Abstract
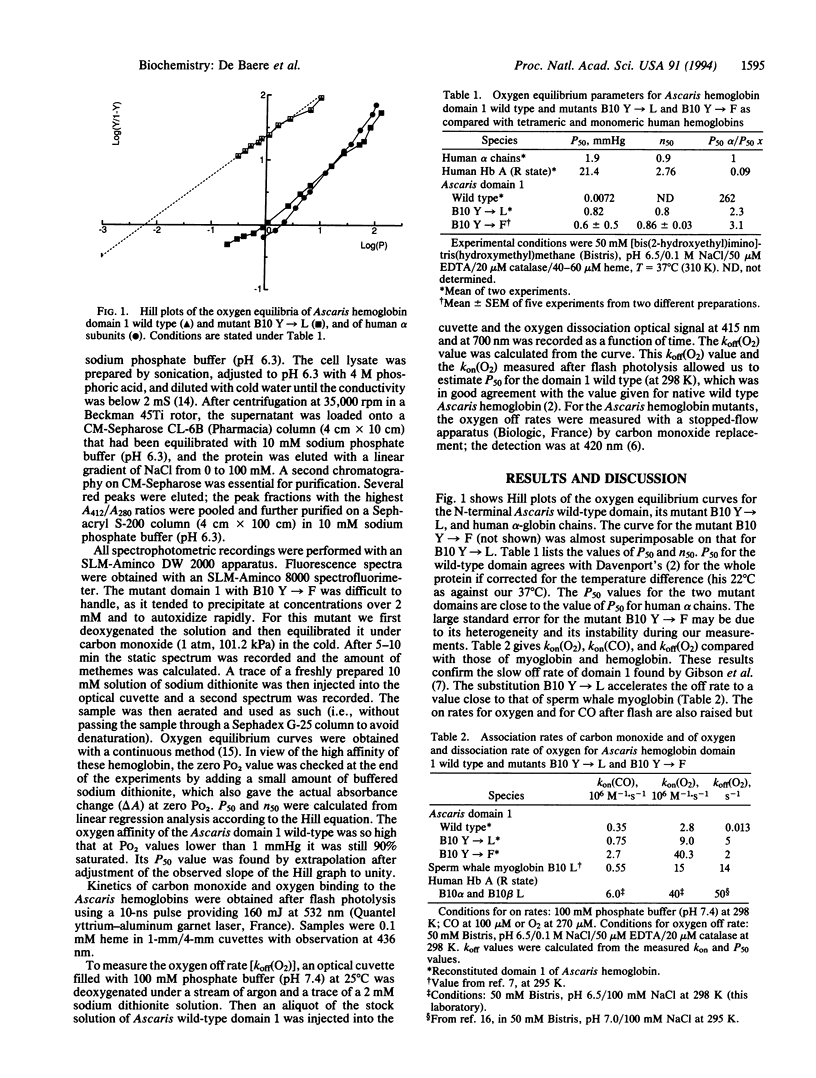
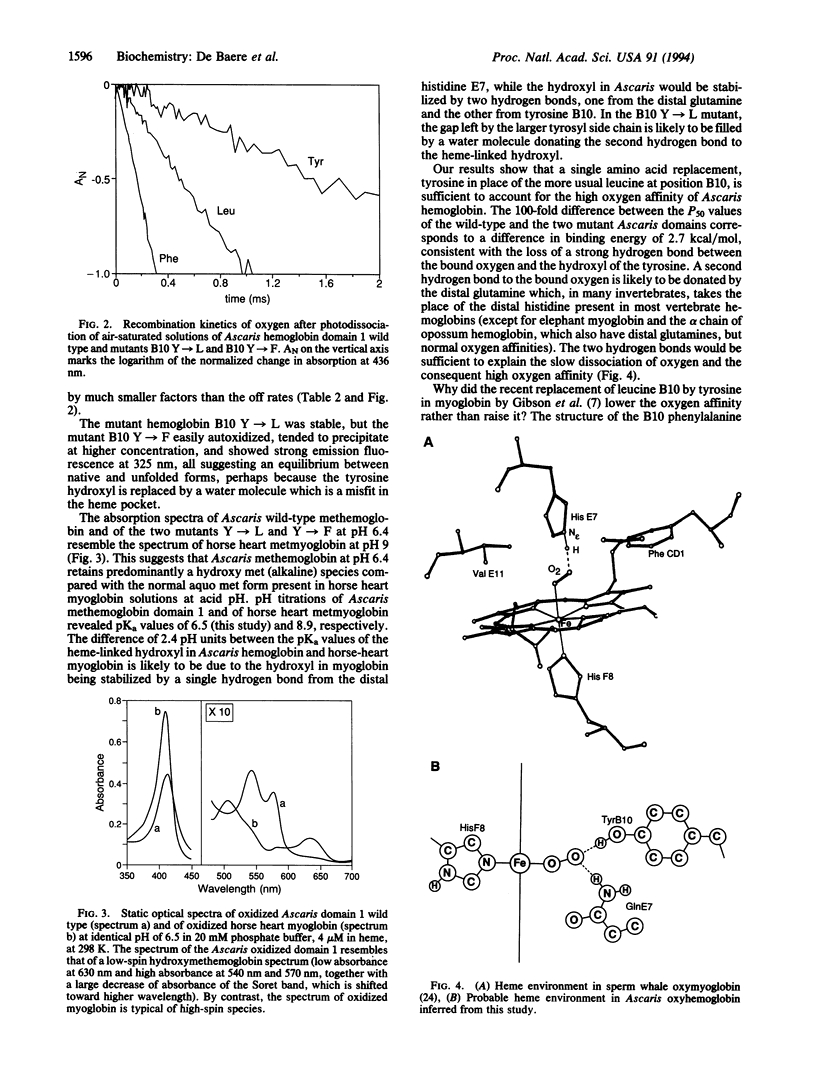
We have tried to find out why Ascaris hemoglobin has such an exceptionally high oxygen affinity (P50 approximately 0.004 mmHg; 1 mmHg = 133 Pa). Following Kloek et al., we have synthesized the N-terminal globin domain of Ascaris hemoglobin in Escherichia coli [Kloek, A. P., Yang, J., Mathews, F. S. & Goldberg, D. (1993) J. Biol. Chem. 268, 17669-17671]. Like Kloek et al., we found its oxygen affinity to be as high as that of native Ascaris hemoglobin. We thought that this high affinity might be due to the heme-bound oxygen molecule being stabilized by two hydrogen bonds from the globin instead of the usual one. Ascaris hemoglobin has a distal glutamine instead of the more usual histidine as one of the potential hydrogen bond donors. In addition, it contains a tyrosine at position 10 of B helix (B10) in place of the leucine generally found there in vertebrate myoglobins and hemoglobins. Following the discovery of Carver et al. that sperm whale myoglobin with the replacement of leucine B10 by phenylalanine has a raised oxygen affinity, we have replaced tyrosine B10 in the N-terminal domain of Ascaris hemoglobin by either leucine or phenylalanine [Carver, T. E., Brantley, R. E., Jr., Singleton, E. W., Arduini, R. M., Quillin, H. L., Phillips, G. N., Jr., & Olson, J. S. (1992) J. Biol. Chem. 267, 14443-14450]. Either of these replacements lowered the oxygen affinity about 100-fold, to the same level of that of human alpha-globin chains. These results are consistent with a hydrogen bond linking the tyrosine hydroxyl to the heme-linked oxygen, with a bond energy of 2.7 kcal/mol.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carver T. E., Brantley R. E., Jr, Singleton E. W., Arduini R. M., Quillin M. L., Phillips G. N., Jr, Olson J. S. A novel site-directed mutant of myoglobin with an unusually high O2 affinity and low autooxidation rate. J Biol Chem. 1992 Jul 15;267(20):14443–14450. [PubMed] [Google Scholar]
- De Baere I., Liu L., Moens L., Van Beeumen J., Gielens C., Richelle J., Trotman C., Finch J., Gerstein M., Perutz M. Polar zipper sequence in the high-affinity hemoglobin of Ascaris suum: amino acid sequence and structural interpretation. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4638–4642. doi: 10.1073/pnas.89.10.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson Q. H., Regan R., Olson J. S., Carver T. E., Dixon B., Pohajdak B., Sharma P. K., Vinogradov S. N. Kinetics of ligand binding to Pseudoterranova decipiens and Ascaris suum hemoglobins and to Leu-29-->Tyr sperm whale myoglobin mutant. J Biol Chem. 1993 Aug 15;268(23):16993–16998. [PubMed] [Google Scholar]
- Gray R. D., Gibson Q. H. The effect of inositol hexaphosphate on the kinetics of CO and O 2 binding by human hemoglobin. J Biol Chem. 1971 Dec 10;246(23):7168–7174. [PubMed] [Google Scholar]
- Hockenhull-Johnson J. D., Stern M. S., Martin P., Dass C., Desiderio D. M., Wittenberg J. B., Vinogradov S. N., Walz D. A. The amino acid sequence of hemoglobin II from the symbiont-harboring clam Lucina pectinata. J Protein Chem. 1991 Dec;10(6):609–622. doi: 10.1007/BF01025713. [DOI] [PubMed] [Google Scholar]
- Hockenhull-Johnson J. D., Stern M. S., Wittenberg J. B., Vinogradov S. N., Kapp O. H., Walz D. A. The amino acid sequence of hemoglobin III from the symbiont-harboring clam Lucina pectinata. J Protein Chem. 1993 Jun;12(3):261–277. doi: 10.1007/BF01028189. [DOI] [PubMed] [Google Scholar]
- Kloek A. P., Yang J., Mathews F. S., Goldberg D. E. Expression, characterization, and crystallization of oxygen-avid Ascaris hemoglobin domains. J Biol Chem. 1993 Aug 25;268(24):17669–17671. [PubMed] [Google Scholar]
- Komiyama N. H., Shih D. T., Looker D., Tame J., Nagai K. Was the loss of the D helix in alpha globin a functionally neutral mutation? Nature. 1991 Jul 25;352(6333):349–351. doi: 10.1038/352349a0. [DOI] [PubMed] [Google Scholar]
- Kraus D. W., Wittenberg J. B. Hemoglobins of the Lucina pectinata/bacteria symbiosis. I. Molecular properties, kinetics and equilibria of reactions with ligands. J Biol Chem. 1990 Sep 25;265(27):16043–16053. [PubMed] [Google Scholar]
- Kraus D. W., Wittenberg J. B., Lu J. F., Peisach J. Hemoglobins of the Lucina pectinata/bacteria symbiosis. II. An electron paramagnetic resonance and optical spectral study of the ferric proteins. J Biol Chem. 1990 Sep 25;265(27):16054–16059. [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Mullis K., Faloona F., Scharf S., Saiki R., Horn G., Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):263–273. doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- Olson J. S., Mathews A. J., Rohlfs R. J., Springer B. A., Egeberg K. D., Sligar S. G., Tame J., Renaud J. P., Nagai K. The role of the distal histidine in myoglobin and haemoglobin. Nature. 1988 Nov 17;336(6196):265–266. doi: 10.1038/336265a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Staden R., Moens L., De Baere I. Polar zippers. Curr Biol. 1993 May 1;3(5):249–253. doi: 10.1016/0960-9822(93)90174-m. [DOI] [PubMed] [Google Scholar]
- Phillips S. E. Structure and refinement of oxymyoglobin at 1.6 A resolution. J Mol Biol. 1980 Oct 5;142(4):531–554. doi: 10.1016/0022-2836(80)90262-4. [DOI] [PubMed] [Google Scholar]
- Quillin M. L., Arduini R. M., Olson J. S., Phillips G. N., Jr High-resolution crystal structures of distal histidine mutants of sperm whale myoglobin. J Mol Biol. 1993 Nov 5;234(1):140–155. doi: 10.1006/jmbi.1993.1569. [DOI] [PubMed] [Google Scholar]
- Rohlfs R. J., Mathews A. J., Carver T. E., Olson J. S., Springer B. A., Egeberg K. D., Sligar S. G. The effects of amino acid substitution at position E7 (residue 64) on the kinetics of ligand binding to sperm whale myoglobin. J Biol Chem. 1990 Feb 25;265(6):3168–3176. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman D. R., Kloek A. P., Krishnan B. R., Guinn B., Goldberg D. E. Ascaris hemoglobin gene: plant-like structure reflects the ancestral globin gene. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11696–11700. doi: 10.1073/pnas.89.24.11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]


