Given the widespread use of bevacizumab in clinical practice, it is important to raise clinicians’ awareness of the potential risks of this treatment. The authors aimed to provide an overview of the most common side effects of bevacizumab and to suggest a practical approach for their management.
Keywords: Bevacizumab, Antiangiogenic, Toxicity, Side effects
Abstract
Bevacizumab, currently an option for treatment of different types of tumors including glioblastoma, has a peculiar toxicity profile related to its antiangiogenic effect. Because some bevacizumab-related adverse events can be life threatening, it is important to identify risk factors and to establish treatment protocols to minimize treatment-related morbidity and mortality. In glioblastoma patients, the risk of developing certain side effects, such as gastrointestinal perforation, venous thromboembolism, and intracranial hemorrhages, is slightly higher than in patients treated with bevacizumab for other tumor types. We performed a systematic review of the side effects of bevacizumab and their incidence, causal mechanisms, and available treatments. Finally, we identified risk factors and proposed preventive and therapeutic measures for these adverse events.
Implications for Practice:
Given the widespread use of bevacizumab in clinical practice, it is important to raise clinicians’ awareness of the potential risks of this treatment. Our aim was to provide an overview of the most common side effects of bevacizumab and to suggest a practical approach for their management.
Introduction
Angiogenesis is a fundamental process in cancer growth that involves different proteins and receptors such as vascular endothelial growth factor (VEGF) and VEGF receptor (VEGFR), which have recently become a target for anticancer treatments. A wide variety of agents targeting both VEGF and VEGFR have recently become standard treatments for different tumors. Bevacizumab, a monoclonal antibody, works as a chimeric VEGF receptor, blocking VEGF and preventing it from binding to VEGFR. Several phase III studies have shown its efficacy when combined with chemotherapy for the treatment of colorectal cancer, nonsquamous non-small cell lung cancer (NSCLC), and renal, ovarian, and breast cancer [1–7]. In the glioblastoma setting, data about the efficacy of bevacizumab derive from phase II trials for recurrent disease [8–13]. Recently, two large phase III trials in newly diagnosed glioblastomas have been conducted to compare bevacizumab with placebo in association with temozolomide concomitant and adjuvant to radiotherapy [14, 15]. These studies demonstrated that bevacizumab improves progression-free survival (PFS) but not overall survival (OS).
VEGF influences not only tumoral angiogenesis but also several physiological processes that involve vascular homeostasis, coagulation, wound healing, renal filtration, and blood pressure regulation. The VEGF blockade thus determines several peculiar toxicities, some of which can be life threatening. This review is focused on the main bevacizumab-related adverse events, their risk factors, and possible preventive and therapeutic measures.
Methods
We performed a review of available English literature on the adverse events of bevacizumab in all cancer types, with a focus on glioblastoma multiforme (GBM). Results from phase II and III trials on GBM and mainly from meta-analysis for other cancer types were gathered to compare the incidence of bevacizumab-related adverse events in GBM and other tumors.
Hypertension
Hypertension (HTN) is the most common adverse event associated with bevacizumab (Table 1). In the two phase III studies on GBM, the incidence of grade ≥3 HTN was 4% in the bevacizumab arm and 1% in the control arm of the Radiotherapy Oncology Group (RTOG) 0825 trial [14] and 7.6% and 8%, respectively, in the AVAglio trial [15]. All-grade HTN incidence reported in AVAglio trial was 39% in the bevacizumab arm and 12.7% in the control arm [15], whereas in the RTOG trial, all-grade HTN incidence was not reported. A recent review [16] examined the toxicities of 210 patients included in three phase II trials conducted by the National Cancer Institute (NCI) on bevacizumab alone and in combination with enzastaurin and tandutinib in the treatment of high-grade gliomas and GBM (NCT00271609, NCT00586508, NCT00667394). In this study, the overall incidence of all-grade HTN was 21.9% and grade ≥3 HTN was 9%.
Table 1.
Incidence of hypertension in clinical trials
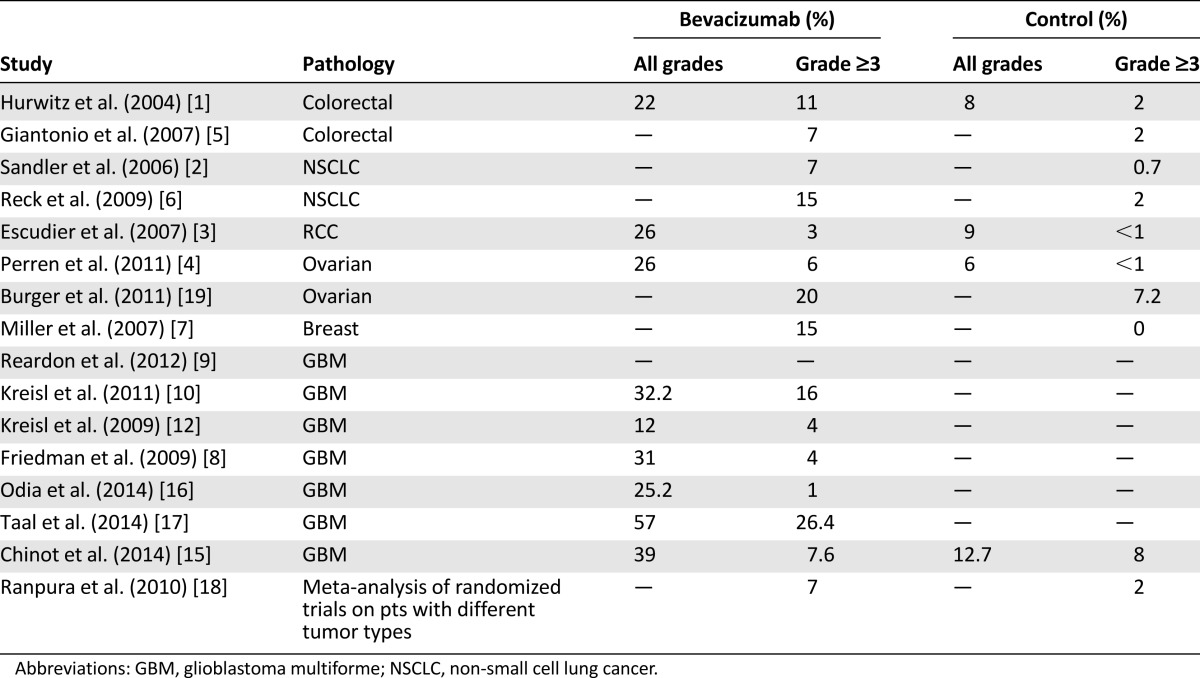
In randomized trials, the incidence of grade 3 and 4 HTN in bevacizumab-treated patients ranged from 3% to 15% compared with 0%–2.0% in controls [1–7].
A meta-analysis of randomized clinical trials of patients with different tumor types showed that low-dose (<10 mg/kg per dose) bevacizumab increased the incidence of any-grade HTN 3-fold and that high-dose (≥10 mg/kg per dose) bevacizumab increased the incidence 7.5-fold [20]. A subsequent meta-analysis [18] found that 24% of patients receiving bevacizumab developed any-grade of HTN and 8% developed grade ≥3 HTN (relative risk: 5.3).
The incidence of HTN in GBM patients treated with bevacizumab is comparable to that reported for other cancer types [14, 15, 18, 20].
Mechanisms
The exact causes of the increase of blood pressure following treatments with bevacizumab have not been adequately clarified. One hypothesis is that VEGF inhibition might reduce the activity of nitric oxide synthase, which could affect vascular smooth muscle cell compliance and renal sodium elimination, resulting in increased arterial blood pressure. Another cause might be decreased microvessel perfusion and microvascular density resulting from VEGF blockade. Because total peripheral resistance is dependent on the arterioles and capillaries, this reduction could lead to increased peripheral resistance [19].
Management
The management of HTN associated with bevacizumab follows the general principles of HTN management. It is important to evaluate the cardiovascular risk of each patient and to maintain blood pressure within the normal range. In low-risk patients, a target pressure lower than 140/90 mmHg can be recommended, whereas in high-risk patients, this should be lowered to 130/80 mmHg [21].
Arterial blood pressure should be measured before each bevacizumab administration, and patients should be instructed to undertake blood pressure monitoring at home. Bevacizumab administration should be delayed and antihypertensive therapy started if blood pressure is >150/100 mmHg. Bevacizumab should be permanently discontinued if HTN is not adequately controlled with appropriate therapy or the patient has a hypertensive crisis or signs of hypertensive encephalopathy.
No controlled studies are available to support the use of a specific antihypertensive agent. Treatment strategies for bevacizumab-related HTN have yet to be defined, but the more traditional antihypertensives, including angiotensin-converting enzyme (ACE) inhibitors, β-blockers, calcium channel blockers, and diuretics, have proven to be effective in the management of bevacizumab-associated HTN in clinical practice. Assessment of the cardiovascular risk of HTN, advisable for all patients, should include evaluation of risk factors such as diabetes mellitus, underlying cardiovascular disease, chronic kidney disease, tobacco use, hyperlipidemia, obesity, family history and advanced age, all of which are warning signs of possible development of treatment-related hypertension or hypertension-related complications.
Proteinuria
Proteinuria is the second most common bevacizumab adverse event. It is dose dependent, and there seems to be a relationship between the duration of treatment and the occurrence of this event [22].
The normal range for urine protein excretion is 40–80 mg daily, with levels >150 mg indicating proteinuria. The Common Terminology Criteria for Adverse Events version 4.0/4 grades proteinuria toxicity from 1 to 3. Grade 1 corresponds to a 1+ urine dipstick with a 24-hour urine protein level of 1 g; grade 2 indicates a 2+ urine dipstick or 24-hour protein level of 1.0–3.4 g, and grade 3 indicates a 24-hour urine protein level of ≥3.5 g.
The incidence of grade 3 proteinuria in bevacizumab arms of randomized trials ranged from 0.8% to 4.0% compared with 0%–1.0% in the control arms [1–7] (Table 2).
Table 2.
Incidence of proteinuria in clinical trials
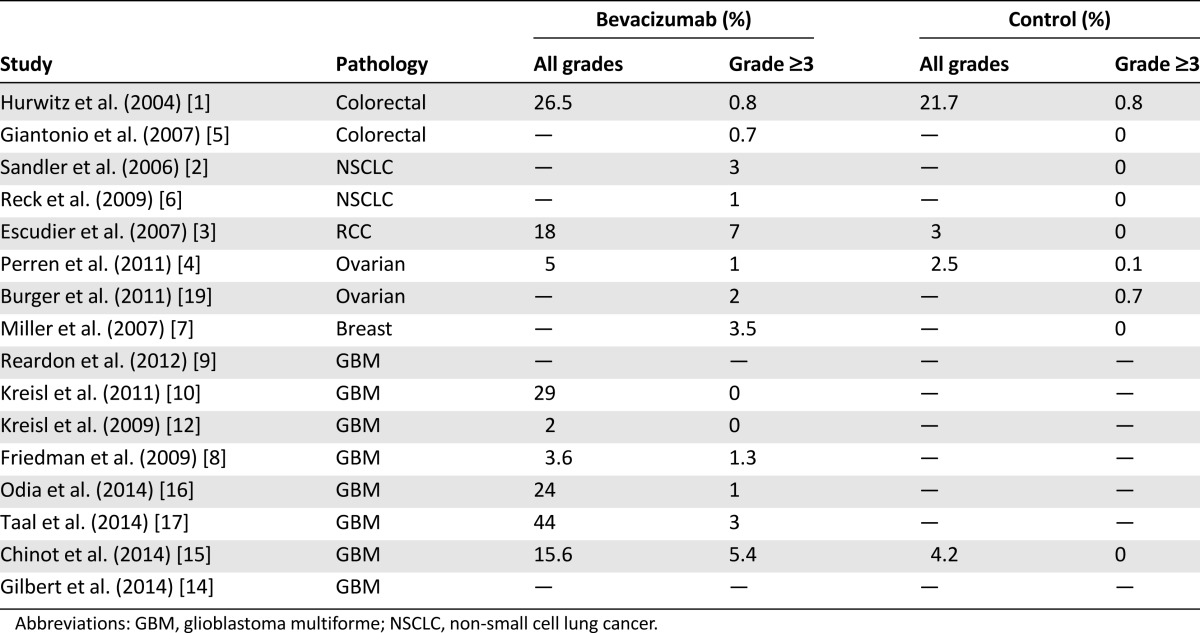
A recent meta-analysis [22] showed that 13% of patients receiving bevacizumab had at least grade 1 proteinuria, and 2.2% had grade ≥3 proteinuria (a 5-fold increase) with respect to patients receiving chemotherapy without bevacizumab. The risk was also found to be dependent on the dose of bevacizumab, with higher doses related to significantly higher incidence of proteinuria [22]. The incidence of bevacizumab-related proteinuria appears to be similar in patients with GBM, with rates at 0%–15% (all grades). In the AVAglio trial [15], all-grade proteinuria was 15.6% in the bevacizumab arm, with 5.4% grade ≥3 events. In phase II trials, incidence ranged from ∼5%–6% [8, 11] to 25%–30% [10, 16].
Mechanisms
The pathophysiology of bevacizumab-related proteinuria is unknown. VEGF plays a role in maintaining endothelial fenestrations, which form part of the glomerular filtration barrier. VEGF inhibition results in loss of fenestrations, endothelial edema, and detachment, leading to barrier integrity impairment [23]. Podocytes, a key constituent of the glomerular filtration mechanism, and glomerular capillary endothelial cells constitutively express VEGF. In mice, the pharmacological inhibition of VEGF in podocytes produces renal injury, including loss of endothelial fenestrations and proteinuria [24].
Renal biopsies from patients with proteinuria showed thrombotic microangiopathy or proliferative membranous glomerulonephritis, probably resulting from glomerular endothelial damage occurring after VEGF inhibition [25]. Most cases of proteinuria resolve once bevacizumab is discontinued, but severe cases can persist.
Management
Given the high frequency of this adverse event, urinary protein excretion assessment should be undertaken before every administration of bevacizumab. The use of a serial urinalysis dipstick is recommended, with a urine dipstick ≥2+ warranting further assessment with 24-hour urine collection for protein. Bevacizumab administration should be temporarily suspended if 24-hour urine protein levels are >2 g and resumed when levels are <2 g. Treatment discontinuation is recommended in cases of nephrotic syndrome (24-hour urine protein >3.5 g).
ACE inhibitors and angiotensin II receptor blockers (ARBs) reduce the severity of proteinuria and the risk of end-stage renal disease. Despite the relative frequency of anti-VEGF agent-induced proteinuria, no studies on these drugs have been conducted to verify their efficacy in the treatment of this adverse event, precluding evidence-based treatment recommendations [26].
Hemorrhage
Bleeding is a frequent complication of bevacizumab (Table 3). Most cases involve low-grade mucocutaneous hemorrhages that can be managed easily and that rarely lead to treatment discontinuation. Epistaxis is the most common of these events. In most of the studies, epistaxis was not distinguished from other types of bleeding; therefore, its exact incidence is not well established. Data from a few studies report rates of grade 1–2 epistaxis ranging from 5% to 26% [10, 16]. Epistaxis is usually of mild to moderate severity and does not require specific treatment. In particularly severe cases, operative hemostatic interventions might be indicated.
Table 3.
Incidence of hemorrhages (all kinds) in clinical trials
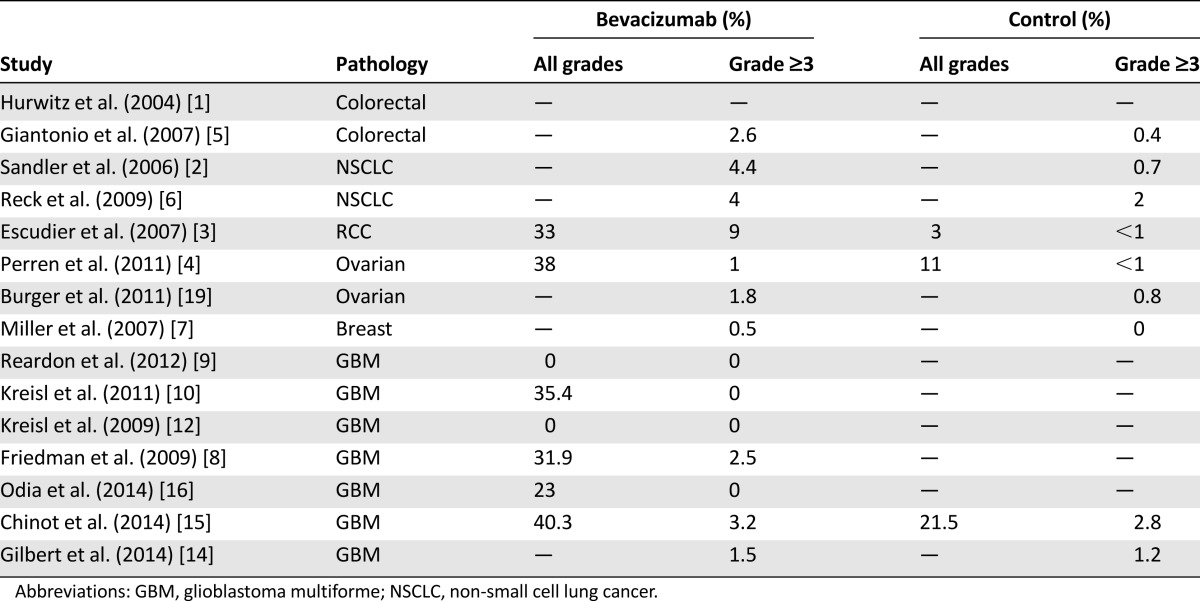
Intracranial hemorrhages (ICHs) are associated with bevacizumab in GBM patients, and systemic bleeding can be a complication of bevacizumab treatment for all types of cancer. Brain tumors, GBM in particular, are associated with spontaneous ICHs, with incidence ranging from 7% to 35% in different series [27]. In patients with GBM treated with bevacizumab, grade ≥3 hemorrhages are uncommon, occurring at a rate of 0%–4% [8, 9, 11, 12, 16]. In the recent RTOG 0825 trial [14], the incidence of grade ≥3 hemorrhages was 1.5% in the bevacizumab arm and 0.3% in the placebo arm. In the AVAglio trial [15], incidence of all-grade cerebral hemorrhages was 3.3% in the bevacizumab arm and 2% in the placebo arm, whereas incidence of grade ≥3 cerebral hemorrhages was 2% in the bevacizumab arm and 0.9% in the placebo arm. In the bevacizumab and placebo arms of the same study, incidence of hemorrhages at other sites was 37% versus 19.6%, respectively, for all grades and 1.3% versus 0.9%, respectively, for grade ≥3. A recent meta-analysis [28] of 12,617 patients from 20 randomized trials with different tumor types suggested that bevacizumab was associated with an increased risk of bleeding (relative risk [RR]: 2.48). Grade ≥3 hemorrhages occurred in 3.5% of patients (RR: 1.91). The risk was greater in patients treated with higher doses of bevacizumab (5 mg/kg per week; RR: 3.02) than in those given lower doses (2.5 mg/kg per week; RR: 2.01). The risk of fatal bleeding was low (0.8%). The highest incidence of high-grade hemorrhages was observed in patients with NSCLC (11.5%), whereas the lowest incidence was found in patients with breast cancer (0.9%).
Data on the occurrence of hemorrhages in GBM, for which incidence is comparable to those reported in the literature for patients with other tumor types, demonstrate that bevacizumab-related hemorrhage risk in patients with brain tumors is no higher than that in patients with other types of cancer.
Mechanisms
The most common risk factor for bleeding is the presence of an adjacent tumor mass or infiltrating vessels. Damage to the vascular wall infiltrated by tumor masses and necrosis of the tumor after treatment can lead to bleeding.
It has been suggested that VEGF might regulate platelet adhesion and activation: therefore, lack of active VEGF could interfere with platelet activation. Another possible mechanism is the deregulation of vascular repair and the growth process. A VEGF block could impair endothelial survival and proliferation, leading to damaged vascular integrity, particularly in tissues with high VEGF dependence such as injured mucosal membrane of the airways. Another theory is that deregulation of nitric oxide, an important molecule for platelet-endothelium interaction, could impair the activation of platelets.
Decreased matrix deposition can determine weakness in the supporting layers of vessels, making them fragile and prone to bleeding. Bevacizumab can also induce thrombocytopenia, contributing to the risk of hemorrhage [29].
Management
In order to minimize the risk of severe hemorrhage, it is of utmost importance to accurately select patients and to evaluate potential risk factors before starting bevacizumab treatment. Particular attention should be paid to the disease site and any signs of initial bleeding. In addition, the presence of active gastric ulcers increases the risk of gastrointestinal hemorrhage. In patients with brain tumors, the major concern is intracranial bleeding. In the presence of signs of recent hemorrhage within the tumor mass, the implications for treatment with bevacizumab should be evaluated carefully. In this context, at magnetic resonance imaging (MRI) of the brain, it is important to distinguish between active bleeding and the presence of hemoglobin-degradation products that indicate previous nonactive hemorrhages.
Thromboembolism
Venous thromboembolism (VTE) is relatively common in cancer patients, especially those with brain tumors (Table 4). A retrospective review [30] on VTE in patients with malignant glioma (9,489 patients) revealed overall incidence of 7.5%. More than 50% of the events occurred within 2 months after neurosurgical procedures. Risk factors for VTE that were identified in this study include age >65 years, diagnosis of GBM, and recent neurosurgical procedures. In a prospective study evaluating the risk of deep vein thrombosis in 77 patients treated with adjuvant radiochemotherapy following surgery for high-grade gliomas, the risk of thrombotic events at 12 and 24 months was 20.8% and 31.7%, respectively, with incidence at its highest within the first 7 months [31].
Table 4.
Incidence of venous thrombo-embolism in clinical trials
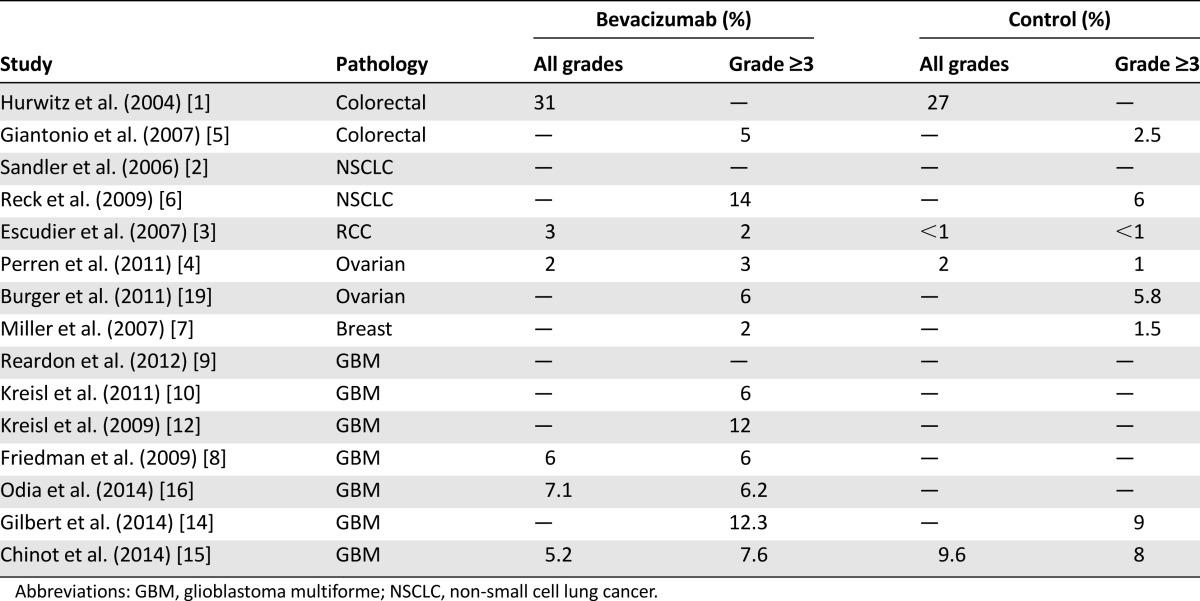
Relatively high rates of VTE have been reported in studies evaluating bevacizumab in recurrent GBM (5% –19%) [8, 11–13, 16]. However, the results reported must be considered in the context of the significant risk of thromboembolism incurred by all GBM patients.
In the recently published RTOG 0825 trial [14], grade ≥3 VTE rates were 12% in the bevacizumab arm and 9% in the placebo arm. In the AVAglio trial, all grades of VTE occurred in 8.2% of patients treated with bevacizumab and 9.6% of those on a placebo. Rates for grade ≥3 VTE were 7.6% and 8%, respectively.
The reported incidence of VTE in bevacizumab-treated patients with different types of cancer ranges from 3% to 23% [32]. A recent meta-analysis demonstrated an increased risk of VTE associated with bevacizumab therapy among cancer patients (RR: 1.33; p < .001) [33].
GBM patients treated with bevacizumab seem to be at slightly higher risk of developing VTE than patients with other cancers treated with bevacizumab, probably because of the intrinsic predisposition of GBM patients to this complication.
GBM patients treated with bevacizumab seem to be at slightly higher risk of developing VTE than patients with other cancers treated with bevacizumab, probably because of the intrinsic predisposition of GBM patients to this complication.
Mechanisms
Under normal conditions, VEGF mediates the repair of damaged endothelial surfaces, and its blockade might result in the disruption of this process. The consequent exposure of subendothelial tissues and apoptosis of endothelial cells trigger the clotting cascade, with subsequent clot formation. Cellular apoptosis could increase procoagulant activity by redistribution of phosphatidylserine, which enhances the activation of factor X. Furthermore, in apoptotic cells, downregulation of anticoagulant factors thrombomodulin and heparan sulfate takes place [29, 34]. The underlying prothrombotic state, characteristic of cancer patients, might exacerbate this process. Anti-VEGF therapy could also induce tissue factor (factor III), which triggers the coagulation process. Pathologic conditions involving the coagulation factors (e.g., factor V Leiden mutation) could increase the predisposition to VTE.
Management
Anticoagulant therapy with low-molecular-weight heparin (LMWH) is the preferred therapeutic option for patients treated with bevacizumab. The main concern expressed about anticoagulant treatments associated with bevacizumab is that they enhance the risk of bleeding. The estimated risk of ICH in brain tumor patients on therapeutic anticoagulation is 2% [35]. In one small retrospective study on 21 glioma patients receiving bevacizumab, it was found that anticoagulation (warfarin or LMWH) produced 5% of symptomatic and 10% of asymptomatic ICHs [36].
In a retrospective study by Norden et al. [37] of 64 patients receiving bevacizumab and anticoagulant therapy (either warfarin or LMWH), the rate of grade ≥3 hemorrhage was 6%. Among 218 bevacizumab-treated patients who did not receive concurrent anticoagulants, the rate of any-grade ICH was 3%, whereas grade ≥3 ICHs occurred in 1% of patients. Because the difference between the incidence of serious ICH in patients on concomitant anticoagulation and bevacizumab and in patients without bevacizumab treatment was negligible, the authors concluded that it was acceptable.
At present, in the vast majority of clinical trials with bevacizumab, therapeutic anticoagulation is not considered a contraindication, and thus it is not an exclusion criterion, as long as the international normalized ratio or partial thromboplastin time is within therapeutic limits and the patient has been on a stable dose of anticoagulants for at least 2 weeks.
If VTE occurs during treatment, bevacizumab should be stopped and an appropriate therapy with LMWH prescribed. Bevacizumab can be resumed after the start of anticoagulant therapy but should be discontinued in cases of grade 4 VTE or recurrent VTEs refractory to anticoagulant treatment.
Arterial Thromboembolic Events
The incidence of arterial thromboembolic events (ATEs), including transient ischemic attacks, cerebral infarction, unstable angina, troponin elevation, and acute myocardial infarction, is reportedly low but statistically significant and higher in bevacizumab-treated patients than in controls; these events are rarely fatal. Randomized studies have reported incidence of 4.4% in bevacizumab-treated patients and 1.9% in non-bevacizumab-treated patients [38]. In a meta-analysis of 1,745 patients [39], the reported incidence of ATEs was 3.8% in patients treated with bevacizumab and 1.7% in patients treated without bevacizumab, with an absolute number of 5.5 ATEs per 100 person-years and a relative risk of ATE with bevacizumab of 2 (p = .03). In the AVAglio trial [15], a significant difference in the incidence of ATEs was observed between the bevacizumab and control arms. All-grade ATEs accounted for 5.9% of events in the patients treated with bevacizumab and 1.6% of those in the placebo arm (p = .001); grade ≥3 events occurred in 3.3% of the patients in the bevacizumab arm and 1.3% of those receiving placebo (p = .0003). In a phase II trial by Friedman et al. [8], all-grade ATEs were reported in 4.8% and 6.3% of the patients treated with bevacizumab and bevacizumab plus irinotecan, respectively, whereas the incidence of grade ≥3 ATEs was 2.4% and 2.5%, respectively. These data also confirm that in glioblastoma patients there is an increased risk of arterial events with bevacizumab treatment. In the recent paper from the NCI group [16], although the data on the incidence of ATEs occurred in the study were not specified, one case of deadly cerebrovascular ischemic stroke was reported (0.5% of all the patients included in the study).
Mechanisms
The mechanisms underlying ATEs are similar to those described for VTEs (the effects of bevacizumab on vessels and coagulation system). Increased risk of ATEs in GBM patients treated with bevacizumab also could be represented by radiation-induced vessels damage. It is known that radiotherapy can induce dilation of the blood vessel lumen, thickening of the blood vessel wall, enlargement of endothelial cell nuclei, and arterial sclerosis [40] that might lead to a predisposition for developing thrombotic events, especially during treatment with bevacizumab.
Management
All patients on bevacizumab should be considered at risk of ATEs, and extra caution is recommended when prescribing the agent to patients aged >65 years with a history of ATE or conditions predisposing the patient to vascular diseases.
Bevacizumab treatment, which should be considered partially responsible for any ATE that occurs during treatment, should be discontinued permanently if these adverse events occur. Patients should be informed about warning signs and encouraged to seek prompt emergency care should they appear. Patients should have consultations with appropriate specialists (e.g., cardiology, neurology) for guidance on the evaluation and management of ATE [41].
Patients with a recent ATE should not be treated with bevacizumab for at least 6 months, and bevacizumab should be started only if the patient is stable and asymptomatic.
Gastrointestinal Perforation
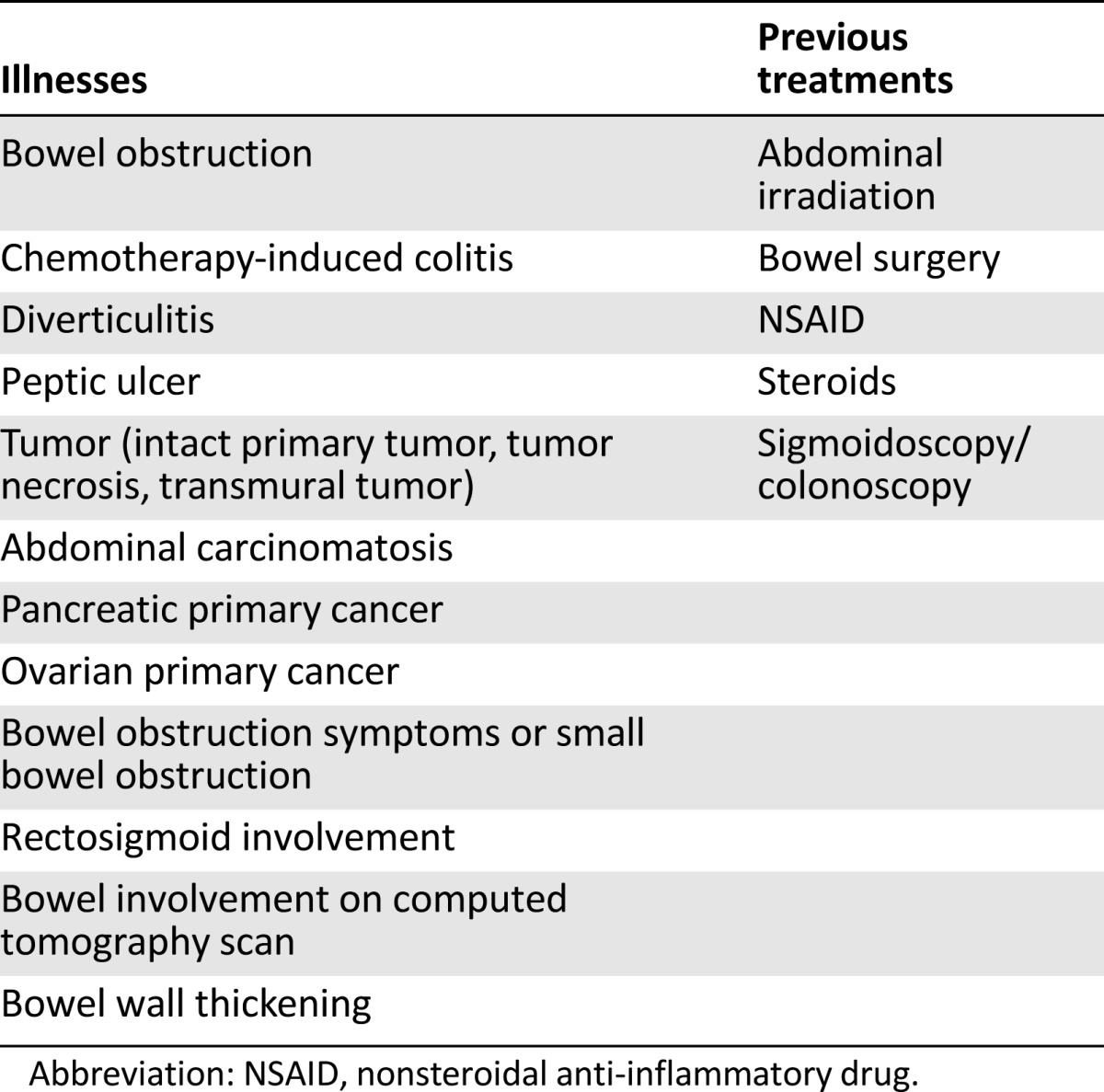
Spontaneous perforation of the gastrointestinal tract is a potential risk in patients receiving bevacizumab. Although risk factors for bevacizumab-associated bowel perforation have been identified, (Table 5) this complication can also occur in patients without clear risk factors.
Table 5.
Risk factors related to gastrointestinal perforation with bevacizumab
Few studies address the risk of bowel perforation in patients with GBM (Table 6). In a single-arm phase II trial [9] testing bevacizumab combined with irinotecan and carboplatin in 40 recurrent GBM patients, the bowel perforation rate was 7.5%, with 1 fatal case (2.5%). In a phase II trial of bevacizumab alone or concomitant with irinotecan, bowel perforation occurred in 3% of the patients in the combined arm but did not occur in the patients given single-agent bevacizumab [8].
Table 6.
Incidence of bowel perforation in clinical trials
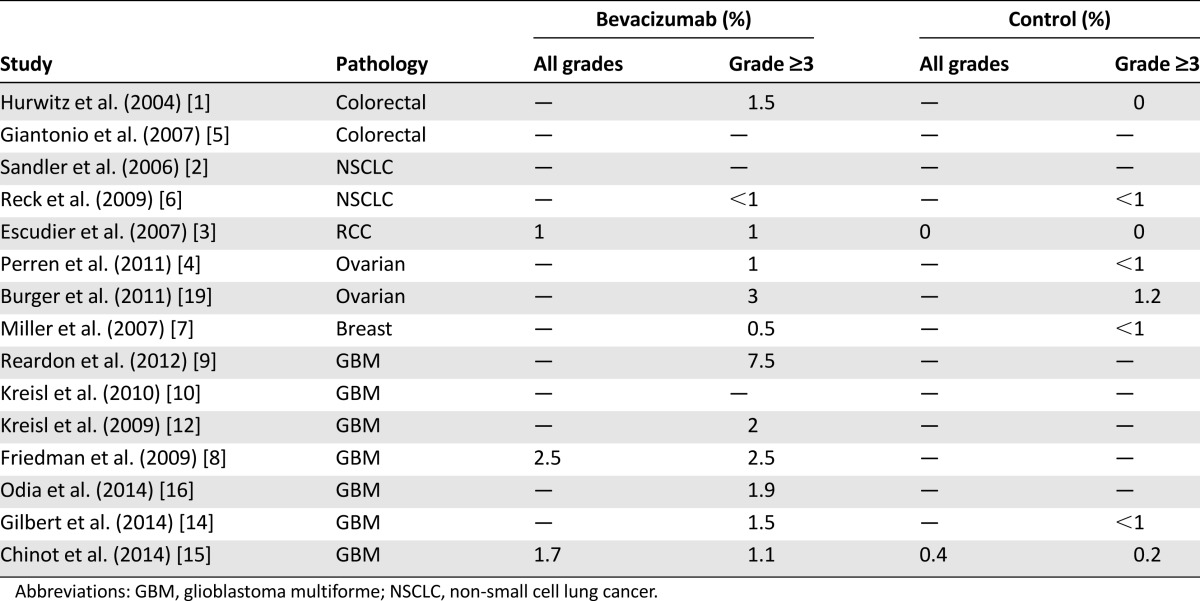
In other trials, the incidence of this adverse event ranged from 0% to 3% [10–13], with the majority of cases occurring when bevacizumab was given in combination with chemotherapy [8, 9]. In the RTOG 0825 trial [14], grade ≥3 bowel perforation rates were 1.5% in the bevacizumab arm and <1% in the placebo arm. In the AVAglio trial [15], bowel perforation occurred in 1.7% of the patients in the bevacizumab arm and in 0.4% of those in the placebo arm. In the recent NCI study [16], the incidence of gastrointestinal perforation was 1.9%, with no fatal event.
Among other cancer types, the highest incidence of gastrointestinal perforation was found in colorectal and ovarian cancers: incidence was 0.9% in patients with colorectal cancer [42] and up to 11% in a phase II study with ovarian cancer patients [43]; however, in two phase III trials, the incidence rates were 1% and 3%, respectively [4, 19].
Available data suggest that the incidence of gastrointestinal perforation is slightly higher in GBM than in other cancer types. In particular, of tumors without abdominal involvement, GBM incurs the highest rate of gastrointestinal perforation. It is likely that the concomitant use of corticosteroids might contribute to this increased incidence.
Of tumors without abdominal involvement, GBM incurs the highest rate of gastrointestinal perforation. It is likely that the concomitant use of corticosteroids might contribute to this increased incidence.
Mechanisms
Various mechanisms have been proposed in the attempt to explain the relationship between bevacizumab and bowel perforation. VEGF inhibition might cause thrombosis of smaller splanchnic and mesenteric vessels, leading to bowel ischemia and perforation [44]. Another theory is that alterations occur in the mechanisms governing intestinal wall maintenance and healing, bowel wall health being dependent on microcirculation, protection with nitrous oxide, prostacyclins, and normal platelet function, all of which are regulated by VEGF. The intestinal mucosa could be susceptible to ulcers and perforation as a result of VEGF inhibition by bevacizumab, especially when the agent is used in association with nonsteroidal anti-inflammatory drugs [45]. Another possible causative mechanism is the regression of normal blood vessels as a result of VEGF inhibition; this could lead to bowel wall hypoperfusion, leading to cell damage and necrosis [46]. Last, the presence of a tumor invading the intestinal wall could predispose the organ to perforation.
Management
In order to reduce the incidence of bowel perforation, complete assessment of risk factors should be performed before starting treatment with bevacizumab (Table 5). The presence of active colitis, diverticulitis, peptic ulcers, extensive bowel involvement, or obstruction contraindicates bevacizumab use. Nevertheless, given the limited therapeutic options for GBM, comorbidities such as diverticulosis could be relative contraindications and bevacizumab treatment could be considered as long as appropriate preventive measures are taken. In patients with diverticulosis, medical therapy could reduce the risk of diverticulitis, which can lead to bowel perforation during treatment with bevacizumab. Given the high risk of recurrence after an episode of acute diverticulitis, maintenance pharmacological therapy is often prescribed to reduce that risk.
A widely used approach is the combined administration of antibiotics (e.g., rifaximin), mesalamine, and probiotics [47, 48]. Monthly courses of antibiotics (rifaximin) may be prescribed, but their beneficial effects appear to be short term. Mesalamine, a bowel-specific aminosalicylate drug with local action, appears promising for the prevention of recurrence, whereas the role of probiotics is still unclear. For the prevention of diverticulitis, we suggest courses of 200 mg rifaximin for 4 days every 2 weeks throughout bevacizumab treatment.
Corticosteroid use is associated with increased risk of gastrointestinal perforation and bleeding because it alters the homeostasis of the gastroduodenal mucosa, favoring the onset of gastritis and ulcers. In a recent systematic review [49], it was estimated that the incidence of steroid-related perforation was 2.9% and that steroids increased the risk of perforation by 40%. The increase in risk was considered significant only for hospitalized patients and not for ambulatory patients. It is common practice to prescribe antiacid agents, mostly proton pump inhibitors, along with steroids as prophylaxis for gastritis, although no controlled trial demonstrates their efficacy in terms of prophylaxis for this indication [49].
Wound Healing
Angiogenesis is a fundamental step in cicatrization, and by interfering with angiogenesis, bevacizumab also interferes with wound healing. The frequency of wound breakdown of all grades in patients with GBM treated with bevacizumab ranges from 0% to 6% [26].
Mechanism
The inhibition of angiogenesis prevents new vessels forming in the wound site and thus interferes with the physiological process of cicatrization. Furthermore, because VEGF inhibition can impair the activation of platelets, which play a crucial role in promoting wound healing, any such inactivation might be responsible for bevacizumab-related wound dehiscence.
Management
Though there are no clearly established data, current consensus from physicians suggests that bevacizumab should be stopped for 6 to 8 weeks before major surgery and for at least 4 weeks after surgery to reduce the risk of wound-healing complications.
In the case of wound breakdown, treatment with bevacizumab should be stopped. Rechallenge could be considered and evaluated for each case, depending on severity of the complication.
Reversible Posterior Leukoencephalopathy Syndrome
Reversible posterior leukoencephalopathy syndrome (RPLS) is a rare neurological condition associated with anti-VEGF/VEGFR agents that has been reported in <0.1% of patients [29]. No cases of RPLS were reported in the RTOG 0825 and AVAglio trials [14, 15]. The clinical syndrome typically consists of a relatively acute onset of headache, seizures, confusion, and cortical blindness. Most patients have associated HTN. MRI typically reveals T2/FLAIR hyperintensive alterations without contrast enhancement in the white matter. Typically, these lesions are localized in the posterior cerebral hemispheres but also may involve the anterior regions and the posterior fossa structures. The risk of permanent brain injuries arises if the diagnosis is delayed and bevacizumab administration is not interrupted.
Mechanisms
The two main theories on the pathogenesis implicate the failure of cerebral vasomotor autoregulation because of HTN and primary endothelial damage that may alter the integrity of the blood-brain barrier.
Management
RPLS is a rare but serious condition that should be recognized promptly and suspected in cases of sudden and unexplained neurological deterioration in patients receiving bevacizumab who have no brain metastases and whose primary brain tumor shows signs of progression. Suspension of bevacizumab and pressure control are the only therapeutic measures to take: if prompt, they can lead to complete recovery from the disease. After neurological recovery, bevacizumab resumption should be discouraged [50].
Conclusion
Bevacizumab is part of standard treatment for many cancer types, including GBM. Anti-VEGF agents are characterized by peculiar toxicities, some of which might represent major causes of morbidity and mortality.
The toxicity profile of bevacizumab in GBM is similar to that reported in clinical trials on other types of cancer. Cases of life-threatening adverse events have been reported in phase II and III studies on GBMs with bevacizumab treatment, and the incidence of some fatal events was no higher than incidence reported in studies on other tumors, whereas other fatal events occurred at slightly increased frequency in GBM patients.
Bowel perforation in bevacizumab-treated patients with GBM was reported in various studies with an incidence of up to 8% and is frequently related to concomitant steroid treatment. Despite the potent antiedema and corticosteroid-sparing effect of bevacizumab [51], a high number of patients with GBM need steroid therapy; therefore, it is important to identify risk factors for gastrointestinal perforation, such as diverticulosis, to take appropriate preventive measures.
GBM is one of the tumors with the highest incidence of thromboembolic events (independent of the type of treatment), and it has been demonstrated that bevacizumab increases the risk of VTE in patients with this tumor type. In most of the studies on bevacizumab in GBM, the incidence rate of VTE was within acceptable ranges (5%–12%) and was comparable to the rates observed in control arms. Anticoagulant treatments do not seem to increase the risk of severe bleeding in association with bevacizumab; therefore, concomitant treatment with LMWH and bevacizumab could be considered in cases of VTE.
A slight increase in the incidence of ATEs has been associated with bevacizumab; however, in the case of ischemic strokes, no correlation with bevacizumab could be clearly demonstrated because other predisposing factors can be related to the adverse event, such as postradiation vasculitis and vessel damage from surgery.
A major concern regarding the use of bevacizumab in patients with brain tumors is the risk of ICHs. In clinical trials, it has been demonstrated that the rates of ICH are relatively low in GBM patients treated with bevacizumab.
Safety data from various studies showed that hypertension and proteinuria could be dose dependent; therefore, their incidence could be increased by longer exposure to bevacizumab. In glioblastoma studies, data regarding this issue are contradictory because high incidence of proteinuria and hypertension has been recorded in phase III trials [14, 15] (in which exposure to bevacizumab was longer) and phase II trials [17] (in which exposure was shorter). Further studies should address this issue to determine the risks of prolonged treatment with bevacizumab. In particular, the ongoing phase III clinical trial TAMIGA (NCT01860638) could provide interesting data about this issue [52].
Some bevacizumab-related adverse events are potentially life threatening, although fatalities are rare. It is well known that some of the toxicities are related to the presence of risk factors. In such cases, preventive measures should be taken to minimize complications related to the use of bevacizumab. Meticulous screening for risk factors and accurate selection of candidates for bevacizumab treatment, with thorough evaluation of the risk-benefit ratio, is crucial for reducing the incidence of serious complications [53].
Further research on predictive factors is warranted to select the patients most likely to benefit from antiangiogenic treatments and to spare unsuitable candidates from exposure to potentially life-threatening risks.
The side effects of bevacizumab are well known, as are the risk factors and predisposing conditions. It is hoped that internal guidelines will be drawn up for the prevention and treatment of these complications, in the interest of patient safety.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Author Contributions
Conception/Design: Alba A. Brandes
Data analysis and interpretation: Alba A. Brandes, Marco Bartolotti, Enrico Franceschi
Manuscript writing: Alba A. Brandes, Marco Bartolotti, Alicia Tosoni, Rosalba Poggi, Enrico Franceschi
Final approval of manuscript: Alba A. Brandes, Marco Bartolotti, Alicia Tosoni, Rosalba Poggi, Enrico Franceschi
Disclosures
The authors indicated no financial relationships.
References
- 1.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 2.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 4.Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 5.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 6.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 7.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 8.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 9.Reardon DA, Desjardins A, Peters KB, et al. Phase II study of carboplatin, irinotecan, and bevacizumab for bevacizumab naïve, recurrent glioblastoma. J Neurooncol. 2012;107:155–164. doi: 10.1007/s11060-011-0722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreisl TN, Zhang W, Odia Y, et al. A phase II trial of single-agent bevacizumab in patients with recurrent anaplastic glioma. Neuro Oncol. 2011;13:1143–1150. doi: 10.1093/neuonc/nor091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vredenburgh JJ, Desjardins A, Herndon JE, II, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 12.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai A, Filka E, McGibbon B, et al. Phase II pilot study of bevacizumab in combination with temozolomide and regional radiation therapy for up-front treatment of patients with newly diagnosed glioblastoma multiforme: Interim analysis of safety and tolerability. Int J Radiat Oncol Biol Phys. 2008;71:1372–1380. doi: 10.1016/j.ijrobp.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert MR, Sulman EP, Mehta MP. Bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:2048–2049. doi: 10.1056/NEJMc1403303. [DOI] [PubMed] [Google Scholar]
- 15.Chinot OL, Wick W, Cloughesy T. Bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:2049. doi: 10.1056/NEJMc1403303. [DOI] [PubMed] [Google Scholar]
- 16.Odia Y, Shih JH, Kreisl TN, et al. Bevacizumab-related toxicities in the National Cancer Institute malignant glioma trial cohort. J Neurooncol. 2014;120:431–440. doi: 10.1007/s11060-014-1571-6. [DOI] [PubMed] [Google Scholar]
- 17.Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): A randomised controlled phase 2 trial. Lancet Oncol. 2014;15:943–953. doi: 10.1016/S1470-2045(14)70314-6. [DOI] [PubMed] [Google Scholar]
- 18.Ranpura V, Pulipati B, Chu D, et al. Increased risk of high-grade hypertension with bevacizumab in cancer patients: A meta-analysis. Am J Hypertens. 2010;23:460–468. doi: 10.1038/ajh.2010.25. [DOI] [PubMed] [Google Scholar]
- 19.Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 20.Zhu X, Wu S, Dahut WL, et al. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: Systematic review and meta-analysis. Am J Kidney Dis. 2007;49:186–193. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 21.Maitland ML, Bakris GL, Black HR, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. 2010;102:596–604. doi: 10.1093/jnci/djq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu S, Kim C, Baer L, et al. Bevacizumab increases risk for severe proteinuria in cancer patients. J Am Soc Nephrol. 2010;21:1381–1389. doi: 10.1681/ASN.2010020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gurevich F, Perazella MA. Renal effects of anti-angiogenesis therapy: Update for the internist. Am J Med. 2009;122:322–328. doi: 10.1016/j.amjmed.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 24.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugimoto H, Hamano Y, Charytan D, et al. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem. 2003;278:12605–12608. doi: 10.1074/jbc.C300012200. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong TS, Wen PY, Gilbert MR, et al. Management of treatment-associated toxicites of anti-angiogenic therapy in patients with brain tumors. Neuro Oncol. 2012;14:1203–1214. doi: 10.1093/neuonc/nor223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velander AJ, DeAngelis LM, Navi BB. Intracranial hemorrhage in patients with cancer. Curr Atheroscler Rep. 2012;14:373–381. doi: 10.1007/s11883-012-0250-3. [DOI] [PubMed] [Google Scholar]
- 28.Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: A meta-analysis. Lancet Oncol. 2009;10:559–568. doi: 10.1016/S1470-2045(09)70112-3. [DOI] [PubMed] [Google Scholar]
- 29.Higa GM, Abraham J. Biological mechanisms of bevacizumab-associated adverse events. Expert Rev Anticancer Ther. 2009;9:999–1007. doi: 10.1586/era.09.68. [DOI] [PubMed] [Google Scholar]
- 30.Semrad TJ, O’Donnell R, Wun T, et al. Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg. 2007;106:601–608. doi: 10.3171/jns.2007.106.4.601. [DOI] [PubMed] [Google Scholar]
- 31.Brandes AA, Scelzi E, Salmistraro G, et al. Incidence of risk of thromboembolism during treatment high-grade gliomas: A prospective study. Eur J Cancer. 1997;33:1592–1596. doi: 10.1016/s0959-8049(97)00167-6. [DOI] [PubMed] [Google Scholar]
- 32.Zangari M, Fink LM, Elice F, et al. Thrombotic events in patients with cancer receiving antiangiogenesis agents. J Clin Oncol. 2009;27:4865–4873. doi: 10.1200/JCO.2009.22.3875. [DOI] [PubMed] [Google Scholar]
- 33.Nalluri SR, Chu D, Keresztes R, et al. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: A meta-analysis. JAMA. 2008;300:2277–2285. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 34.Bombeli T, Karsan A, Tait JF, et al. Apoptotic vascular endothelial cells become procoagulant. Blood. 1997;89:2429–2442. [PubMed] [Google Scholar]
- 35.Wen PY, Schiff D, Kesari S, et al. Medical management of patients with brain tumors. J Neurooncol. 2006;80:313–332. doi: 10.1007/s11060-006-9193-2. [DOI] [PubMed] [Google Scholar]
- 36.Nghiemphu PL, Green RM, Pope WB, et al. Safety of anticoagulation use and bevacizumab in patients with glioma. Neuro Oncol. 2008;10:355–360. doi: 10.1215/15228517-2008-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norden AD, Bartolomeo J, Tanaka S, et al. Safety of concurrent bevacizumab therapy and anticoagulation in glioma patients. J Neurooncol. 2012;106:121–125. doi: 10.1007/s11060-011-0642-1. [DOI] [PubMed] [Google Scholar]
- 38.Randall LM, Monk BJ. Bevacizumab toxicities and their management in ovarian cancer. Gynecol Oncol. 2010;117:497–504. doi: 10.1016/j.ygyno.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–1239. doi: 10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- 40.Reinhold HS, Calvo W, Hopewell JW, et al. Development of blood vessel-related radiation damage in the fimbria of the central nervous system. Int J Radiat Oncol Biol Phys. 1990;18:37–42. doi: 10.1016/0360-3016(90)90264-k. [DOI] [PubMed] [Google Scholar]
- 41.Girardi F, Franceschi E, Brandes AA. Cardiovascular safety of VEGF-targeting therapies: Current evidence and handling strategies. The Oncologist. 2010;15:683–694. doi: 10.1634/theoncologist.2009-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hapani S, Sher A, Chu D, et al. Increased risk of serious hemorrhage with bevacizumab in cancer patients: A meta-analysis. Oncology. 2010;79:27–38. doi: 10.1159/000314980. [DOI] [PubMed] [Google Scholar]
- 43.Cannistra SA, Matulonis UA, Penson RT, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25:5180–5186. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 44.Sliesoraitis S, Tawfik B. Bevacizumab-induced bowel perforation. J Am Osteopath Assoc. 2011;111:437–441. [PubMed] [Google Scholar]
- 45.Roodhart JM, Langenberg MH, Witteveen E, et al. The molecular basis of class side effects due to treatment with inhibitors of the VEGF/VEGFR pathway. Curr Clin Pharmacol. 2008;3:132–143. doi: 10.2174/157488408784293705. [DOI] [PubMed] [Google Scholar]
- 46.Kamba T, Tam BYY, Hashizume H, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 47.Tursi A. Advances in the management of colonic diverticulitis. CMAJ. 2012;184:1470–1476. doi: 10.1503/cmaj.120580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin ST, Stocchi L. New and emerging treatments for the prevention of recurrent diverticulitis. Clin Exp Gastroenterol. 2011;4:203–212. doi: 10.2147/CEG.S15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Narum S, Westergren T, Klemp M. Corticosteroids and risk of gastrointestinal bleeding: A systematic review and meta-analysis. BMJ Open. 2014;4:e004587. doi: 10.1136/bmjopen-2013-004587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seet RC, Rabinstein AA. Clinical features and outcomes of posterior reversible encephalopathy syndrome following bevacizumab treatment. QJM. 2012;105:69–75. doi: 10.1093/qjmed/hcr139. [DOI] [PubMed] [Google Scholar]
- 51.Vredenburgh JJ, Cloughesy T, Samant M, et al. Corticosteroid use in patients with glioblastoma at first or second relapse treated with bevacizumab in the BRAIN study. The Oncologist. 2010;15:1329–1334. doi: 10.1634/theoncologist.2010-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brandes AA, Mason W, Pichler J, et al. Can bevacizumab prolong survival for glioblastoma patients through multiple lines of therapy? Future Oncol. 2014;10:1137–1145. doi: 10.2217/fon.14.75. [DOI] [PubMed] [Google Scholar]
- 53.Chi AS, Chamberlain MC. Is there a role for bevacizumab in the treatment of glioblastoma? The Oncologist. 2013;18:1080–1082. doi: 10.1634/theoncologist.2013-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]


