Abstract
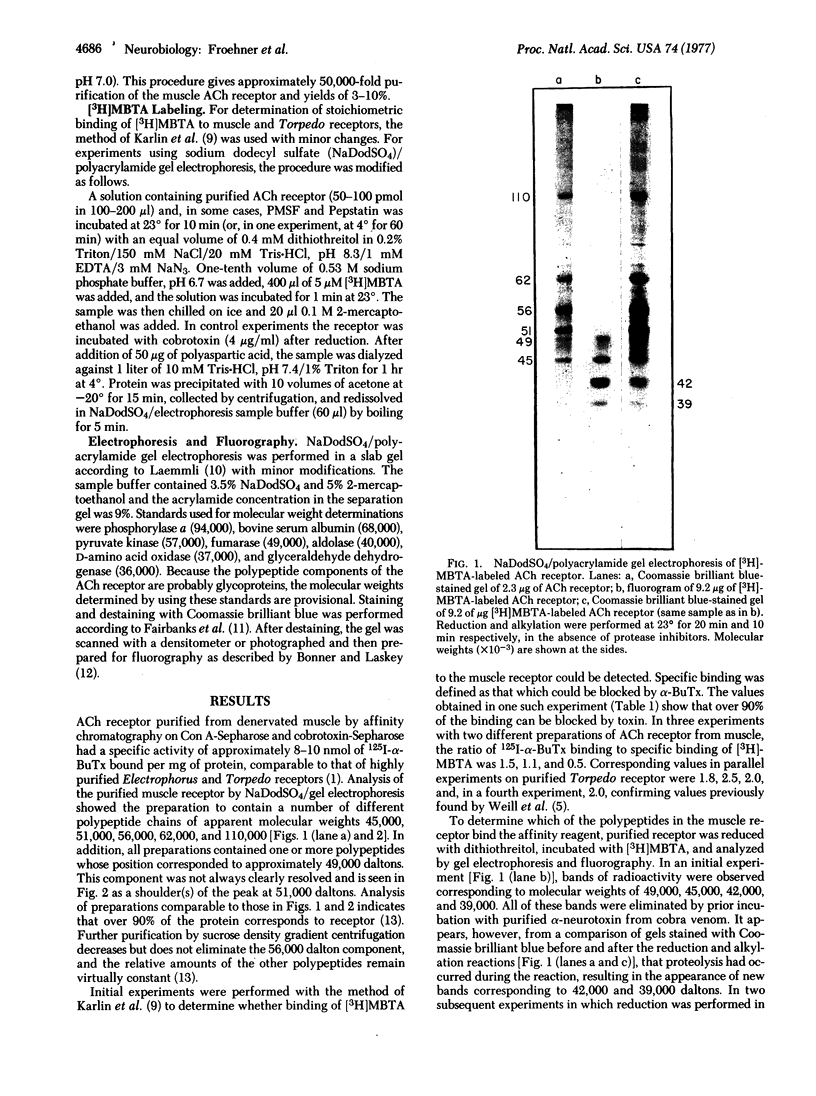
The acetylcholine receptor from denervated rat skeletal muscle was purified by affinity chromatography and, after reduction, was treated with the affinity alkylating agent 4-(N-maleimido)benzyltri[3H]methylammonium iodide. The receptor specifically incorporated approximately 1 mol of alkylating agent per mol of 125I-labeled alpha-bungarotoxin bound. Analysis of the labeled receptor by polyacrylamide gel electrophoresis in sodium dodecyl sulfate showed that two subunits were labeled; their apparent molecular weights were 45,000 and 49,000. These results suggest that the affinity reagent labels a second site for acetylcholine binding in the muscle receptor that is not labeled in receptors from Electrophorus or Torpedo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Haim D., Landau E. M., Silman I. The role of a reactive disulphide bond in the function of the acetylcholine receptor at the frog neuromuscular junction. J Physiol. 1973 Oct;234(2):305–325. doi: 10.1113/jphysiol.1973.sp010347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker G. Molecular properties of the cholinergic receptor purified from Electrophorus electricus. Biochemistry. 1973 Oct 23;12(22):4403–4409. doi: 10.1021/bi00746a017. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brockes J. P., Hall Z. W. Acetylcholine receptors in normal and denervated rat diaphragm muscle. I. Purification and interaction with [125I]-alpha-bungarotoxin. Biochemistry. 1975 May 20;14(10):2092–2099. doi: 10.1021/bi00681a008. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Benedetti L., Bourgeois J. P., Brisson A., Cartaud J., Devaux P., Grünhagen H., Moreau M., Popot J. L., Sobel A. Some structural properties of the cholinergic receptor protein in its membrane environmental relevant to its function as a pharmacological receptor. Cold Spring Harb Symp Quant Biol. 1976;40:211–230. doi: 10.1101/sqb.1976.040.01.023. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Eldefraw M. E., Eldefrawi M. E. Molecular and functional properties of the acetylcholine-receptor. Ann N Y Acad Sci. 1975 Dec 30;264:183–202. doi: 10.1111/j.1749-6632.1975.tb31483.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gordon A., Bandini G., Hucho F. Investigation of the Naja naja siamensis toxin binding site of the cholinergic receptor protein from Torpedo electric tissue. FEBS Lett. 1974 Oct 15;47(2):204–208. doi: 10.1016/0014-5793(74)81012-4. [DOI] [PubMed] [Google Scholar]
- Karlin A., Cowburn D. The affinity-labeling of partially purified acetylcholine receptor from electric tissue of Electrophorus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3636–3640. doi: 10.1073/pnas.70.12.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A., McNamee M. G., Cowburn D. A. Assay of the acetylcholine receptor by affinity labeling. Anal Biochem. 1976 Dec;76(2):442–451. doi: 10.1016/0003-2697(76)90339-0. [DOI] [PubMed] [Google Scholar]
- Karlin A., Prives J., Deal W., Winnik M. Affinity labeling of the acetylcholine receptor in the electroplax. J Mol Biol. 1971 Oct 14;61(1):175–188. doi: 10.1016/0022-2836(71)90214-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. M., Singer S. J., Lennox E. S. The effects of reducing and alkylating agents on the acetylcholine receptor activity of frog sartorius muscle. J Membr Biol. 1973 Feb 27;11(3):217–226. doi: 10.1007/BF01869823. [DOI] [PubMed] [Google Scholar]
- Raftery M. A., Vandlen R. L., Reed K. L., Lee T. Characterization of Torpedo californica acetylcholine receptor: its subunit composition and ligand-binding properties. Cold Spring Harb Symp Quant Biol. 1976;40:193–202. doi: 10.1101/sqb.1976.040.01.021. [DOI] [PubMed] [Google Scholar]
- Reiter M. J., Cowburn D. A., Prives J. M., Karlin A. Affinity labeling of the acetylcholine receptor in the electroplax: electrophoretic separtion in sodium dodecyl sulfate. Proc Natl Acad Sci U S A. 1972 May;69(5):1168–1172. doi: 10.1073/pnas.69.5.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. M., Wassarman P. M. [3H]Dansyl chloride: a useful reagent for the quantitation and molecular weight determination of nanogram amounts of protein. Anal Biochem. 1977 Jan;77(1):25–32. doi: 10.1016/0003-2697(77)90286-x. [DOI] [PubMed] [Google Scholar]
- Silman I., Karlin A. Acetylcholine receptor: covalent attachment of depolarizing groups at the active site. Science. 1969 Jun 20;164(3886):1420–1421. doi: 10.1126/science.164.3886.1420. [DOI] [PubMed] [Google Scholar]
- Weill C. L., McNamee M. G., Karlin A. Affinity-labeling of purified acetylcholine receptor from Torpedo californica. Biochem Biophys Res Commun. 1974 Dec 11;61(3):997–1003. doi: 10.1016/0006-291x(74)90254-x. [DOI] [PubMed] [Google Scholar]



