Abstract
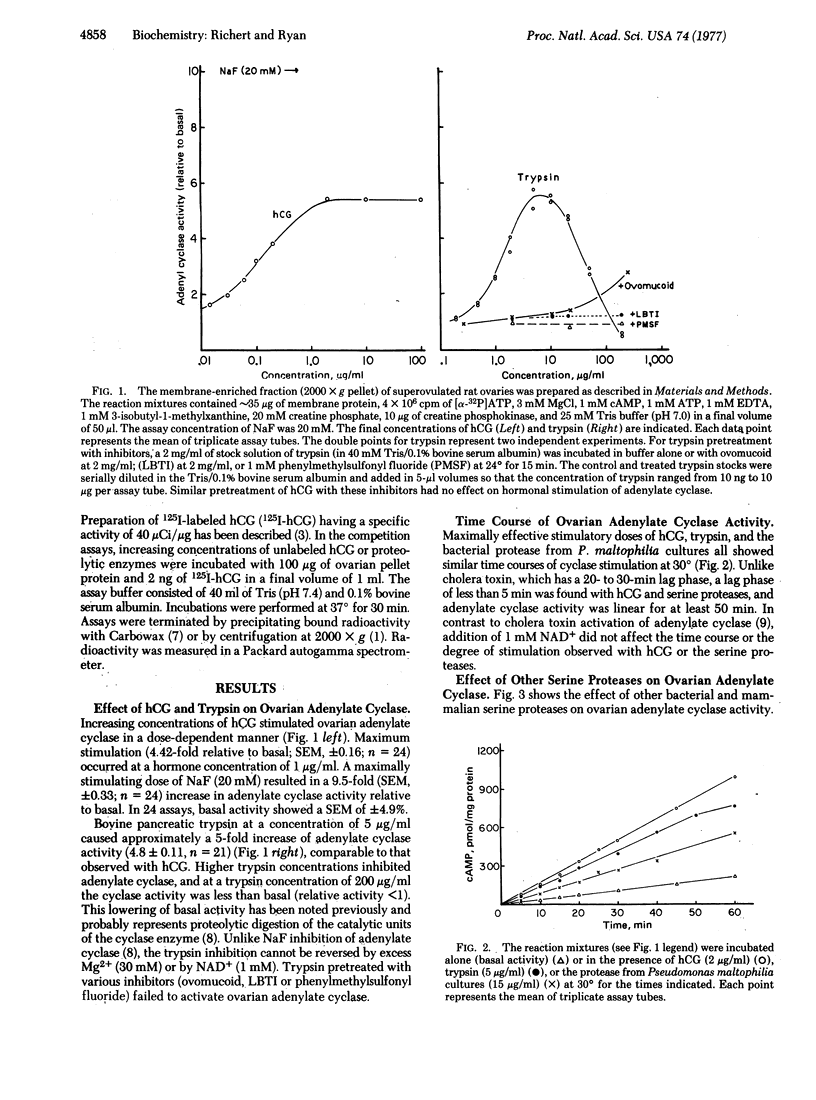
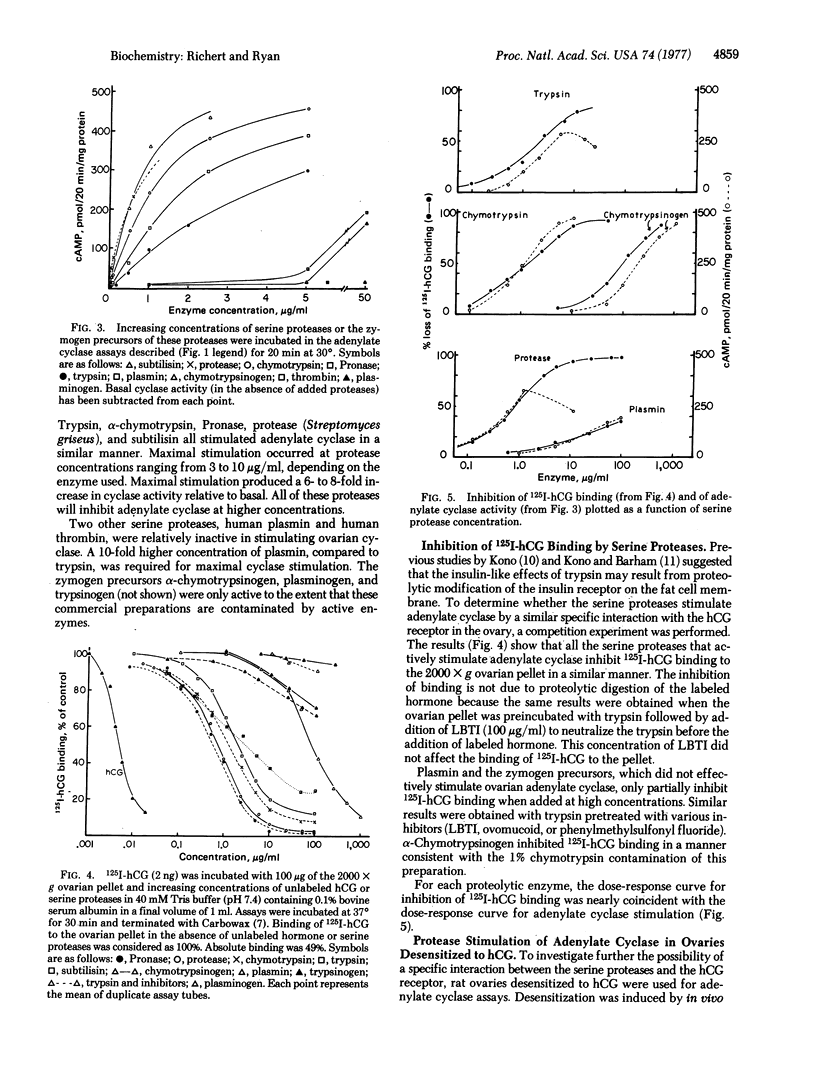
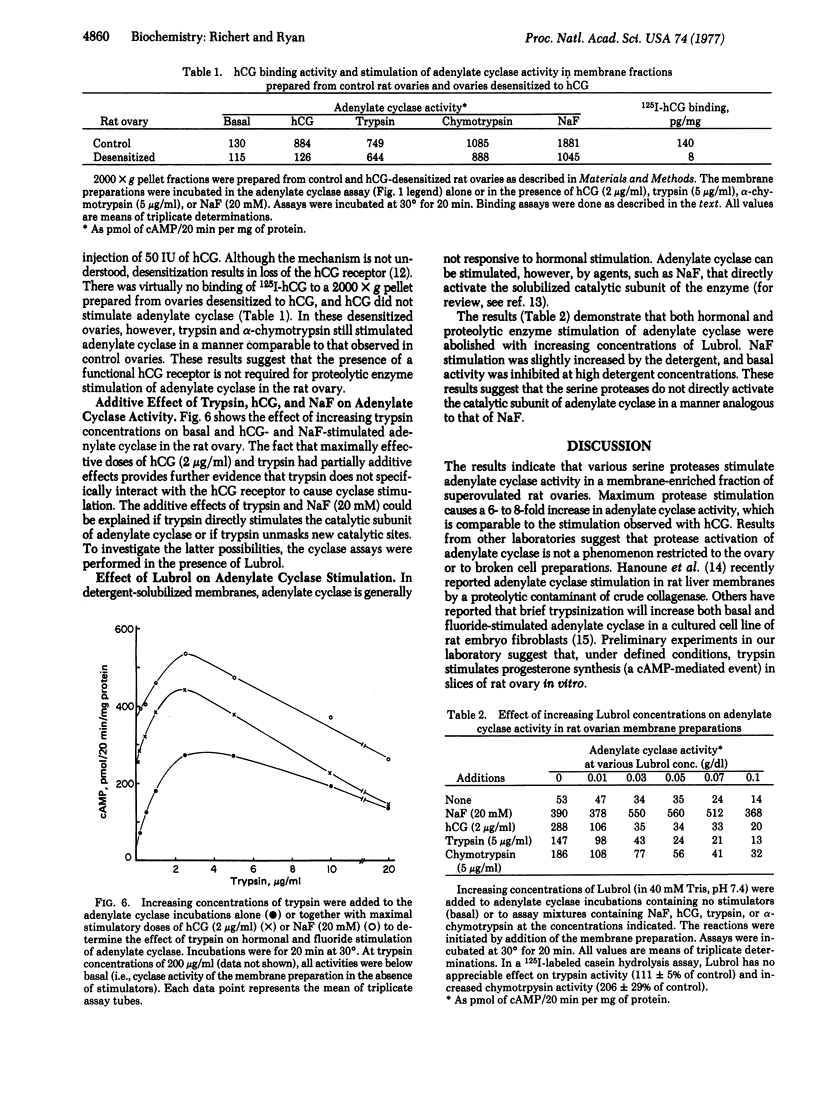
Various serine proteases (e.g., trypsin, α-chymotrypsin, Pronase, and subtilisin) stimulate adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] activity in a membrane-enriched fraction of the rat ovary. Maximum stimulation is observed at protease concentrations ranging from 3 to 10 μg/ml. Higher protease concentrations inhibit ovarian adenylate cyclase in a dose-dependent manner. Protease stimulation causes a 6- to 8-fold increase in adenylate cyclase activity, which is comparable to the stimulation observed with human chorionic gonadotropin. Combinations of trypsin plus hormone or trypsin plus NaF stimulate ovarian adenylate cyclase activity to a greater extent than does any one of these alone.
The mechanism of protease stimulation of adenylate cyclase involves limited proteolysis because zymogen precursors fail to activate the cyclase as does trypsin pretreated with trypsin inhibitors. Unlike cholera toxin, the serine protease stimulation is immediate (within the first 5 min) and requires no additional factors (e.g., NAD+). It is unlikely that protease stimulation of adenylate cyclase results from a proteolytic modification of the hormone receptor on the cell surface, because of the additive effects noted above and because protease stimulation is also observed in ovaries desensitized to hormone that lack this hormone receptor. Results with Lubrol-treated membranes also suggest that proteolytic enzymes do not directly activate the catalytic subunit of the cyclase or unmask new catalytic sites because the protease effect (like hormonal stimulation) is abolished by the detergent, whereas fluoride stimulation is enhanced. Other data suggest that serine protease and chorionic gonadotropin stimulation of adenylate cyclase result from activation of a membrane protease that then regulates adenylate cyclase in the ovary.
Keywords: human chorionic gonadotropin, trypsin, serine proteases, cyclic AMP, luteinized rat ovaries
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L., Pohl S. L., Rodbell M. Adenyl cyclase in fat cells. 1. Properties and the effects of adrenocorticotropin and fluoride. J Biol Chem. 1969 Jul 10;244(13):3468–3476. [PubMed] [Google Scholar]
- Birnbaumer L., Yang P. C., Hunzicker-Dunn M., Bockaert J., Duran J. M. Adenylyl cyclase activities in ovarian tissues. I. Homogenization and conditions of assay in graafian follicles and corpora lutea of rabbits, rats, and pigs: regulation by ATP, and some comparative properties. Endocrinology. 1976 Jul;99(1):163–184. doi: 10.1210/endo-99-1-163. [DOI] [PubMed] [Google Scholar]
- Gill D. M. Involvement of nicotinamide adenine dinucleotide in the action of cholera toxin in vitro. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2064–2068. doi: 10.1073/pnas.72.6.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayzel A. I., Hatcher V. B., Lazarus G. S. Protease activity of normal and PHA stimulated human lymphocytes. Cell Immunol. 1975 Jul;18(1):210–219. doi: 10.1016/0008-8749(75)90049-0. [DOI] [PubMed] [Google Scholar]
- Hanoune J., Stengel D., Lacombe M. L., Feldmann G., Coudrier E. Proteolytic activation of rat liver adenylate cyclase by a contaminant of crude collagenase from Clostridium histolyticum. J Biol Chem. 1977 Mar 25;252(6):2039–2045. [PubMed] [Google Scholar]
- Kono T., Barham F. W. Insulin-like effects of trypsin on fat cells. Localization of the metabolic steps and the cellular site affected by the enzyme. J Biol Chem. 1971 Oct 25;246(20):6204–6209. [PubMed] [Google Scholar]
- Kono T. Destruction and restoration of the insulin effector system of isolated fat cells. J Biol Chem. 1969 Nov 10;244(21):5777–5784. [PubMed] [Google Scholar]
- Kurosky A., Markel D. E., Peterson J. W., Fitch W. M. Primary structure of cholera toxin beta-chain: a glycoprotein hormone analog? Science. 1977 Jan 21;195(4275):299–301. doi: 10.1126/science.831277. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee C. Y., Ryan R. J. Interaction of ovarian receptors with human luteinizing hormone and human chorionic gonadotropin. Biochemistry. 1973 Nov 6;12(23):4609–4615. doi: 10.1021/bi00747a011. [DOI] [PubMed] [Google Scholar]
- Lee C. Y. The porcine ovarian follicle: III. Development of chorionic gonadotropin receptors associated with increase in adenyl cyclase activity during follicle maturation. Endocrinology. 1976 Jul;99(1):42–48. doi: 10.1210/endo-99-1-42. [DOI] [PubMed] [Google Scholar]
- Richert N. D., Ryan R. J. Protease inhibitors block hormonal activation of adenylate cyclase. Biochem Biophys Res Commun. 1977 Sep 23;78(2):799–805. doi: 10.1016/0006-291x(77)90250-9. [DOI] [PubMed] [Google Scholar]
- Richert N. D., Ryan R. J. Specific gonadotropin binding to Pseudomonas maltophilia. Proc Natl Acad Sci U S A. 1977 Mar;74(3):878–882. doi: 10.1073/pnas.74.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan W. L., Short N. A., Curtis G. L. Adenylate cyclase stimulation by trypsin. Proc Soc Exp Biol Med. 1975 Dec;150(3):699–702. doi: 10.3181/00379727-150-39109. [DOI] [PubMed] [Google Scholar]
- Saito M., Aoyagi T., Umezawa H., Nagai Y. Bestatin, a new specific inhibitor of aminopeptidases, enhances activation of small lymphocytes by concanavalin A. Biochem Biophys Res Commun. 1976 May 23;76(2):526–533. doi: 10.1016/0006-291x(77)90756-2. [DOI] [PubMed] [Google Scholar]
- Saito M., Yoshizawa T., Aoyagi T., Nagai Y. Involvement of proteolytic acivity in early events in lymphocyte transformation by phytohemagglutinin. Biochem Biophys Res Commun. 1973 May 15;52(2):569–575. doi: 10.1016/0006-291x(73)90750-x. [DOI] [PubMed] [Google Scholar]


