Abstract
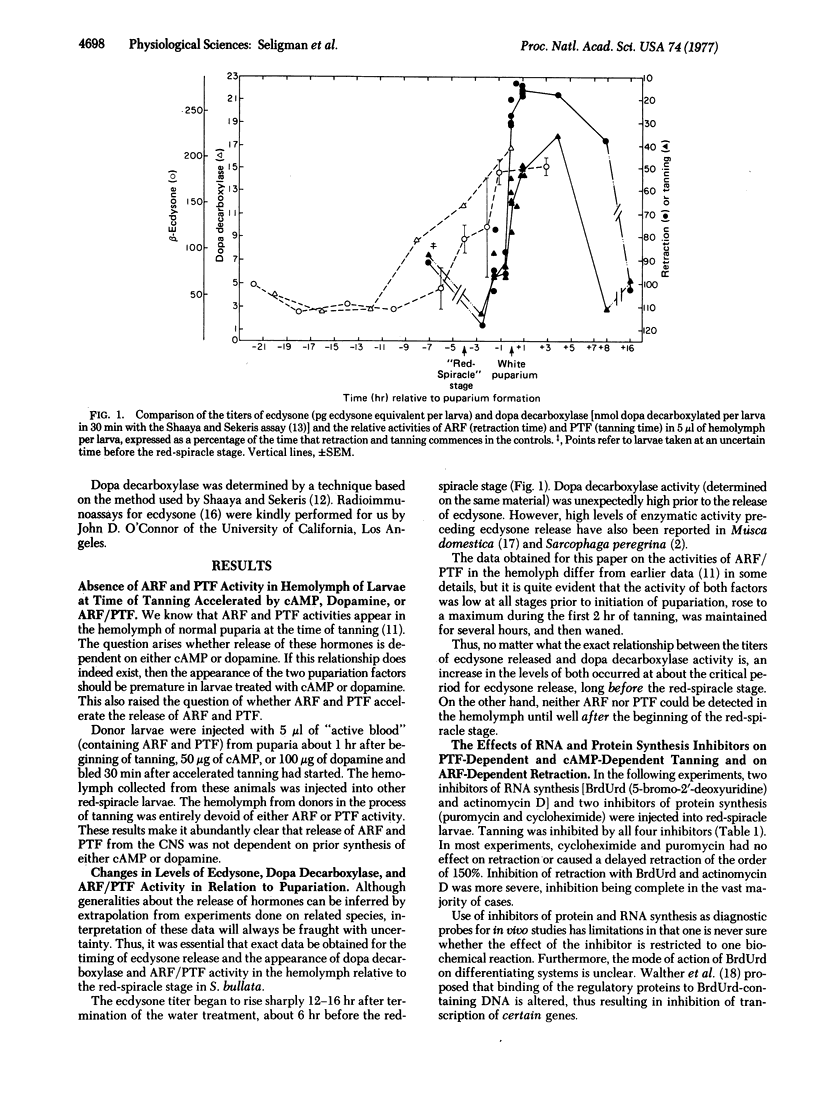
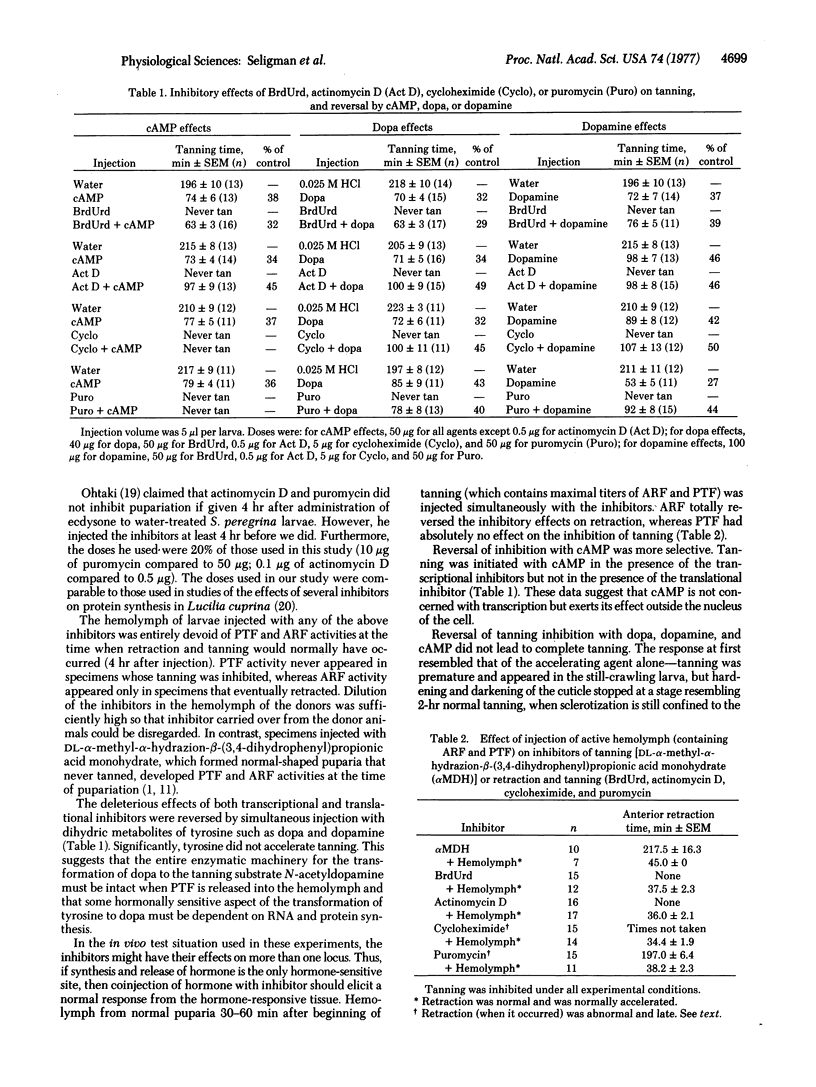
Two pupariation factors, anterior retraction factor (ARF) and puparium tanning factor (PTF), are absent from the hemolymph of larvae at the time of tanning accelerated by ARF/PTF, cyclic AMP, or dopamine. ARF and PTF are not involved in derepression of dopa decarboxylase (aromatic L-amino-acid decarboxylase, aromatic L-amino-acid carboxy-lyase, EC 4.1.1.28) synthesis initiated by ecdysone. Tanning is entirely inhibited by injection of two transcriptional inhibitors, actinomycin and BrdUrd, and two translational inhibitors, puromycin and cycloheximide. Retraction activity is more severely inhibited by the transcriptional than by the translational inhibitors. A tanning response is initiated by cyclic AMP in the presence of the transcriptional but not the translational inhibitors. Dihydric tanning substances (dopa, dopamine) initiate tanning in the presence of both types of inhibitors. Release of ARF and PTF from the central nervous system is inhibited by the four inhibitors. ARF totally reverses the inhibitory effects on retraction, whereas PTF does not reverse inhibition of tanning. These data are interpreted to mean that PTF is concerned with the regulation of two components of the tanning response: (i) acceleration of synthesis of a particular protein (associated with the tyrosine hydroxylation complex), and (ii) activation via cyclic AMP of a component of the tyrosine hydroxylating system.
Keywords: tyrosine hydroxylation, transcriptional and translational inhibitors, anterior retraction factor, pupariation tanning factor
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borst D. W., Bollenbacher W. E., O'Connor J. D., King D. S., Fristrom J. W. Ecdysone levels during metamorphosis of Drosophila melanogaster. Dev Biol. 1974 Aug;39(2):308–316. doi: 10.1016/0012-1606(74)90242-5. [DOI] [PubMed] [Google Scholar]
- Borst D. W., O'Connor J. D. Trace analysis of ecdysones by gas-liquid chromatography, radioimmunoassay and bioassay. Steroids. 1974 Nov;24(5):637–656. doi: 10.1016/0039-128x(74)90017-8. [DOI] [PubMed] [Google Scholar]
- Campbell A. J., Birt L. M. The effect of inhibitors in vivo on protein synthesis and the amino acid pool in the sheep blowfly, Lucilia cuprina. Biochem J. 1975 Aug;150(2):227–234. doi: 10.1042/bj1500227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel G., Blechl A., Blechl J., Herman P., Seligman M. I. 3':5'-cyclic AMP and hormonal control of puparium formation in the fleshfly Sarcophaga bullata. Proc Natl Acad Sci U S A. 1977 May;74(5):2182–2186. doi: 10.1073/pnas.74.5.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoulis E. G., Sekeris C. E. Translation of mRNA for 3,4-dihydroxyphenylalanine decarboxylase isolated from epidermis tissue of Calliphora vicina R. -D. in a heterologous system. Dependence of mRNA concentration on the insect steroid hormone ecdysone. Eur J Biochem. 1975 Feb 3;51(1):305–316. doi: 10.1111/j.1432-1033.1975.tb03930.x. [DOI] [PubMed] [Google Scholar]
- KARLSON P., SCHWEIGER A. [On tyrosine metabolism by insects. IV. The phenol oxidase system of Calliphora and its modification by the hormone ecdysone]. Hoppe Seylers Z Physiol Chem. 1961 May 3;323:199–210. doi: 10.1515/bchm2.1961.323.1.199. [DOI] [PubMed] [Google Scholar]
- SHAAYA E., SEKERIS C. E. ECDYSONE DURING INSECT DEVELOPMENT. 3. ACTIVITIES OF SOME ENZYMES OF TYROSINE METABOLISM IN COMPARISON WITH ECDYSONE TITER DURING THE DEVELOPMENT OF THE BLOWFLY, CALLIPHORA ERYTHROCEPHIA MEIG. Gen Comp Endocrinol. 1965 Feb;5:35–39. doi: 10.1016/0016-6480(65)90066-3. [DOI] [PubMed] [Google Scholar]
- Sivasubramanian P., Friedman S., Fraenkel G. Nature and role of proteinaceous hormonal factors acting during puparium formation in flies. Biol Bull. 1974 Aug;147(1):163–185. doi: 10.2307/1540576. [DOI] [PubMed] [Google Scholar]
- Walther B. T., Pictet R. L., David J. D., Rutter W. J. On the mechanism of 5-bromodeoxyuridine inhibition of exocrine pancreas differentiation. J Biol Chem. 1974 Mar 25;249(6):1953–1964. [PubMed] [Google Scholar]
- Zdarek J., Fraenkel G. Overt and covert effects of endogenous and exogenous ecdysone in puparium formation of flies. Proc Natl Acad Sci U S A. 1970 Sep;67(1):331–337. doi: 10.1073/pnas.67.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]


