Abstract
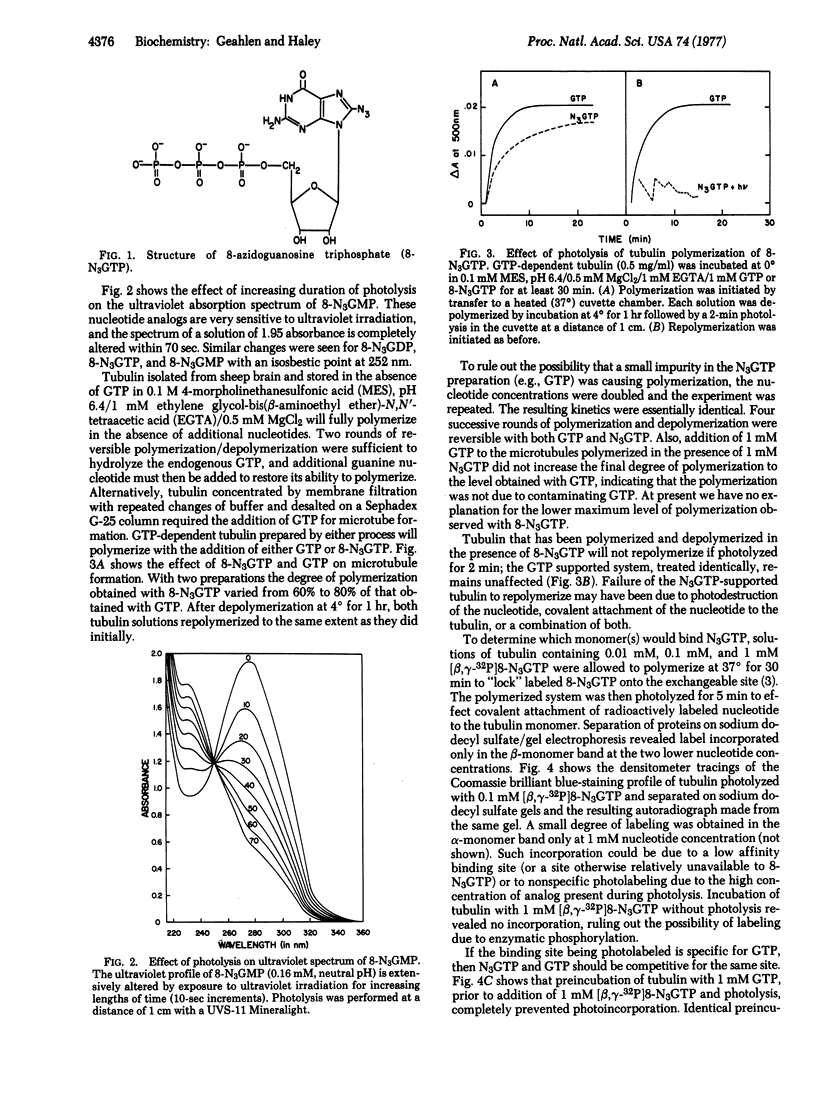
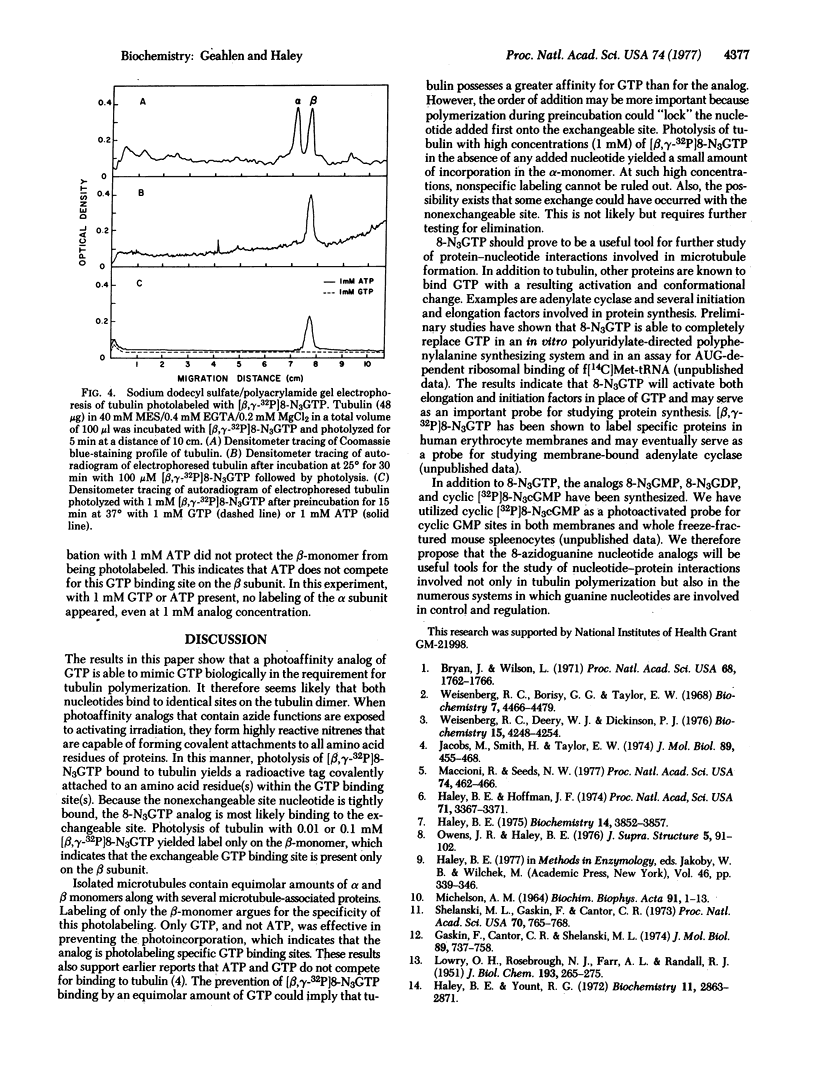
Tubulin dimers isolated from brain contain two GTP binding sites, a nonexchangeable site and an exchangeable site. To localize the exchangeable site, we used a photoaffinity analog of GTP, 8-azidoguanosine triphosphate (8-N3GTP), which supports tubulin polymerization in the absence of activating light. Photolysis of tubulin polymerized in the presence of 0.01 to 0.1 mM [beta, gamma-32P]8-N3GTP resulted in covalent incorporation of radioactivity only onto the beta monomer. Photolysis with 8-N3GTP also prevented any further repolymerization of the tubulin whereas like treatment in the presence of GTP had no effect. Preincubation of tubulin with GTP prevented photo-incorporation of [beta, gamma-32P]8-N3GTP whereas preincubation with ATP did not.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryan J., Wilson L. Are cytoplasmic microtubules heteropolymers? Proc Natl Acad Sci U S A. 1971 Aug;68(8):1762–1766. doi: 10.1073/pnas.68.8.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin F., Cantor C. R., Shelanski M. L. Turbidimetric studies of the in vitro assembly and disassembly of porcine neurotubules. J Mol Biol. 1974 Nov 15;89(4):737–755. doi: 10.1016/0022-2836(74)90048-5. [DOI] [PubMed] [Google Scholar]
- Haley B. E., Hoffman J. F. Interactions of a photo-affinity ATP analog with cation-stimulated adenosine triphosphatases of human red cell membranes. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3367–3371. doi: 10.1073/pnas.71.9.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley B. E. Photoaffinity labeling of adenosine 3',5'-cyclic monophosphate binding sites of human red cell membranes. Biochemistry. 1975 Aug 26;14(17):3852–3857. doi: 10.1021/bi00688a018. [DOI] [PubMed] [Google Scholar]
- Haley B., Yount R. G. -Fluoroadenosine triphosphate. Synthesis, properties, and interaction with myosin and heavy meromyosin. Biochemistry. 1972 Jul 18;11(15):2863–2871. doi: 10.1021/bi00765a020. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Smith H., Taylor E. W. Tublin: nucleotide binding and enzymic activity. J Mol Biol. 1974 Nov 5;89(3):455–468. doi: 10.1016/0022-2836(74)90475-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MICHELSON A. M. SYNTHESIS OF NUCLEOTIDE ANHYDRIDES BY ANION EXCHANGE. Biochim Biophys Acta. 1964 Sep 11;91:1–13. doi: 10.1016/0926-6550(64)90164-1. [DOI] [PubMed] [Google Scholar]
- Maccioni R., Seeds N. W. Stoichiometry of GTP hydrolysis and tubulin polymerization. Proc Natl Acad Sci U S A. 1977 Feb;74(2):462–466. doi: 10.1073/pnas.74.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J. R., Haley B. E. A study of adenosine 3'-5' cyclic monophosphate binding sites of human erythrocyte membranes using 8-azidoadenosine 3'-5' cyclic monophosphate, a photoaffinity probe. J Supramol Struct. 1976;5(1):91–102. doi: 10.1002/jss.400050110. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C., Borisy G. G., Taylor E. W. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968 Dec;7(12):4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Deery W. J., Dickinson P. J. Tubulin-nucleotide interactions during the polymerization and depolymerization of microtubules. Biochemistry. 1976 Sep 21;15(19):4248–4254. doi: 10.1021/bi00664a018. [DOI] [PubMed] [Google Scholar]



