Abstract
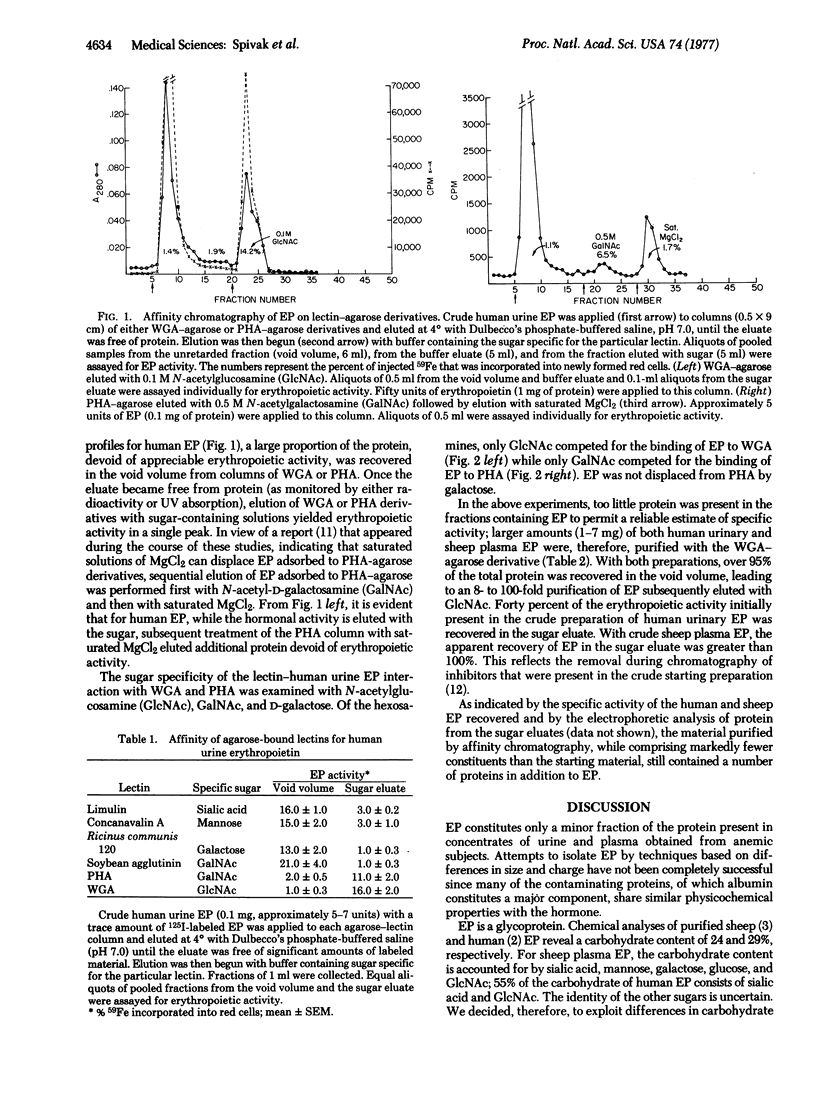
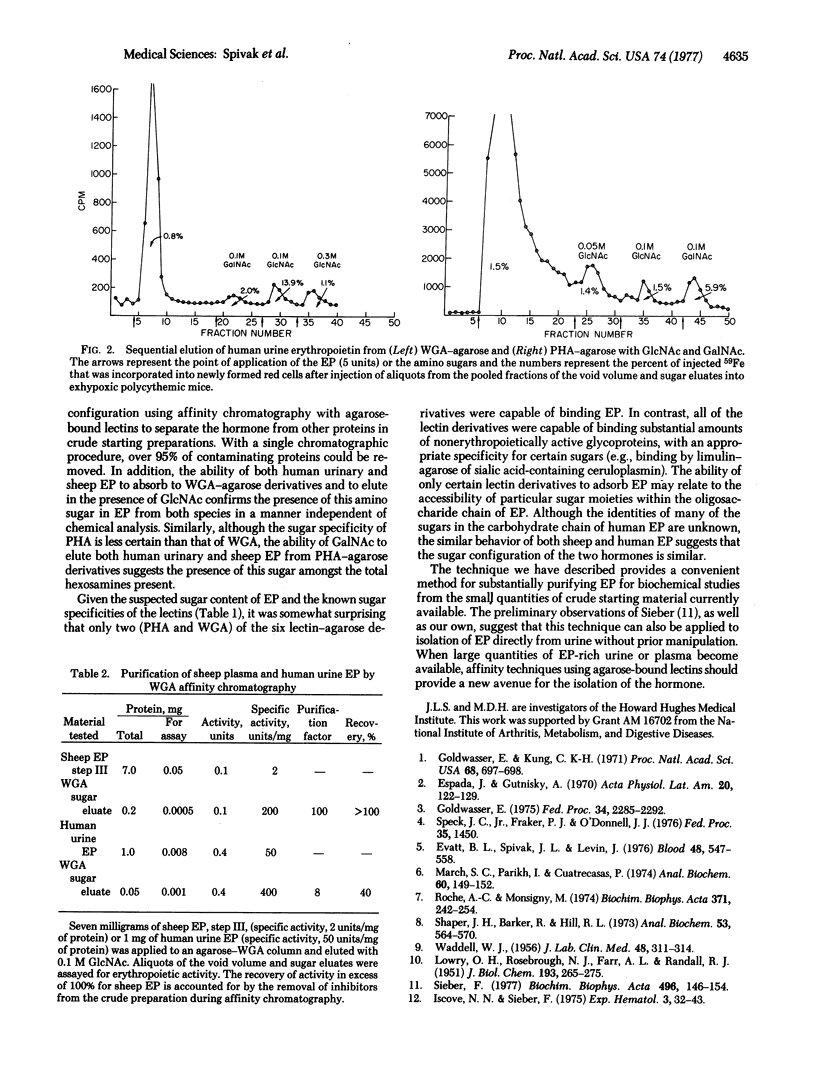
Affinity chromatography using agarose-bound lectins was used to isolate erythropoietin from crude preparations of sheep plasma and human urinary erythropoietin. On the basis of previous estimates of the sugar content of the hormone, six lectins (wheat germ agglutinin, phytohemagglutinin, Ricinus communis 120, soybean agglutinin, concanavalin A, and limulin) were chosen for study. Only wheat germ agglutinin-agarose and phytohemagglutinin-agarose derivatives had significant affinity for erythropoietin. By use of wheat germ aggutinin-agarose columns, erythropoietin could be separated from over 95% of the initial starting protein, resulting in an 8-to 100-fold purification and a recovery of at least 40% depending on the source of the hormone. Affinity chromatography with agarose-bound lectins provides a simple rapid method for isolating erythropoietin from crude preparations of the hormone.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Espada J., Gutnisky A. Purificación de eritropoyetina urinaria humana. Acta Physiol Lat Am. 1970;20(2):122–129. [PubMed] [Google Scholar]
- Evatt B. L., Spivak J. L., Levin J. Relationships between thrombopoiesis and erythropoiesis: with studies of the effects of preparations of thrombopoietin and erythropoietin. Blood. 1976 Oct;48(4):547–558. [PubMed] [Google Scholar]
- Goldwasser E. Erythropoietin and the differentiation of red blood cells. Fed Proc. 1975 Dec;34(13):2285–2292. [PubMed] [Google Scholar]
- Goldwasser E., Kung C. K. Purification of erythropoietin. Proc Natl Acad Sci U S A. 1971 Apr;68(4):697–698. doi: 10.1073/pnas.68.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscove N. N., Sieber F. Erythroid progenitors in mouse bone marrow detected by macroscopic colony formation in culture. Exp Hematol. 1975 Jan;3(1):32–43. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Roche A. C., Monsigny M. Purification and properties of limulin: a lectin (agglutinin) from hemolymph of Limulus polyphemus. Biochim Biophys Acta. 1974 Nov 5;371(1):242–254. doi: 10.1016/0005-2795(74)90174-3. [DOI] [PubMed] [Google Scholar]
- Shaper J. H., Barker R., Hill R. L. Purification of wheat germ agglutinin by affinity chromatography. Anal Biochem. 1973 Jun;53(2):564–570. doi: 10.1016/0003-2697(73)90107-3. [DOI] [PubMed] [Google Scholar]
- Sieber F. Chromatography of human urinary erythropoietin and granulocyte colony-stimulating factor on insolubilized phytohaemagglutinin. Biochim Biophys Acta. 1977 Jan 24;496(1):146–154. doi: 10.1016/0304-4165(77)90122-2. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]


