Abstract
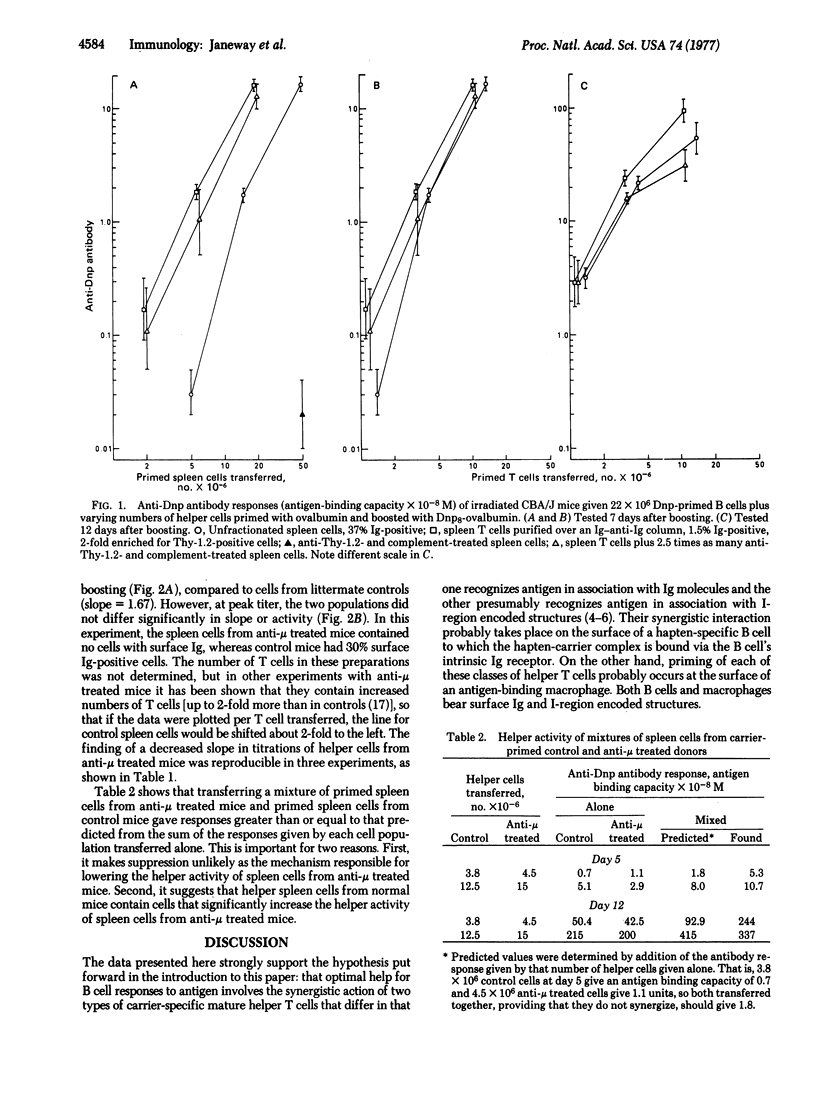
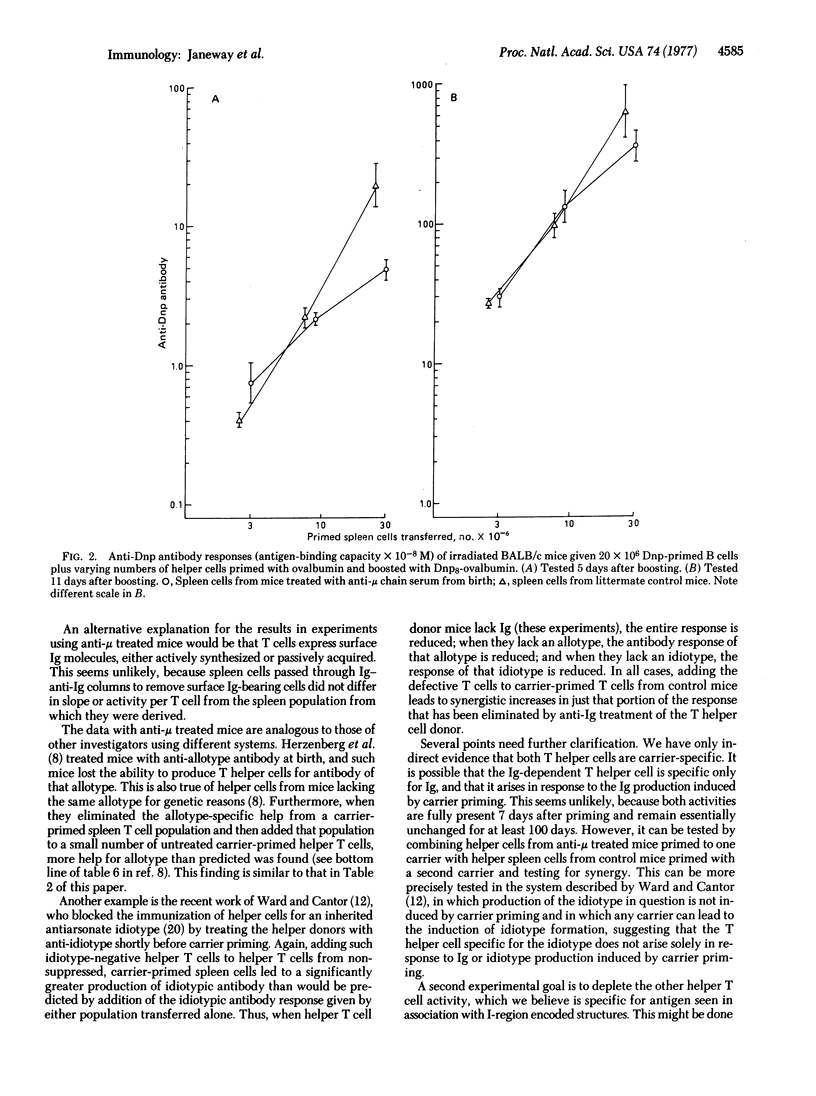
Evidence from various systems suggests that thymus-derived lymphocytes can affect the quality of antibody responses by recognizing various portions of the immunoglobulin receptor of bone-marrow-derived thymus-independent lymphocytes. A model for this process is proposed involving two antigen-specific mature T helper cells, one of which also is specific for immunoglobulin determinants. These two cells act synergistically. Evidence from adoptive secondary antibody responses demonstrates that both cells are antigen-specific T cells and that the immunoglobulin-recognizing T helper cell is absent from experimentally agammaglobulinemic mice. This cell is termed an "immunoglobulin-dependent T cell" because its activation requires the presence of immunoglobulin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cunningham A. J., Lafferty K. J. A simple conservative explanation of the H-2 restriction of interactions between lymphocytes. Scand J Immunol. 1977;6(1-2):1–6. doi: 10.1111/j.1365-3083.1977.tb00317.x. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Paul W. E. Effect of thymus-derived lymphocytes on amount and affinity of anti-hapten antibody. J Immunol. 1971 Mar;106(3):872–874. [PubMed] [Google Scholar]
- Herzenberg L. A., Okumura K., Cantor H., Sato V. L., Shen F. W., Boyse E. A., Herzenberg L. A. T-cell regulation of antibody responses: demonstration of allotype-specific helper T cells and their specific removal by suppressor T cells. J Exp Med. 1976 Aug 1;144(2):330–344. doi: 10.1084/jem.144.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr Cellular cooperation during in vivo anti-hapten antibody responses. I. The effect of cell number on the response. J Immunol. 1975 Apr;114(4):1394–1401. [PubMed] [Google Scholar]
- Janeway C. A., Jr Cellular cooperation during in vivo anti-hapten antibody responses. II. The effect of in vivo and in vitro x-irradiation on T and B cells. J Immunol. 1975 Apr;114(4):1402–1407. [PubMed] [Google Scholar]
- Janeway C. A., Jr Cellular cooperation during in vivo anti-hapten antibody responses. III. The helper cell activity of activated thymocytes, of spleen cells treated with anti-theta serum, and of spleen cells from anti-thymocyte serum-treated or adult thymectomized donors. J Immunol. 1975 Apr;114(4):1408–1414. [PubMed] [Google Scholar]
- Janeway C. A., Jr The mechanism of a hapten-specific helper effect in mice. J Immunol. 1973 Oct;111(4):1250–1256. [PubMed] [Google Scholar]
- Janeway C. A., Wigzell H., Binz H. Two different VH gene products make up the T-cell receptors. Scand J Immunol. 1976;5(9):993–1001. doi: 10.1111/j.1365-3083.1976.tb03051.x. [DOI] [PubMed] [Google Scholar]
- Karniely Y., Mozes E., Shearer G. M., Sela M. The role of thymocytes and bone marrow cells in defining the response to the dinitrophenyl hapten attached to positively and negatively charged synthetic polypeptide carriers. Cell fractionation over charged columns. J Exp Med. 1973 Jan 1;137(1):183–195. doi: 10.1084/jem.137.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The regulatory influence of activated T cells on B cell responses to antigen. Adv Immunol. 1972;15:1–94. doi: 10.1016/s0065-2776(08)60683-5. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Graves M., Dorf M. E., Dimuzio H., Benacerraf B. Cell interactions between histoincompatible T and B lymphocytes. VII. Cooperative responses between lymphocytes are controlled by genes in the I region of the H-2 complex. J Exp Med. 1975 Jan 1;141(1):263–268. doi: 10.1084/jem.141.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H., Hamaoka T., Dorf M. E., Maurer P. H., Benacerraf B. Cell interactions between histoincompatible T and B lymphocytes. IV. Involvement of the immune response (Ir) gene in the control of lymphocyte interactions in responses controlled by the gene. J Exp Med. 1973 Sep 1;138(3):734–739. doi: 10.1084/jem.138.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T., Ishizaka K. Regulation of antibody response in vitro. VI. Carrier-specific helper cells for IgG and IgE antibody response. J Immunol. 1973 Sep;111(3):720–732. [PubMed] [Google Scholar]
- Kuettner M. G., Wang A. L., Nisonoff A. Quantitative investigations of idiotypic antibodies. VI. Idiotypic specificity as a potential genetic marker for the variable regions of mouse immunoglobulin polypeptide chains. J Exp Med. 1972 Mar 1;135(3):579–595. doi: 10.1084/jem.135.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton A. R., 3rd, Asofsky R., Hylton M. B., Cooper M. D. Suppression of immunoglobulin class synthesis in mice. I. Effects of treatment with antibody to -chain. J Exp Med. 1972 Feb 1;135(2):277–297. doi: 10.1084/jem.135.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison N. A. The carrier effect in the secondary response to hapten-protein conjugates. II. Cellular cooperation. Eur J Immunol. 1971 Jan;1(1):18–27. doi: 10.1002/eji.1830010104. [DOI] [PubMed] [Google Scholar]
- Murgita R. A., Mattioli C. A., Tomasi T. B., Jr Production of a runting syndrome and selective A deficiency in mice by the administration of anti-heavy chain antisera. J Exp Med. 1973 Jul 1;138(1):209–228. doi: 10.1084/jem.138.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura K., Herzenberg L. A., Murphy D. B., McDevitt H. O., Herzenberg L. A. Selective expression of H-2 (i-region) loci controlling determinants on helper and suppressor T lymphocytes. J Exp Med. 1976 Sep 1;144(3):685–698. doi: 10.1084/jem.144.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W. E., Benacerraf B. Functional specificity of thymus- dependent lymphocytes. Science. 1977 Mar 25;195(4284):1293–1300. doi: 10.1126/science.320663. [DOI] [PubMed] [Google Scholar]
- Sela M., Mozes E. Dependence of the chemical nature of antibodies on the net electrical charge of antigens. Proc Natl Acad Sci U S A. 1966 Feb;55(2):445–452. doi: 10.1073/pnas.55.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbaum F. I., Evans C. B., Tigelaar R. E. Immunity to Plasmodium Berghei yoelii in mice. I. The course of infection in T cell and B cell deficient mice. J Immunol. 1976 Nov;117(5 PT2):1999–2005. [PubMed] [Google Scholar]


