Abstract
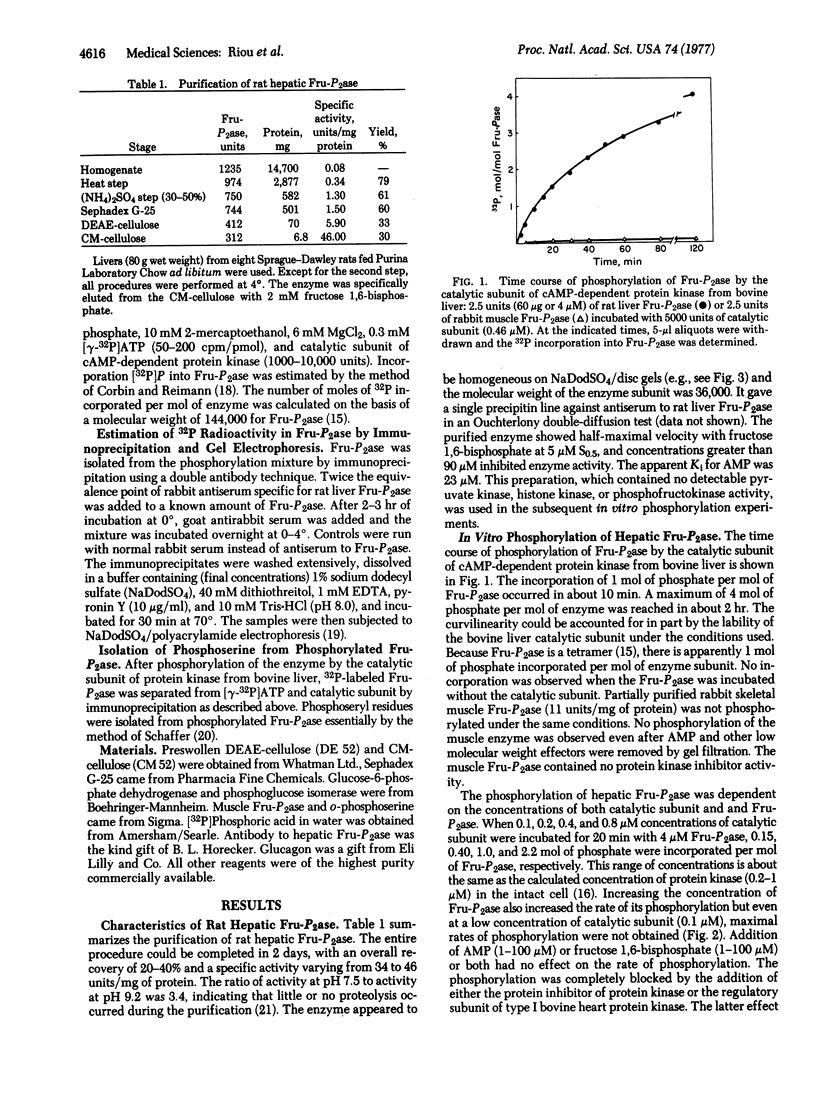
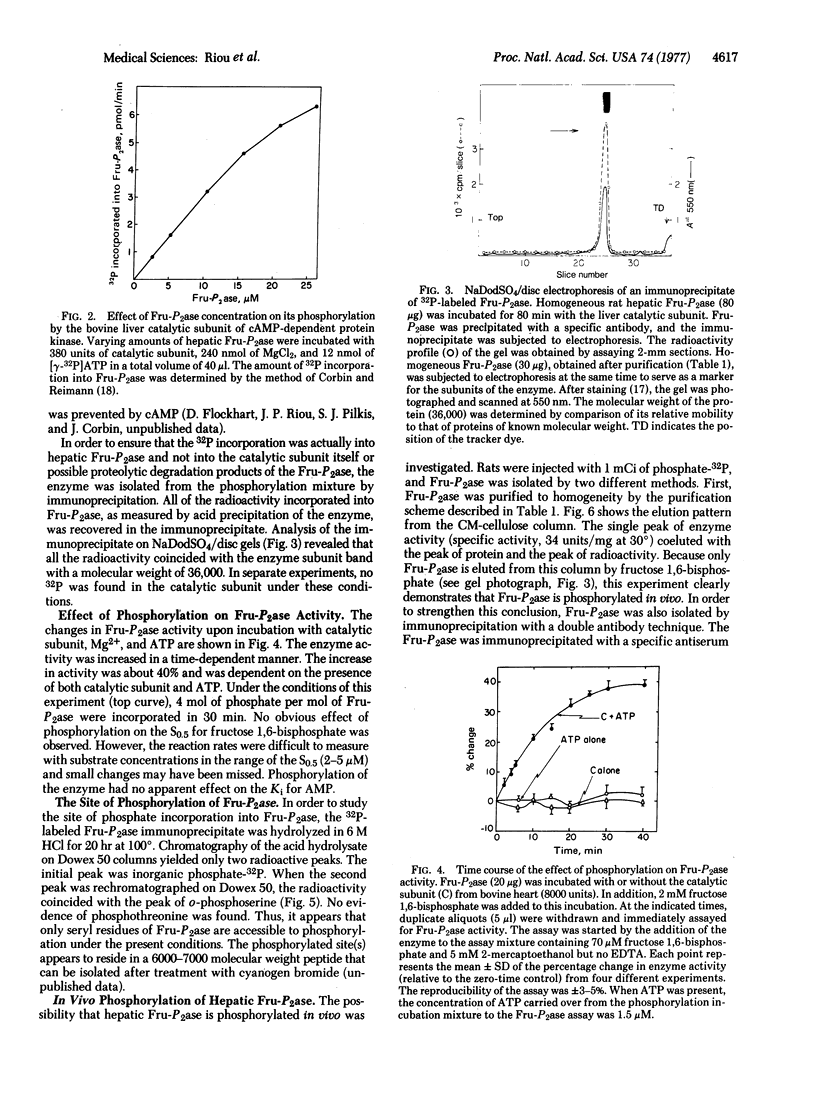
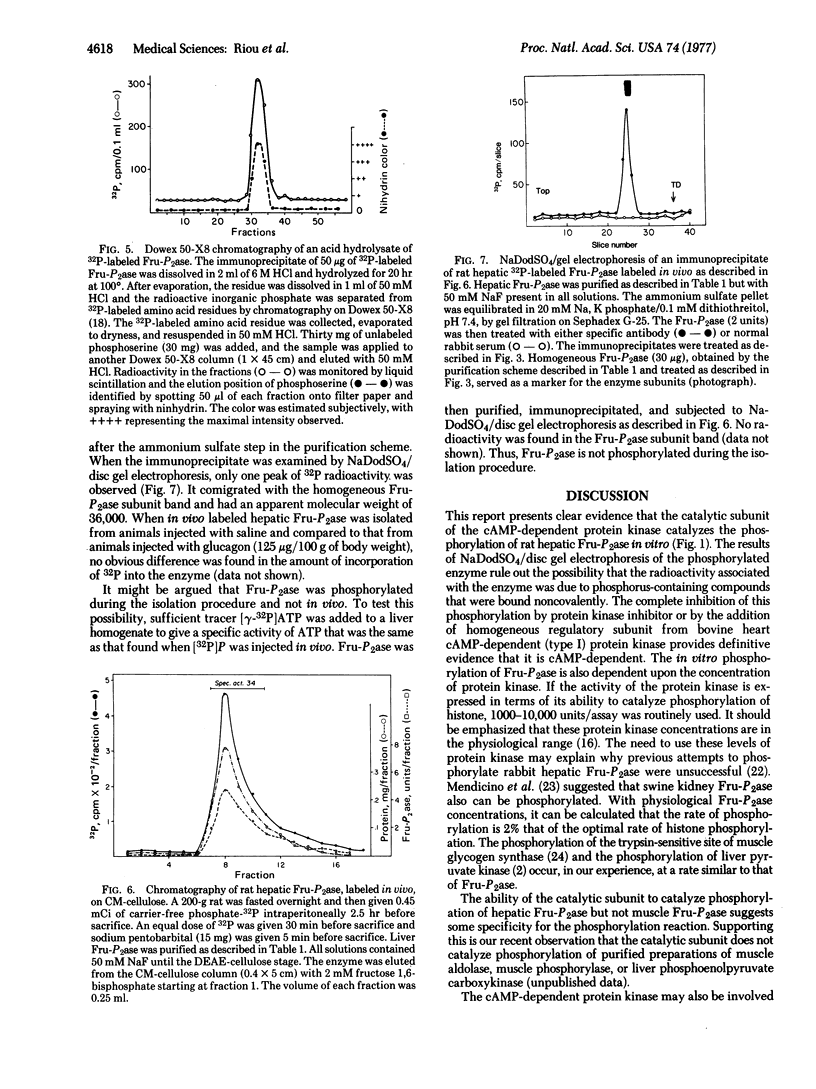
Incorporation of 32P from [gamma-32P]ATP into a homogenous preparation of rat hepatic fructose-1,6-bisphosphatase (D-fructose-1,6-bisphosphatase 1-phosphohydrolase, EC 3.1.3.11) was catalyzed by a homogeneous preparation of the catalytic subunit of cyclic AMP-dependent protein kinase from bovine liver. Approximately 4 mol of phosphate were incorporated per mol of the tetrameric enzyme. This phosphorylation was associated with an increase in enzyme activity. In addition, in vivo phosphorylation of the enzyme was observed after injection of radioactive inorganic phosphate into rats and subsequent isolation of the enzyme by conventional purification methods and by immunoprecipitation. All of the labeled phosphate incorporation into the enzyme, both in vitro and in vivo, was precipitated by antibody specific for the enzyme. Furthermore, the 32Pi counts were coincident with the enzyme subunit band when the immunoprecipitates were examined by sodium dodecyl sulfate/disc gel electrophoresis. Acid hydrolysis of the immunoprecipitated enzyme that was phosphorylated in vitro revealed that only seryl residues were labeled. On the basis of the concentration of protein kinase (0.2-1.0 muM) necessary to phosphorylate physiological amounts of fructose-1,6-bisphosphatase (1.0-4.0 muM), it is suggested that cyclic AMP-dependent protein kinase may catalyze the phosphorylation of fructose-1,6-bisphosphatase in vivo.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beavo J. A., Bechtel P. J., Krebs E. G. Mechanisms of control for cAMP-dependent protein kinase from skeletal muscle. Adv Cyclic Nucleotide Res. 1975;5:241–251. [PubMed] [Google Scholar]
- Blair J. B., Cimbala M. A., Foster J. L., Morgan R. A. Hepatic pyruvate kinase. Regulation by glucagon, cyclic adenosine 3'-5'-monophosphate, and insulin in the perfused rat liver. J Biol Chem. 1976 Jun 25;251(12):3756–3762. [PubMed] [Google Scholar]
- Brand I. A., Söling H. D. Activation and inactivation of rat liver phosphofructokinase by phosphorylation-dephosphorylation. FEBS Lett. 1975 Sep 15;57(2):163–168. doi: 10.1016/0014-5793(75)80707-1. [DOI] [PubMed] [Google Scholar]
- Carlson C. W., Baxter R. C., Ulm E. H., Pogell B. M. Role of oleate in the regulation of "neutral" rabbit liver fructose 1,6-diphosphatase activity. J Biol Chem. 1973 Aug 25;248(16):5555–5561. [PubMed] [Google Scholar]
- Clark M. G., Kneer N. M., Bosch A. L., Lardy H. A. The fructose 1,6-diphosphatase-phosphofructokinase substrate cycle. A site of regulation of hepatic gluconeogenesis by glucagon. J Biol Chem. 1974 Sep 25;249(18):5695–5703. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Feliú J. E., Hue L., Hers H. G. Hormonal control of pyruvate kinase activity and of gluconeogenesis in isolated hepatocytes. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2762–2766. doi: 10.1073/pnas.73.8.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maragoudakis M. E., Hankin H. Partial purification and properties of a cyclic 3',5'-AMP-independent protein kinase from rat liver. Biochim Biophys Acta. 1977 Jan 11;480(1):122–136. doi: 10.1016/0005-2744(77)90327-8. [DOI] [PubMed] [Google Scholar]
- Mendicino J., Beaudreau C., Bhattacharyya R. N. Reversible inactivation of D-fructose 1,6-diphosphatase by adenosine triphosphate and cyclic 3' ,5'-adenosine monophosphate. Arch Biochem Biophys. 1966 Sep 26;116(1):436–445. doi: 10.1016/0003-9861(66)90050-6. [DOI] [PubMed] [Google Scholar]
- Pilkis S. J., Riou J. P., Claus T. H. Hormonal control of [14C]glucose synthesis from [U-14C]dihydroxyacetone and glycerol in isolated rat hepatocytes. J Biol Chem. 1976 Dec 25;251(24):7841–7852. [PubMed] [Google Scholar]
- Pontremoli S., Traniello S., Luppis B., Wood W. A. Fructose diphosphatase from rabbit liver. I. Purification and properties. J Biol Chem. 1965 Sep;240(9):3459–3463. [PubMed] [Google Scholar]
- Riou J. P., Claus T. H., Pilkis S. J. Control of pyruvate kinase activity by glucagon in isolated hepatocytes. Biochem Biophys Res Commun. 1976 Dec 6;73(3):591–599. doi: 10.1016/0006-291x(76)90851-2. [DOI] [PubMed] [Google Scholar]
- Rognstad R., Katz J. Effects of hormones and of ethanol on the fructose 6-P-fructose 1,6-P2 futile cycle during gluconeogenesis in the liver. Arch Biochem Biophys. 1976 Dec;177(2):337–345. doi: 10.1016/0003-9861(76)90447-1. [DOI] [PubMed] [Google Scholar]
- Soderling T. R. Regulation of glycogen synthetase. Specificity and stoichiometry of phosphorylation of the skeletal muscle enzyme by cyclic 3':5'-AMP-dependent protein kinase. J Biol Chem. 1975 Jul 25;250(14):5407–5412. [PubMed] [Google Scholar]
- Sugden P. H., Holladay L. A., Reimann E. M., Corbin J. D. Purification and characterization of the catalytic subunit of adenosine 3':5'-cyclic monophosphate-dependent protein kinase from bovine liver. Biochem J. 1976 Nov;159(2):409–422. doi: 10.1042/bj1590409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketa K., Sarngadharan M. G., Watanabe A., Aoe H., Pogell B. M. Reversible inactivation of rabbit liver fructose 1,6-diphosphatase by adenosine triphosphate and adenosine diphosphate. J Biol Chem. 1971 Sep 25;246(18):5676–5683. [PubMed] [Google Scholar]
- Taunton O. D., Stifel F. B., Greene H. L., Herman R. H. Rapid reciprocal changes in rat hepatic glycolytic enzyme and fructose diphosphatase activities following insulin and glucagon injection. J Biol Chem. 1974 Nov 25;249(22):7228–7239. [PubMed] [Google Scholar]
- Tejwani G. A., Pedrosa F. O., Pontremoli S., Horecker B. L. Dual role of Zn2+ as inhibitor and activator of fructose 1,6-bisphosphatase of rat liver. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2692–2695. doi: 10.1073/pnas.73.8.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejwani G. A., Pedrosa F. O., Pontremoli S., Horecker B. L. The purification of properties of rat liver fructose 1,6-bisphosphatase. Arch Biochem Biophys. 1976 Nov;177(1):253–264. doi: 10.1016/0003-9861(76)90435-5. [DOI] [PubMed] [Google Scholar]
- Titanji V. P., Zetterqvist O., Engstroöm L. Regulation in vitro of rat liver pyruvate kinase by phosphorylation-dephosphorylation reactions, catalyzed by cyclic-AMP dependent protein kinases and a histone phosphatase. Biochim Biophys Acta. 1976 Jan 23;422(1):98–108. doi: 10.1016/0005-2744(76)90011-5. [DOI] [PubMed] [Google Scholar]
- Traniello S., Pontremoli S., Tashima Y., Horecker B. L. Fructose 1, 6-diphosphatase from liver: isolation of the native form with optimal activity at neutral pH. Arch Biochem Biophys. 1971 Sep;146(1):161–166. doi: 10.1016/s0003-9861(71)80052-8. [DOI] [PubMed] [Google Scholar]



