Although complete surgical resection of adenocarcinoma of the gastroesophageal junction (GEJ) remains the cornerstone of treatment for resectable disease, long-term outcomes are poor and recurrence rates are high with surgery alone in patients presenting with locally advanced disease. This review discusses why the treatment of locally advanced GEJ tumors remains controversial, discusses various multimodal approaches, the respective pros and cons, evaluates the role of radiation therapy, highlights some ongoing and planned clinical trials, and suggests areas that need further research.
Keywords: Gastroesophageal cancer, Chemotherapy, Radiation, Adenocarcinoma
Abstract
Over the last several decades, the incidence of adenocarcinoma of the gastroesophageal junction (GEJ) has been increasing in developed countries. Although complete surgical resection remains the cornerstone of treatment for resectable disease, long-term outcomes are poor and recurrence rates are high with surgery alone in patients presenting with locally advanced disease. Multimodal therapy has been shown to improve survival; however, the optimal therapeutic approach remains controversial, and practices vary across the world. Preoperative chemoradiotherapy is generally used in the U.S., whereas perioperative chemotherapy without radiation is favored in most European countries. In this review, we discuss why the treatment of locally advanced GEJ tumors remains controversial, examine the evidence for various multimodal approaches, discuss their respective pros and cons, evaluate the role of radiation therapy, highlight some ongoing and planned clinical trials, and suggest areas that need further research.
Abstract
摘要
在过去的几十年中,发达国家的胃食管交界处(GEJ)腺癌发病率不断升高。虽然完全切除手术仍是可切除性疾病的治疗基础,但长期预后不佳,且局部晚期疾病患者接受单纯手术治疗后的复发率较高。研究显示,多元模式治疗可以改善患者的生存情况;但最佳治疗方法仍存在争议,在全世界各地的临床实践中采用的方法各不相同。在美国普遍使用术前同步放化疗,而在大多数欧洲国家更倾向于使用围手术期化疗而不使用放疗。本综述将讨论局部晚期GEJ肿瘤治疗仍存在争议的原因,审阅各种多元模式治疗的证据,讨论各种方法的优点和缺点,评估放疗的作用,重点介绍部分正在进行和计划进行的临床试验,并提出需要进一步研究的领域。The Oncologist 2015;20:134–142
Implications for Practice:
Despite increasing incidence of adenocarcinoma of the gastroesophageal junction in developed countries, the optimal therapeutic approach for locally advanced disease remains controversial. Outcomes are dismal with surgery alone. Multimodal therapy improves survival, but practices vary worldwide. Perioperative chemotherapy is the standard in most European countries, whereas preoperative chemoradiotherapy is favored in the United States. In this review, we discuss reasons for the controversy, examine the data for various multimodal approaches, summarize key ongoing and future trials, and provide evidence-based treatment recommendations.
Introduction
Cancers of the esophagus, gastroesophageal junction (GEJ), and stomach are among the leading causes of cancer-related deaths worldwide [1]. Over the last several decades, there has been a shift in the epidemiology of upper gastrointestinal tract tumors in developed countries. Rates of proximal esophageal and distal gastric cancers have declined, but there has been an alarming increase in the number of distal esophageal, GEJ, and gastric cardia adenocarcinomas [2–4].
Surgery remains the mainstay of treatment for resectable disease, but most patients in Western countries present with locally advanced disease, and 5-year overall survival (OS) rates with surgery alone are dismally at approximately 25% [5]. In an attempt to improve outcomes, multimodal strategies such as neoadjuvant chemotherapy with or without concurrent radiotherapy, perioperative chemotherapy, postoperative chemoradiotherapy, and adjuvant chemotherapy have been investigated with variable results. The optimal management of locally advanced GEJ adenocarcinoma remains controversial, and practices vary across the world. The only consensus is that surgical resection alone is insufficient, and additional therapy must be considered. In this review, we discuss why the management of locally advanced GEJ adenocarcinoma remains a challenge, analyze the evidence for multimodal therapy, summarize recent advances in treatment, and highlight some ongoing and future trials.
The Challenge
One of the main obstacles in determining an optimal therapeutic approach has been the lack of a standardized definition or classification of GEJ adenocarcinomas [6]. Because of their borderline location, GEJ cancers have variably been included in predominantly esophageal or gastric cancer studies. Very few clinical trials have focused on GEJ tumors as a separate entity. Results are difficult to interpret because GEJ tumors comprise only a minority in most studies. Moreover, one has to be mindful of the heterogeneity of patients enrolled. Most esophageal studies included patients with proximal squamous cell carcinomas (SCCs), as well as distal adenocarcinomas; tumors that behave quite differently and are even staged separately in the most recent 2010 seventh edition of the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) staging system [7–9]. Similarly, most gastric cancer studies have not differentiated between adenocarcinomas of the distal stomach or the cardia, two dissimilar diseases with unique risk factors and prognoses [10].
There are also key geographical differences in the etiology, stage at presentation, treatment tolerability, patient comorbidities, and outcomes of these malignancies [11, 12]. Results from one region cannot necessarily be extrapolated to another. Significantly better outcomes have consistently been reported from Eastern countries compared with the West [13–15].
In an attempt to harmonize staging, the latest AJCC/UICC seventh edition included GEJ cancers with esophageal adenocarcinomas [8]. This includes tumors arising from the distal 5 cm of the esophagus (Siewert type I), the GEJ (Siewert type II), or the cardia of the stomach (within 5 cm of the GEJ) with extension into the GEJ or esophagus (Siewert type III) [7]. Tumors arising from the stomach greater than 5 cm distal to the GEJ or those within 5 cm but without extension into the GEJ or esophagus are staged as gastric cancers. This revision was based on worse survival seen among GEJ cancer patients compared with other gastric cancers [8]. However, several investigators disagree with this oversimplified classification of GEJ cancers, and staging remains a subject of debate. In a large series of patients, Siewert et al. [16] reported that GEJ adenocarcinoma may be a heterogeneous disease with variable outcomes based upon the exact location with respect to the GEJ (i.e., Siewert type). Others have proposed diverse carcinogenic and molecular pathways for GEJ and esophageal adenocarcinomas [17, 18]. The Siewert classification remains very helpful for determining the appropriate surgical approach but does not help guide pre-, post-, or perioperative treatment.
Multimodal Treatment of Locally Advanced Gastroesophageal Junction Adenocarcinoma
In GEJ cancers, most researchers have focused on either neoadjuvant or perioperative strategies to reduce the risk of recurrence, eradicate micrometastatic disease, and improve outcomes after complete surgical resection. In addition to the ability to evaluate therapeutic response in vivo, preoperative therapy offers several other advantages. It affords the possibility of downsizing tumors and improving rates of R0 resection. Multiple trials have shown that R0 resection is associated with improved survival [19, 20]. Secondly, patients can tolerate intensive therapy prior to surgery, but a significant number of patients are unable to receive postoperative treatment because of poor tolerance or postsurgical complications. For instance, in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial, only 41.6% of all patients randomized to perioperative chemotherapy and 49.5% of all patients who completed preoperative chemotherapy and surgery were able to complete postoperative chemotherapy. Thirdly, preoperative therapy may allow modification of treatment based upon response. Currently, the effects of such an approach on outcomes are not fully known but trials using pre- and post-treatment positron emission tomography (PET) scans to assess response and alter treatment accordingly are ongoing [21].
Chemotherapy
Perioperative Chemotherapy
Two accepted approaches for the treatment of locally advanced GEJ cancer are perioperative chemotherapy, which is mostly used in Europe, and preoperative chemoradiotherapy commonly used in the United States. Although a few smaller studies are available, three large phase III trials have addressed perioperative chemotherapy. Two trials showed a survival advantage, but the third did not (Table 1) [5, 22, 23].
Table 1.
Outcomes of selected trials comparing perioperative chemotherapy with surgery alone for locally advanced gastroesophageal cancers
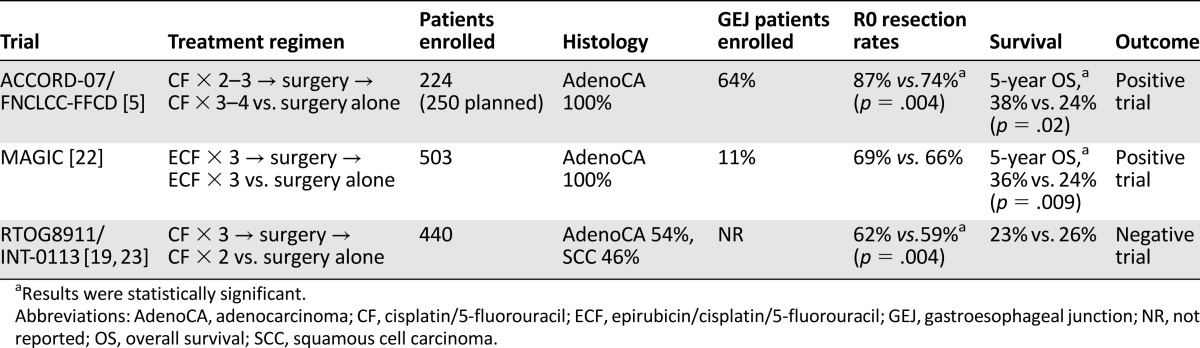
The strongest evidence for perioperative chemotherapy in GEJ adenocarcinomas comes from the French ACCORD-07 study [5]. Unlike other trials, it was dominated by GEJ tumors. 224 of the planned 250 patients were enrolled before it was closed for slow accrual. 64% had GEJ tumors, 24% had gastric adenocarcinomas, and 11% had lower esophageal adenocarcinomas. Patients were randomized to surgery alone or cisplatin/fluorouracil before and after surgery in responding patients. Compared with surgery alone, perioperative chemotherapy improved both 5-year OS and 5-year disease-free survival (34% vs. 19%; p = .003). Curative R0 resection rates were significantly higher with chemotherapy. Although all tumor sites benefited from perioperative chemotherapy, GEJ tumors benefited the most (hazard ratio [HR] 0.57; 95% confidence interval [CI] 0.39–0.83; p = .0145). Only half of the patients who underwent surgery after chemotherapy were able to receive postoperative chemotherapy. This study provides high-level evidence for use of perioperative chemotherapy.
The other randomized trial to show a survival advantage of perioperative chemotherapy was the MAGIC trial conducted in the U.K. [22]. This large study primarily focused on gastric cancer (76%), but 11% of the 503 enrolled subjects had GEJ tumors, and 14% had lower esophageal adenocarcinomas. Patients were randomized either to three cycles of chemotherapy (epirubicin, cisplatin, fluorouracil) before and after surgery or to surgery alone. Perioperative chemotherapy significantly improved 5-year OS (36% vs. 23%; p = .009) and progression-free survival (PFS) (HR 0.66; 95% CI 0.53–0.81; p < .001). Benefit was seen irrespective of the site of the primary tumor. Of all patients, 42% were able to complete all planned cycles of chemotherapy. Although this trial had a smaller number of GEJ patients, it was reported earlier than the French study and formed the initial basis for use of perioperative chemotherapy for gastric and GEJ adenocarcinomas in Europe. Interestingly, the 5-year survival rates in the two arms of the MAGIC trial were very similar to the corresponding arms of the ACCORD-07 trial.
The earliest phase III study to compare perioperative chemotherapy to surgery alone was the U.S. Radiation Therapy Oncology Group (RTOG) 8911/Intergroup (INT)-0113 trial [23]. Patients with resectable esophageal or GEJ cancer were randomized to either surgery alone or cisplatin/fluorouracil before and after surgery. Of the 440 eligible patients, 54% had adenocarcinoma, and 46% had SCC. The percentage of GEJ patients was not reported. No difference in median OS or R0 resection rates was noted between the two arms. There were no differences in outcomes based on histology. These results were reaffirmed in an update in which only R0 resection, irrespective of the use of perioperative chemotherapy, was associated with improved survival [19]. Several possible explanations for the negative results have been postulated by the authors [19, 23].
The INT-0113 trial did not report the exact number of GEJ tumors but included both SCCs and adenocarcinomas involving the esophagus. Other factors could have also impacted results of the INT-0113 trial; for instance, 20% of patients in the perioperative chemotherapy group did not undergo surgical resection compared with only 4% in the surgery arm. Secondly, although postoperative radiation was not part of the treatment plan, it was permitted for patients in either arm who underwent less than R0 resection. Most patients who received radiation also received concomitant chemotherapy, which could have further diluted any benefit of perioperative chemotherapy.
Preoperative Chemotherapy
Neoadjuvant chemotherapy followed by surgery has been investigated, but results are conflicting. The largest study was the U.K. Medical Research Council Esophageal Cancer trial (MRC-OEO2), which randomized 802 patients with operable disease to surgery alone or two cycles of cisplatin/fluorouracil followed by surgery [24]. Both adenocarcinomas (66%) and SCCs were included. 10% had tumors of the cardia, and 64% of the distal esophagus. Regardless of tumor histology or location, OS was significantly improved with preoperative chemotherapy, and R0 resection rates were higher with preoperative chemotherapy (60% vs. 54%; p < .0001). Survival benefit persisted with long-term follow-up (5-year OS, 23% vs. 17%; p = .03) [20]. However, these results conflict with those of the smaller European Organization for Research and Treatment of Cancer (EORTC) 40954 trial, which randomized 144 patients to surgery with or without preoperative chemotherapy using a different regimen of cisplatin/fluorouracil [25]. Almost equal numbers of GEJ (53%) and gastric adenocarcinomas were included. Chemotherapy significantly improved rates of R0 resection (82% vs. 68%; p = .036), but no difference in survival was seen between the two arms. Statistical power of this trial was limited because only 40% of planned patients were enrolled before it was closed because of poor accrual. Superior outcomes in the surgery-alone arm compared with other trials could have also contributed to the lack of benefit from chemotherapy. Although approximately equal numbers of GEJ patients were enrolled in both studies, a direct comparison between the two is difficult because the MRC-OEO2 was predominantly an esophageal cancer trial and the EORTC 40954 was a gastric trial.
A meta-analysis identified eight clinical trials comparing neoadjuvant chemotherapy to surgery alone in patients with esophagogastric cancer and showed an absolute improvement in survival of 7% at 2 years with preoperative chemotherapy [26]. Survival benefit was only significant for patients with adenocarcinoma (HR 0.78; 95% CI 0.64–0.95; p = .014). Benefit was reconfirmed in a recent update of the same meta-analysis [27]. Utility of preoperative chemotherapy pertaining to GEJ adenocarcinomas remains unclear from this meta-analysis because trials evaluating perioperative chemotherapy were also included.
Postoperative Chemotherapy
Adjuvant chemotherapy has been shown to improve survival in gastric cancer [28]; however, its role in GEJ cancer is unknown because there are no large randomized trials comparing postoperative chemotherapy to surgery alone. Most adjuvant chemotherapy trials for gastric cancer were conducted in Eastern countries, and very few, if any, patients with GEJ adenocarcinomas were included [28, 29].
Adjuvant chemotherapy has been shown to improve survival in gastric cancer; however, its role in GEJ cancer is unknown because there are no large randomized trials comparing postoperative chemotherapy to surgery alone.
Chemoradiotherapy
Preoperative Chemoradiotherapy
Despite adequate resection, a significant number of patients with GEJ adenocarcinoma fail locoregionally [30]. Preoperative chemoradiotherapy can reduce local recurrences and improve rates of pathologic complete response, a variable associated with improved survival [30–32]. Until recently, results have been mixed and the utility of radiotherapy has been controversial because most early trials either had small numbers of patients or suffered from other shortcomings.
Six trials are most relevant because they enrolled predominantly adenocarcinomas of the esophagus and GEJ, although most of them also included at least some patients with SCCs (Table 2). Walsh et al. [33] showed a survival advantage for multimodal therapy (3-year OS 32% vs. 6%; p = .01), whereas Urba et al. [34] failed to show any survival advantage for preoperative chemoradiotherapy, likely because of insufficient statistical power. A subsequent larger (n = 265) phase III Australasian study that included GEJ tumors reported significantly improved R0 resection rates with chemoradiotherapy (80% vs. 59%; p = .0002) but no improvement in PFS or OS [35]. Almost 37% subjects had SCC, and subgroup analysis suggested that SCC patients had better PFS with chemoradiotherapy.
Table 2.
Outcomes of selected trials comparing preoperative chemoradiotherapy with surgery alone for locally advanced gastroesophageal cancers
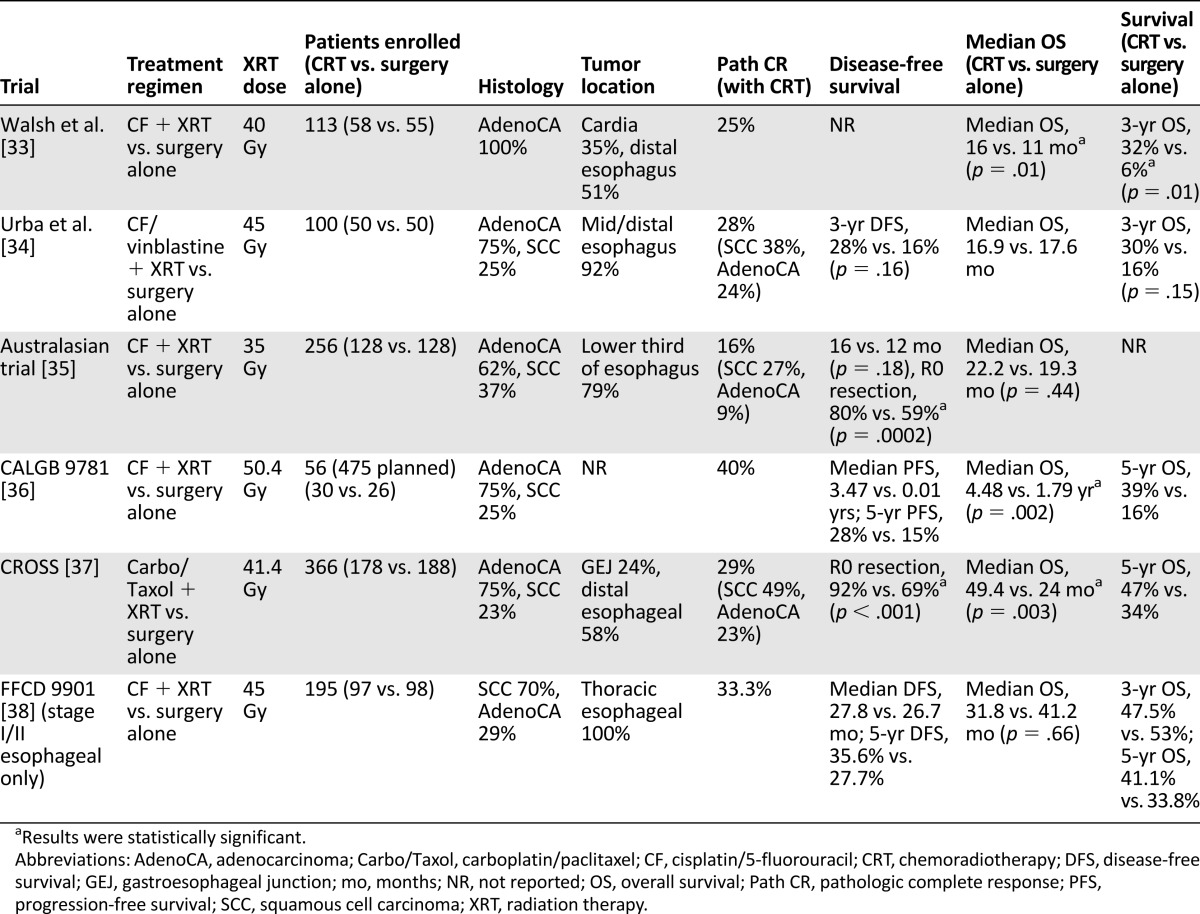
The Cancer and Leukemia Group B (CALGB) designed a phase III trial comparing surgery with or without preoperative chemoradiotherapy for gastroesophageal cancer and showed an improvement in median OS (4.48 vs. 1.79 years; p = .002) with chemoradiotherapy. Although this trial closed prematurely because of poor accrual (n = 56) [36], it provides some data for the utility of trimodality therapy because it was a well-designed multicenter prospective study with adequate follow-up.
Perhaps the strongest evidence for neoadjuvant chemoradiotherapy comes from the Chemoradiotherapy for Esophageal Cancer Followed by Surgery Study (CROSS) [37]. This trial randomized 366 patients with resectable esophageal or GEJ tumors to preoperative radiation (41.4 Gy in 23 fractions) combined with chemotherapy (weekly carboplatin/paclitaxel) or surgery alone. Most patients had adenocarcinoma (75%), and the majority of tumors were located in the distal esophagus (58%) or the GEJ (24%). Radiation was delivered using three-dimensional conformal radiation therapy (3D-CRT), specifying that the gross tumor volume and any enlarged regional nodes be delineated prior to a set expansion for the planning target volume. There was no mention of a specific clinical target volume (CTV). Chemoradiotherapy was associated with increased rates of R0 resection (92% vs. 69%) and, more importantly, a meaningful improvement in median OS from 24 to 49.4 months (p = .003), corresponding to a 5-year OS rate of 47% compared with 35% with surgery alone. The survival benefit of chemoradiotherapy persisted irrespective of the tumor histology, but SCCs derived more benefit and had higher pathologic complete response rates (49% vs. 23%; p = .008). Postoperative morbidity and early mortality were not increased with preoperative chemoradiotherapy. Longer follow-up of the CROSS phase III and its predecessor phase II trial confirmed that chemoradiotherapy considerably reduced locoregional recurrences, peritoneal carcinomatosis, and, to a lesser extent, distant recurrences [30]. CROSS is most relevant because it was a large prospective study that included a substantial number of GEJ tumors.
In contrast, the French FFCD 9901 randomized early stage esophageal cancer patients (predominantly SCC) to surgery with or without preoperative chemoradiation and reported no improvement in survival with chemoradiotherapy [38]. Postoperative mortality was higher in the chemoradiotherapy group, and the trial was stopped early for futility. The results were likely negative because this trial exclusively enrolled early stage (I and II) patients, who are least expected to benefit from such an aggressive approach.
A recent updated meta-analysis of 12 randomized esophageal cancer trials comparing neoadjuvant chemoradiotherapy to surgery alone (including CROSS) concluded that chemoradiotherapy was associated with a significant improvement in OS (HR 0.78, 95% CI 0.70–0.88; p < .0001) [27]. This corresponds to an absolute survival benefit of 8.7% at 2 years and a number needed to treat of 11. Survival benefit was seen in both SCCs (HR 0.80; 95% CI 0.68–0.93 p = .004) and adenocarcinomas (HR 0.75; 95% CI 0.59–0.95 p = .02), dispelling the notion that only SCCs benefit from chemoradiotherapy. In the same meta-analysis, although both neoadjuvant chemoradiotherapy and preoperative chemotherapy improved survival compared with surgery alone, greater benefit was seen with chemoradiotherapy.
Postoperative Chemoradiotherapy
Based on the INT-0116, adjuvant chemoradiotherapy is considered a standard approach for resected gastric cancer in the U.S. In this large phase III trial, 556 patients with gastric (80%) or GEJ (20%) adenocarcinomas were randomized to postoperative chemoradiotherapy or observation after resection [39]. Three-year OS was significantly improved with postoperative chemoradiotherapy (50% vs. 41%; p = .005), as was the 3-year relapse-free survival (48% vs. 31%; p < .001). The survival benefit persisted after 10 years of follow-up [40]. One of the major criticisms of this study has been that only 10% of patients underwent D2 lymphadenectomy, so chemoradiation may have only compensated for suboptimal surgery. Although recent data have underscored the importance of adequate surgery [41], postoperative chemoradiotherapy remains a valid option for patients with high risk GEJ tumors who undergo surgical resection without preoperative treatment.
Preoperative Chemoradiotherapy Versus Chemotherapy
Whereas there is evidence to support both preoperative chemoradiotherapy and perioperative chemotherapy over surgery alone, studies comparing the two approaches head to head are lacking. The Preoperative Chemotherapy or Radiochemotherapy in Esophagogastric Adenocarcinoma Trial (POET) is the only phase III study to exclusively enroll GEJ tumors to answer this question [42]. One hundred and nineteen patients with locally advanced tumors (uT3-4NxM0) were randomized to either preoperative induction chemotherapy (cisplatin/fluorouracil/leucovorin) or induction chemotherapy followed by chemoradiotherapy (cisplatin/etoposide combined with 30 Gy in 15 fractions of radiation) and then surgery. The investigators specified a CTV that encompassed elective nodes (left and right cardiac, left gastric, lesser curvature, and along the celiac, splenic, and hepatic arteries). From a radiation biology standpoint, this dose schema is lower than the 45–50.4 Gy in 25–28 fractions, which has traditionally been the recommended dose to control microscopic disease. Chemoradiotherapy significantly improved rates of pathologic complete response (15.6% vs. 2.0%; p = .03) and tumor-free lymph nodes (64.4% vs. 37.7%; p = .01) at resection. Unfortunately, the trial was closed prematurely because of poor accrual, and statistical significance could not be achieved, but there was a trend toward improved 3-year survival with neoadjuvant chemoradiotherapy (47.4% vs. 27.7%; p = .07).
Reviewing trials comparing the benefits of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy, the Australasian meta-analysis was only able to identify one other small trial, which also closed prematurely [27]. It finally concluded that a clear advantage of preoperative chemoradiotherapy over chemotherapy could not be established.
Response-Directed Therapy
Not all patients with GEJ tumors respond to preoperative chemotherapy [5, 22]. By identifying such patients, the toxicity of futile treatment can be spared, and alternative effective therapy can be recommended. Currently, no such biomarkers are available; however, data are emerging that metabolic information from interim 18-fluorodeoxyglucose PET may be prognostic and predictive [43, 44]. In a phase II study, metabolic responses were evaluated using PET after 2 weeks of induction chemotherapy with cisplatin/fluorouracil in 110 patients with locally advanced GEJ adenocarcinoma [43]. Metabolic responders continued chemotherapy for 12 weeks and then underwent surgery, whereas nonresponders immediately proceeded to surgical resection. After 2.3 years of follow-up, median OS was not reached for responders, whereas it was 25.8 months for nonresponders (HR 2.13; 95% CI 1.14–3.99 p = .015). Responders also had improved median event-free survival (29.7 vs. 14.1 months; p = .002) and major histologic remission rates (58% vs. 0%; p = .001). Median survival of PET nonresponders referred for early surgery seemed to be comparable or even superior to metabolic nonresponders (25.8 vs. 18 months) in a previous prospective study in which all patients received two cycles of preoperative chemotherapy irrespective of PET response [45]. Although comparisons across trials should be interpreted with caution, compared with these historical controls, early termination of chemotherapy did not seem to negatively impact survival in PET nonresponders.
The same investigators conducted another study to evaluate a PET-guided treatment algorithm in 56 patients in which responders continued preoperative chemotherapy and nonresponders underwent salvage chemoradiotherapy followed by surgery [44]. Histologic response rates improved with salvage chemoradiotherapy, but survival remained poor in PET nonresponders. Although this may indicate the unique aggressiveness of nonresponders irrespective of therapy used, the dose of radiation used was relatively low (32 Gy), and the same chemotherapeutic agents deemed ineffective by PET response were continued in combination with radiotherapy. The ongoing CALGB 80803 trial evaluating the utility of PET-directed selection of chemotherapeutic agents to combine with neoadjuvant radiotherapy may be a more robust and appropriate evaluation of this response-directed strategy [21].
In addition to the commonly reported decline in maximum standardized uptake value (SUV) parameter, there may be other useful predictors of response such as spatial-temporal features that can be extracted from an initial and post-treatment PET/computed tomography (CT) [46]. In one study, declines in mean SUV and skewness, as well as three textural features, were found to be associated with response in a series of 20 patients who were treated with trimodality therapy [46].
Discussion
Treatment of locally advanced GEJ adenocarcinoma remains challenging. Although there are data to support several strategies, none has emerged as a clear winner. Based on the ACCORD-07 and MAGIC trials, perioperative chemotherapy has become the standard in most European countries, whereas preoperative chemoradiotherapy is favored in the United States. In the absence of a study comparing the two approaches directly, one can make an argument for either. Clearly, a preoperative treatment approach should be used because it is not only most tolerable before surgery but also improves rates of R0 resection and pathologic complete response, both of which correlate with improved survival.
As mentioned earlier, initial trials evaluating chemoradiotherapy had several limitations. In these trials, a variety of radiation techniques and doses were used that could have impacted the results, especially because the way radiotherapy is planned, imaged, and delivered has changed dramatically over the last 30 years. Prior to the integration of 3D-CRT, in which beams are designed to improve normal tissue sparing, two dimensional plans were the standard such that a fluoroscopic image was generated with the patient swallowing contrast to delineate the primary tumor [47]. Current practice options offer 3D-CRT and intensity-modulated radiation therapy (IMRT) techniques, which rely on modern imaging for tumor target delineation with CT, PET/CT, and four-dimensional CT scanning, which can determine the amount of tumor-associated respiratory motion. Moreover, endoscopic oncologists can also place radio-opaque markers called fiducial markers, above and below the tumor so that the radiation oncologist can visualize the extent of disease as the patient breathes and thus rely on image-guided radiation therapy to confidently irradiate the daily target [48]. In the preoperative setting, avoidance of tissue injury to the lung and heart is of particular importance, and these improvements may be associated with improved outcomes, such as the significantly improved OS, locoregional control, and non-cancer-related death seen in the M.D. Anderson Cancer Center series of patients treated with IMRT compared with 3D-CRT [49]. Moreover, the daily and total dose of radiation has differed across the trials, with a schema of 45–50.4 Gy delivered in 25–28 fractions common in the U.S. versus a variety of daily and total dose regimens elsewhere. Finally, although consensus guidelines have been adopted across many gastrointestinal sites, there has not yet been agreement on a uniform GEJ treatment volume [50–52]. The extent of tissue irradiated is influenced by the elective nodal target, which forms the basis for the CTV; the Gastrointestinal Working Party of the Radiation Oncology Group of the EORTC has developed guidelines for the neoadjuvant radiation for GEJ adenocarcinoma, but these guidelines have not been widely adopted elsewhere [53]. In other sites, such as pancreatic cancer, failure of radiation field design to incorporate specific guidelines has resulted in reduced survival and a trend toward increased nonhematologic toxicity [54].
Based on available data, there remains a strong rationale to consider neoadjuvant radiotherapy, despite the heterogeneity in dose, technique, and volume of neoadjuvant irradiation used. Although results were not reported by tumor location in the CROSS, a substantial number of patients had GEJ tumors (24%), and improved survival was reported for all patients. The POET, which exclusively enrolled GEJ tumors, reported higher rates of pathologic complete response and lymph-node negative status at surgery with chemoradiotherapy compared with chemotherapy. Unlike the CROSS, elective nodal regions were included in the POET, in which even at a dose of only 30 Gy in 15 fractions, there was a significantly higher tumor-free lymph node rate. This may be more relevant for Western patients with larger, more advanced tumors. Moreover, the sequence strategies differed, with the CROSS delivering upfront concurrent chemoradiation versus induction chemotherapy followed by concurrent chemoradiation. Unfortunately, POET did not reach statistical significance because it failed to complete accrual, but there was a trend toward improved survival with chemoradiotherapy. The updated Australasian meta-analysis was only able to identify one other small trial besides POET and failed to identify a clear advantage of chemoradiotherapy over chemotherapy; however, the absolute survival benefit at 2 years seemed better with chemoradiotherapy (8.7% vs. 5.1%) [27]. Similarly the number needed to treat was less with chemoradiotherapy than with chemotherapy (11 vs. 19) [27]. Perioperative chemotherapy and postoperative chemoradiotherapy are also reasonable options, but only half the patients are able to receive treatment after surgery.
Based on available data, there remains a strong rationale to consider neoadjuvant radiotherapy, despite the heterogeneity in dose, technique, and volume of neoadjuvant irradiation used.
Future Perspectives
Survival rates of locally advanced GEJ adenocarcinomas remain far from satisfactory, and improved therapies are needed. Targeted agents, which appear promising for metastatic disease, warrant prospective studies in the adjuvant setting. GEJ tumors often express epidermal growth factor receptor (EGFR); however, the anti-EGFR antibodies cetuximab and panitumumab have failed to improve outcomes in unselected patients with advanced disease [55, 56]. Similarly, both the SCOPE1 and RTOG 0436 phase III studies were stopped early for futility because they showed no benefit of adding cetuximab to concurrent chemoradiotherapy for locally advanced disease [57, 58].
On the contrary, trastuzumab, a monoclonal antibody against human epidermal growth factor receptor-2 (HER2), prolonged survival when combined with chemotherapy in HER2-overexpressing metastatic gastric or GEJ adenocarcinomas and is now approved for this indication [59]. Almost 30% of GEJ tumors express HER2 and adjuvant trastuzumab has been shown to improve survival in other HER2-expressing malignancies [60, 61]. The phase II TOXAG study is assessing the safety and efficacy of trastuzumab with postoperative chemoradiotherapy in resected HER-2-positive gastric and GEJ tumors [62]. More importantly, the ongoing randomized phase III RTOG 1010 trial is evaluating the benefit of adding trastuzumab to neoadjuvant chemoradiotherapy with carboplatin/paclitaxel followed by completion of 1 year of trastuzumab after curative resection of HER-2-overexpressing adenocarcinomas of the esophagus and GEJ [63].
Another key pathway involved in tumorigenesis is the angiogenic pathway. The phase III Avastin in Gastric Cancer trial (AVAGAST) failed to show an improvement in survival with the addition of bevacizumab (a monoclonal antibody against vascular endothelial growth factor) to chemotherapy in patients with advanced gastric or GEJ cancer, but preplanned subgroup analysis showed a survival benefit in patients from North and South America [64]. Results are awaited from the MAGIC-B trial comparing perioperative chemotherapy with or without bevacizumab in resectable gastric and GEJ cancers [65].
Studies directly comparing perioperative chemotherapy with chemoradiotherapy are lacking; however, several ongoing trials aim to answer this very important question. The phase II/III TOPGEAR study is comparing perioperative chemotherapy with or without preoperative chemoradiotherapy in patients with resectable gastric and GEJ adenocarcinoma [66]. Similarly, the phase III Neo-AEGIS (ICORG 10-14) trial is randomizing patients with adenocarcinoma of the esophagus and the GEJ to either perioperative chemotherapy (modified MAGIC regimen) or neoadjuvant chemoradiotherapy as per the CROSS protocol [67]. This Ireland-led study has now expanded to the U.K. and is powered to detect a 10% survival difference at 3 years with a sample size of 592.
Trials evaluating chemoradiotherapy have combined various chemotherapeutic agents with radiation, and the optimal regimen remains unknown. The NeoSCOPE randomized phase II trial seeks to compare two preoperative chemoradiotherapy regimens (oxaliplatin/capecitabine and carboplatin/paclitaxel) to determine the most appropriate regimen to be taken forward to a phase III trial against neoadjuvant chemotherapy [68]. A key component of this trial is the strict radiotherapy quality assurance, which will ensure that the radiotherapy given is consistent across all centers.
The debate regarding the role of radiotherapy could potentially be clarified if consensus volume guidelines can be incorporated that would optimize the extent of local irradiation. Quality assurance design could then address whether uniform adherence to field, dose, and beam considerations impacts outcome. Prospective dose trials with imaging biomarkers could also help determine how to maximize the pathologic response of both the primary, involved nodes, and regional nodes harboring occult microscopic disease.
In addition to evaluating targeted agents, novel regimens, and newer radiation techniques, future studies need to focus on GEJ adenocarcinoma as a separate entity to remove the ambiguity created by previous trials and to provide more robust data.
Conclusion
Surgery remains the mainstay of treatment for resectable GEJ tumors, but outcomes with surgery alone for locally advanced disease remain dismal. Although there is a lack of global consensus regarding the optimal management of locally advanced GEJ adenocarcinoma, combined modality therapy improves survival, and all patients with resectable disease should be treated by an experienced multidisciplinary team. Preoperative therapies are favored because of better patient tolerability, increased rates of R0 resection, and higher complete pathologic responses. Based on currently available data, we recommend neoadjuvant chemoradiotherapy for patients with T2 or more advanced tumors and lymph node-positive locoregional disease. Perioperative chemotherapy is also a reasonable option, particularly for patients at high risk for systemic metastases and distant recurrences. For patients who present after surgical resection, postoperative chemoradiotherapy should be offered because it has shown to improve survival compared with surgery alone. Further studies focusing on GEJ adenocarcinomas are needed to determine the optimal therapeutic strategy and the ideal sequence of chemotherapy and radiation with respect to surgery, to evaluate the efficacy of response-directed therapy, to identify novel predictive biomarkers, and to clarify the role of emerging targeted therapies in resectable GEJ adenocarcinomas.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Footnotes
For Further Reading: Patrick M. Forde, Ronan J. Kelly. Genomic Alterations in Advanced Esophageal Cancer May Lead to Subtype-Specific Therapies. The Oncologist 2013;18:823–832.
Implications for Practice: The disease burden of esophageal cancer is increasing in the United States and worldwide, primarily driven by higher rates of adenocarcinoma risk factors, including obesity and Barrett's esophagus. Chemotherapy has moderate efficacy for locally advanced and metastatic esophageal cancer, but new approaches to treatment are urgently needed. This article focuses on potential oncogenic targets in esophageal cancer and comprehensively reviews the current state of the art in targeted therapy for esophageal and gastroesophageal junction tumors. Anti-human epidermal growth factor receptor-2 therapy has provided benefit for a small proportion of patients; however, despite signs of efficacy in early phase clinical trials, results with anti-epidermal growth factor receptor and anti-vascular endothelial growth factor therapy have been generally disappointing. Experience to date with targeted agents suggests that collaborative trials of target-specific agents in those subgroups of patients who have potential oncogenic drivers represent the best opportunity for bringing novel agents to the clinic.
Author Contributions
Conception/Design: Noman Ashraf, Sarah Hoffe, Richard Kim
Provision of study material or patients: Noman Ashraf, Sarah Hoffe, Richard Kim
Collection and/or assembly of data: Noman Ashraf, Sarah Hoffe, Richard Kim
Data analysis and interpretation: Noman Ashraf, Sarah Hoffe, Richard Kim
Manuscript writing: Noman Ashraf, Sarah Hoffe, Richard Kim
Final approval of manuscript: Noman Ashraf, Sarah Hoffe, Richard Kim
Disclosures
Richard Kim: Bayer (C/A, RF); Bristol-Myers Squibb, Roche (C/A, H); Lilly (C/A); Novartis (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Ferlay JSI, Ervik M, Dikshit R et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. 2012. Available at http://globocan.iarc.fr. Accessed April 20, 2014.
- 2.Bosetti C, Levi F, Ferlay J, et al. Trends in oesophageal cancer incidence and mortality in Europe. Int J Cancer. 2008;122:1118–1129. doi: 10.1002/ijc.23232. [DOI] [PubMed] [Google Scholar]
- 3.Trivers KF, Sabatino SA, Stewart SL. Trends in esophageal cancer incidence by histology, United States, 1998–2003. Int J Cancer. 2008;123:1422–1428. doi: 10.1002/ijc.23691. [DOI] [PubMed] [Google Scholar]
- 4.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 5.Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 6.Mariette C, Piessen G, Briez N, et al. Oesophagogastric junction adenocarcinoma: Which therapeutic approach? Lancet Oncol. 2011;12:296–305. doi: 10.1016/S1470-2045(10)70125-X. [DOI] [PubMed] [Google Scholar]
- 7.Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: Esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 8.Rice TW, Rusch VW, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction: Data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116:3763–3773. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 9.Siewert JR, Ott K. Are squamous and adenocarcinomas of the esophagus the same disease? Semin Radiat Oncol. 2007;17:38–44. doi: 10.1016/j.semradonc.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Hansen S, Melby KK, Aase S, et al. Helicobacter pylori infection and risk of cardia cancer and non-cardia gastric cancer. A nested case-control study. Scand J Gastroenterol. 1999;34:353–360. doi: 10.1080/003655299750026353. [DOI] [PubMed] [Google Scholar]
- 11.Shim JH, Song KY, Jeon HM, et al. Is gastric cancer different in Korea and the United States? Impact of tumor location on prognosis. Ann Surg Oncol. 2014;21:2332–2339. doi: 10.1245/s10434-014-3608-7. [DOI] [PubMed] [Google Scholar]
- 12.Verdecchia A, Mariotto A, Gatta G, et al. Comparison of stomach cancer incidence and survival in four continents. Eur J Cancer. 2003;39:1603–1609. doi: 10.1016/s0959-8049(03)00360-5. [DOI] [PubMed] [Google Scholar]
- 13.Noguchi Y, Yoshikawa T, Tsuburaya A, et al. Is gastric carcinoma different between Japan and the United States? Cancer. 2000;89:2237–2246. [PubMed] [Google Scholar]
- 14.Bickenbach K, Strong VE. Comparisons of Gastric Cancer Treatments: East vs. West. J Gastric Cancer. 2012;12:55–62. doi: 10.5230/jgc.2012.12.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashraf N, Hoffe S, Kim R. Adjuvant treatment for gastric cancer: Chemotherapy versus radiation. The Oncologist. 2013;18:1013–1021. doi: 10.1634/theoncologist.2012-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siewert JR, Feith M, Stein HJ. Biologic and clinical variations of adenocarcinoma at the esophago-gastric junction: Relevance of a topographic-anatomic subclassification. J Surg Oncol. 2005;90:139–146; discussion 146. doi: 10.1002/jso.20218. [DOI] [PubMed] [Google Scholar]
- 17.Carneiro F, Chaves P. Pathologic risk factors of adenocarcinoma of the gastric cardia and gastroesophageal junction. Surg Oncol Clin N Am. 2006;15:697–714. doi: 10.1016/j.soc.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Lin J, Beerm DG. Molecular biology of upper gastrointestinal malignancies. Semin Oncol. 2004;31:476–486. doi: 10.1053/j.seminoncol.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): A random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25:3719–3725. doi: 10.1200/JCO.2006.10.4760. [DOI] [PubMed] [Google Scholar]
- 20.Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–5067. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 21.Goodman KA, Cancer and Leukemia Group B (CALGB). PET scan imaging in assessing response in patients with esophageal cancer receiving combination chemotherapy. Available at http://clinicaltrials.gov/ct2/show/study/NCT01333033. Accessed May 4, 2014.
- 22.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 23.Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1979–1984. doi: 10.1056/NEJM199812313392704. [DOI] [PubMed] [Google Scholar]
- 24.Medical Research Council Oesophageal Cancer Working Group Surgical resection with or without preoperative chemotherapy in oesophageal cancer: A randomised controlled trial. Lancet. 2002;359:1727–1733. doi: 10.1016/S0140-6736(02)08651-8. [DOI] [PubMed] [Google Scholar]
- 25.Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210–5218. doi: 10.1200/JCO.2009.26.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: A meta-analysis. Lancet Oncol. 2007;8:226–234. doi: 10.1016/S1470-2045(07)70039-6. [DOI] [PubMed] [Google Scholar]
- 27.Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: An updated meta-analysis. Lancet Oncol. 2011;12:681–692. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 28.Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 29.Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 30.Oppedijk V, van der Gaast A, van Lanschot JJ, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol. 2014;32:385–391. doi: 10.1200/JCO.2013.51.2186. [DOI] [PubMed] [Google Scholar]
- 31.Fields RC, Strong VE, Gönen M, et al. Recurrence and survival after pathologic complete response to preoperative therapy followed by surgery for gastric or gastrooesophageal adenocarcinoma. Br J Cancer. 2011;104:1840–1847. doi: 10.1038/bjc.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donahue JM, Nichols FC, Li Z, et al. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg. 2009;87:392–398; discussion 398–399. doi: 10.1016/j.athoracsur.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–467. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 34.Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305–313. doi: 10.1200/JCO.2001.19.2.305. [DOI] [PubMed] [Google Scholar]
- 35.Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: A randomised controlled phase III trial. Lancet Oncol. 2005;6:659–668. doi: 10.1016/S1470-2045(05)70288-6. [DOI] [PubMed] [Google Scholar]
- 36.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–1092. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 38.Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: Final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32:2416–2422. doi: 10.1200/JCO.2013.53.6532. [DOI] [PubMed] [Google Scholar]
- 39.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 40.Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: A phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30:2327–2333. doi: 10.1200/JCO.2011.36.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samples JE, Stitzenberg KB, Meyers MO. Lymph node yield and survival in gastric carcinoma. J Clin Oncol. 2014;32(suppl):4012a. [Google Scholar]
- 42.Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851–856. doi: 10.1200/JCO.2008.17.0506. [DOI] [PubMed] [Google Scholar]
- 43.Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: The MUNICON phase II trial. Lancet Oncol. 2007;8:797–805. doi: 10.1016/S1470-2045(07)70244-9. [DOI] [PubMed] [Google Scholar]
- 44.zum Büschenfelde CM, Herrmann K, Schuster T, et al. (18)F-FDG PET-guided salvage neoadjuvant radiochemotherapy of adenocarcinoma of the esophagogastric junction: The MUNICON II trial. J Nucl Med. 2011;52:1189–1196. doi: 10.2967/jnumed.110.085803. [DOI] [PubMed] [Google Scholar]
- 45.Ott K, Weber WA, Lordick F, et al. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol. 2006;24:4692–4698. doi: 10.1200/JCO.2006.06.7801. [DOI] [PubMed] [Google Scholar]
- 46.Tan S, Kligerman S, Chen W, et al. Spatial-temporal [18F]FDG-PET features for predicting pathologic response of esophageal cancer to neoadjuvant chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2013;85:1375–1382. doi: 10.1016/j.ijrobp.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fuks Z, Leibel SA, Kutcher GJ, et al. Three-dimensional conformal treatment: A new frontier in radiation therapy. Important Adv Oncol. 1991:151–172. [PubMed] [Google Scholar]
- 48.Fernandez DC, Hoffe SE, Barthel JS, et al. Stability of endoscopic ultrasound-guided fiducial marker placement for esophageal cancer target delineation and image-guided radiation therapy. Pract Radiat Oncol. 2013;3:32–39. doi: 10.1016/j.prro.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Lin SH, Wang L, Myles B, et al. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;84:1078–1085. doi: 10.1016/j.ijrobp.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huguet F, Goodman KA, Azria D, et al. Radiotherapy technical considerations in the management of locally advanced pancreatic cancer: American-French consensus recommendations. Int J Radiat Oncol Biol Phys. 2012;83:1355–1364. doi: 10.1016/j.ijrobp.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 51.Goodman KA, Regine WF, Dawson LA, et al. Radiation Therapy Oncology Group consensus panel guidelines for the delineation of the clinical target volume in the postoperative treatment of pancreatic head cancer. Int J Radiat Oncol Biol Phys. 2012;83:901–908. doi: 10.1016/j.ijrobp.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Myerson RJ, Garofalo MC, El Naqa I, et al. Elective clinical target volumes for conformal therapy in anorectal cancer: A radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys. 2009;74:824–830. doi: 10.1016/j.ijrobp.2008.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matzinger O, Gerber E, Bernstein Z, et al. EORTC-ROG expert opinion: Radiotherapy volume and treatment guidelines for neoadjuvant radiation of adenocarcinomas of the gastroesophageal junction and the stomach. Radiother Oncol. 2009;92:164–175. doi: 10.1016/j.radonc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 54.Abrams RA, Winter KA, Regine WF, et al. Failure to adhere to protocol specified radiation therapy guidelines was associated with decreased survival in RTOG 9704: A phase III trial of adjuvant chemotherapy and chemoradiotherapy for patients with resected adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 2012;82:809–816. doi: 10.1016/j.ijrobp.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): A randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490–499. doi: 10.1016/S1470-2045(13)70102-5. [DOI] [PubMed] [Google Scholar]
- 56.Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): A randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:481–489. doi: 10.1016/S1470-2045(13)70096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crosby T, Hurt CN, Falk S, et al. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): A multicentre, phase 2/3 randomised trial. Lancet Oncol. 2013;14:627–637. doi: 10.1016/S1470-2045(13)70136-0. [DOI] [PubMed] [Google Scholar]
- 58.Suntharalingam M, Winter K, Ilson DH, et al. The initial report of RTOG 0436: A phase III trial evaluating the addition of cetuximab to paclitaxel, cisplatin, and radiation for patients with esophageal cancer treated without surgery. J Clin Oncol. 2014;32(suppl 3):LBA6a. [Google Scholar]
- 59.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 60.Bang Y, Chung H, Xu J, et al. Pathological features of advanced gastric cancer (GC): Relationship to human epidermal growth factor receptor 2 (HER2) positivity in the global screening programme of the ToGA trial. J Clin Oncol. 2009;27(suppl):4556a. [Google Scholar]
- 61.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.A Study of the Combination of Oxaliplatin, Capecitabine and Herceptin (Trastuzumab) and Chemoradiotherapy in The Adjuvant Setting in Operated Patients With HER2+ Gastric or Gastro-Esophageal Junction Cancer (TOXAG Study). Available at http://clinicaltrials.gov/ct2/show/NCT01748773. Accessed May 30, 2014.
- 63.RTOG. Radiation Therapy, Paclitaxel, and Carboplatin With or Without Trastuzumab in Treating Patients With Esophageal Cancer. Available at http://clinicaltrials.gov/ct2/show/study/NCT01196390. Accessed November 9, 2014.
- 64.Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: A randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–3976. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 65.Smyth EC, Langley RE, Stenning SP, et al. ST03: A randomized trial of perioperative epirubicin, cisplatin plus capecitabine (ECX) with or without bevacizumab (B) in patients (pts) with operable gastric, esophagogastric junction (OGJ), or lower esophageal adenocarcinoma. J Clin Oncol. 2013;31(suppl):TPS4156a. [Google Scholar]
- 66.Leong T, Smithers M, Michael M, et al. TOPGEAR: An international randomized phase III trial of preoperative chemoradiotherapy versus preoperative chemotherapy for resectable gastric cancer (AGITG/TROG/EORTC/NCIC CTG) J Clin Oncol. 2012;30(suppl):TPS4141a. [Google Scholar]
- 67.Keegan N, Keane F, Cuffe S, et al. ICORG 10-14: Neo-AEGIS: A randomized clinical trial of neoadjuvant and adjuvant chemotherapy (modified MAGIC regimen) versus neoadjuvant chemoradiation (CROSS protocol) in adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol. 2014;32(suppl):TPS4145a. [Google Scholar]
- 68.Crosby T, Mukherjee S, Hurt C, et al. NeoSCOPE: A phase II randomized comparison of neoadjuvant oxaliplatin/capecitabine versus carboplatin/paclitaxel-based chemoradiation in operable esophageal cancer. J Clin Oncol. 2014;32(suppl):TPS4144a. [Google Scholar]


