of thin sections reveal that septation is defective apparently owing to the inability to synthesize chitin, which forms the primary septum in S. cerevisiae. The viability of such cultures drops rapidly after 3-5 hr, a fact attributable to lysis of the cells through wall defects in the septum region where gross disorganization is apparent. When the mutant cells grown on YEPD plus D-glucosamine are transferred to sporulation medium (1% potassium acetate), they proceed through meiosis to produce viable spores that appear to be altered only in the nature of the spore wall. The spores lack a dark-staining surface layer that is visible in thin sections prepared from wild-type cells, they are notably less hydrophobic than wild-type spores, and they are digested and lysed by glucanases that do not affect normal spores. All of these properties suggest that D-glucosamine is required for spore maturation and is used to synthesize a glucanase-resistant hydrophobic surface layer on the primary glucan spore wall. In agreement with this postulate, D-glucosamine synthesis and the activity of the isomerase do not appear until late in meiosis when tetranucleate cells are abundantly present in the sporulation culture.
Keywords: mitosis, meiosis, glucan, mannoprotein, chitin
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cabib E., Bowers B. Chitin and yeast budding. Localization of chitin in yeast bud scars. J Biol Chem. 1971 Jan 10;246(1):152–159. [PubMed] [Google Scholar]
- Cabib E., Farkas V. The control of morphogenesis: an enzymatic mechanism for the initiation of septum formation in yeast. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2052–2056. doi: 10.1073/pnas.68.9.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Ulane R., Bowers B. A molecular model for morphogenesis: the primary septum of yeast. Curr Top Cell Regul. 1974;8(0):1–32. doi: 10.1016/b978-0-12-152808-9.50008-0. [DOI] [PubMed] [Google Scholar]
- Freese E. B., Cole R. M., Klofat W., Freese E. Growth, sporulation, and enzyme defects of glucosamine mutants of Bacillus subtilis. J Bacteriol. 1970 Mar;101(3):1046–1062. doi: 10.1128/jb.101.3.1046-1062.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHOSH S., BLUMENTHAL H. J., DAVIDSON E., ROSEMAN S. Glucosamine metabolism. V. Enzymatic synthesis of glucosamine 6-phosphate. J Biol Chem. 1960 May;235:1265–1273. [PubMed] [Google Scholar]
- Haber J. E., Halvorson H. O. Methods in sporulation and germination of yeasts. Methods Cell Biol. 1975;11:45–69. doi: 10.1016/s0091-679x(08)60316-7. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Saccharomyces cerevisiae cell cycle. Bacteriol Rev. 1974 Jun;38(2):164–198. doi: 10.1128/br.38.2.164-198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane S. M., Roth R. Carbohydrate metabolism during ascospore development in yeast. J Bacteriol. 1974 Apr;118(1):8–14. doi: 10.1128/jb.118.1.8-14.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller F. A., Cabib E. Chitin and yeast budding. Properties of chitin synthetase from Saccharomyces carlsbergensis. J Biol Chem. 1971 Jan 10;246(1):160–166. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Manners D. J., Masson A. J. The structures of two glucans from yeast-cell walls. FEBS Lett. 1969 Jul;4(2):122–124. doi: 10.1016/0014-5793(69)80211-5. [DOI] [PubMed] [Google Scholar]
- Moens P. B., Esposito R. E., Esposito M. S. Aberrant nuclear behavior at meiosis and anucleate spore formation by sporulation-deficient (SPO) mutants of Saccharomyces cerevisiae. Exp Cell Res. 1974 Jan;83(1):166–174. doi: 10.1016/0014-4827(74)90700-9. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Ballou C. E. Structure of the linkage region between the polysaccharide and protein parts of Saccharomyces cerevisiae mannan. J Biol Chem. 1974 Dec 10;249(23):7685–7694. [PubMed] [Google Scholar]
- Slater M. L. Rapid nuclear staining method for Saccharomyces cerevisiae. J Bacteriol. 1976 Jun;126(3):1339–1341. doi: 10.1128/jb.126.3.1339-1341.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Ballou C. E. Sporulation in D-glucosamine auxotrophs of Saccharomyces cerevisiae: meiosis with defective ascospore wall formation. J Bacteriol. 1975 Dec;124(3):1545–1557. doi: 10.1128/jb.124.3.1545-1557.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]