Abstract
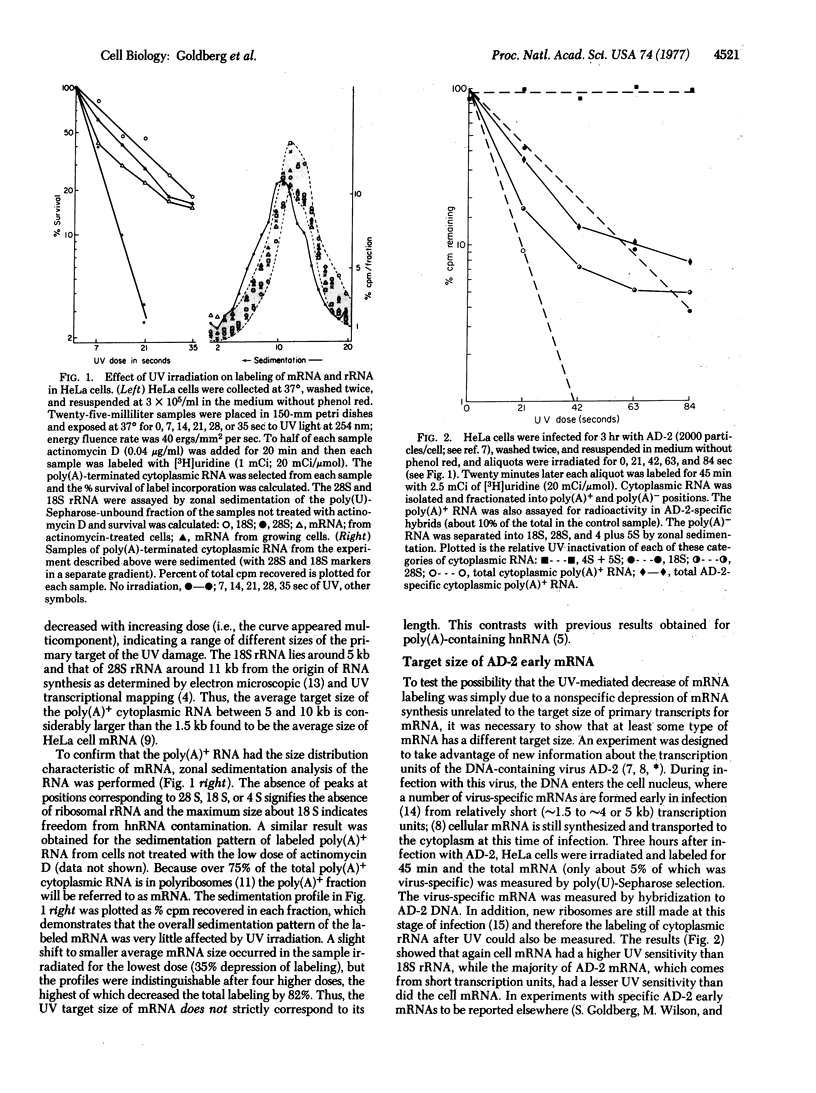
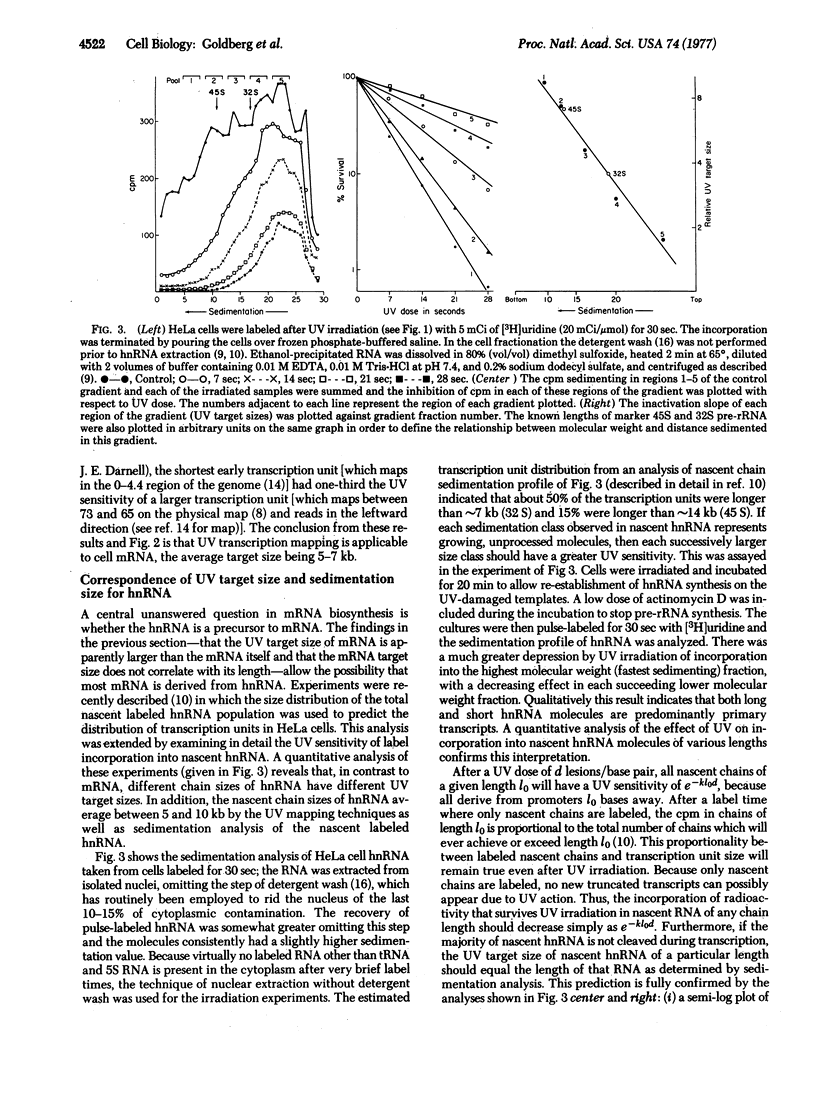
The effects of UV irradiation on the incorporation of [3H]uridine in HeLa (human) cell mRNA, rRNA, heterogeneous nuclear RNA (hnRNA) and early mRNA from adenovirus type 2 have been compared. The UV target size of cell mRNA is at least 3 times larger than the average size of the mRNA itself and larger than the adenovirus-2 early mRNA, which is known to derive from transcription units of about 1.5-5.0 kilobases. The UV target size of hnRNA, in contrast, is about the same as its size determined by sedimentation and overlaps with the target size of mRNA. It is concluded that most mRNA derives from a higher molecular weight hnRNA molecule.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachenheimer S., Darnell J. E. Adenovirus-2 mRNA is transcribed as part of a high-molecular-weight precursor RNA. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4445–4449. doi: 10.1073/pnas.72.11.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):442–446. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos R. N., Aviv H. Globin RNA precursor molecules: biosynthesis and process in erythroid cells. Cell. 1977 Jul;11(3):641–650. doi: 10.1016/0092-8674(77)90081-2. [DOI] [PubMed] [Google Scholar]
- Bräutigam A. R., Sauerbier W. Transcription unit mapping in bacteriophage T7. I. In vivo transcription by Escherichia coli RNA polymerase. J Virol. 1973 Oct;12(4):882–886. doi: 10.1128/jvi.12.4.882-886.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman E., Goldberg S., Darnell J. E., Jr hnRNA in HeLa cells: distribution of transcript sizes estimated from nascent molecule profile. Cell. 1976 Nov;9(3):465–472. doi: 10.1016/0092-8674(76)90091-x. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Flint J. The topography and transcription of the adenovirus genome. Cell. 1977 Feb;10(2):153–166. doi: 10.1016/0092-8674(77)90211-2. [DOI] [PubMed] [Google Scholar]
- Giorno R., Sauerbier W. A radiological analysis of the transcription units for heterogeneous nuclear RNA in cultured murine cells. Cell. 1976 Dec;9(4 Pt 2):775–783. doi: 10.1016/0092-8674(76)90140-9. [DOI] [PubMed] [Google Scholar]
- Goldberg S., Weber J., Darnell J. E., Jr The definition of a large viral transcription unit late in Ad2 infection of HeLa cells: mapping by effects of ultraviolet irradiation. Cell. 1977 Apr;10(4):617–621. doi: 10.1016/0092-8674(77)90094-0. [DOI] [PubMed] [Google Scholar]
- Hackett P. B., Sauerbier W. Radiological mapping of the ribosomal RNA transcription unit in E. coli. Nature. 1974 Oct 18;251(5476):639–641. doi: 10.1038/251639a0. [DOI] [PubMed] [Google Scholar]
- Hackett P. B., Sauerbier W. The transcriptional organization of the ribosomal RNA genes in mouse L cells. J Mol Biol. 1975 Jan 25;91(3):235–256. doi: 10.1016/0022-2836(75)90378-2. [DOI] [PubMed] [Google Scholar]
- Jelinek W., Adesnik M., Salditt M., Sheiness D., Wall R., Molloy G., Philipson L., Darnell J. E. Further evidence on the nuclear origin and transfer to the cytoplasm of polyadenylic acid sequences in mammalian cell RNA. J Mol Biol. 1973 Apr 15;75(3):515–532. doi: 10.1016/0022-2836(73)90458-0. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Philipson L., Pettersson U., Lindberg U., Tibbetts C., Vennström B., Persson T. RNA synthesis and processing in adenovirus-infected cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):447–456. doi: 10.1101/sqb.1974.039.01.057. [DOI] [PubMed] [Google Scholar]
- Ross J. A precursor of globin messenger RNA. J Mol Biol. 1976 Sep 15;106(2):403–420. doi: 10.1016/0022-2836(76)90093-0. [DOI] [PubMed] [Google Scholar]
- Salditt-Georgieff M., Jelinek W., Darnell J. E., Furuichi Y., Morgan M., Shatkin A. Methyl labeling of HeLa cell hnRNA: a comparison with mRNA. Cell. 1976 Feb;7(2):227–237. doi: 10.1016/0092-8674(76)90022-2. [DOI] [PubMed] [Google Scholar]
- Sauerbier W., Millette R. L., Hackett P. B., Jr The effects of ultraviolet irradiation on the transcription of T4 DNA. Biochim Biophys Acta. 1970;209(2):368–386. doi: 10.1016/0005-2787(70)90735-5. [DOI] [PubMed] [Google Scholar]
- Weber J., Jelinek W., Darnell J. E., Jr The definition of a large viral transcription unit late in Ad2 infection of HeLa cells: mapping of nascent RNA molecules labeled in isolated nuclei. Cell. 1977 Apr;10(4):611–616. doi: 10.1016/0092-8674(77)90093-9. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. Secondary structure maps of RNA: processing of HeLa ribosomal RNA. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2827–2831. doi: 10.1073/pnas.70.10.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]


